Understanding the Fate of Applied Nitrogen in Pine Plantations of the Southeastern United States Using 15N Enriched Fertilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Site Description
2.3. Fertilizer Treatments
2.4. Field Sampling
2.5. Laboratory Procedures
2.6. Calculation of Fertilizer N Recovery
2.7. Statistical Analysis
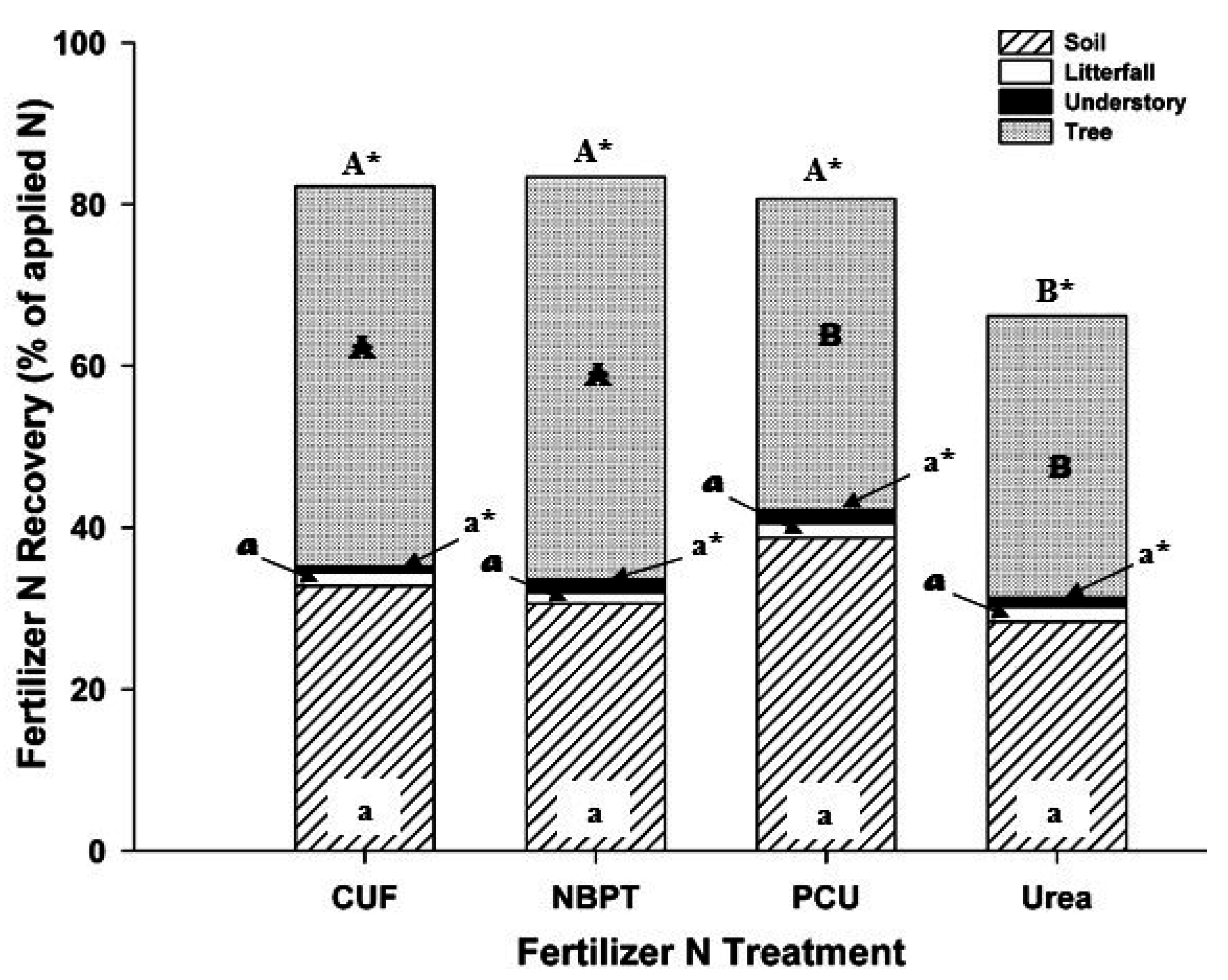
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schultz, R.P. The Ecology and Culture of Loblolly Pine (Pinus taeda L.); Agriculture Handbook #713; USDA Forest Service: Washington DC, USA, 1997; p. 493.
- US Department of Agriculture (USDA), Forest Service. Future of America’s Forest and Rangelands: Forest Service 2010 Resources Planning Act Assessment; US Department of Agriculture (USDA), Forest Service: Washington DC, USA, 2012; p. 198. Available online: http://www.fs.fed.us/research/publications/gtr/gtr_wo87.pdf (accessed on 5 February 2016).
- Fox, T.R.; Jokela, E.J.; Allen, H.L. The development of pine plantation silviculture in the southern United States. J. For. 2007, 105, 337–347. [Google Scholar]
- Allen, H.L. Forest fertilizers: Nutrient amendment, and productivity, and environmental impact. J. For. 1987, 85, 37–46. [Google Scholar]
- Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Tree nutrition and forest fertilization of pine plantations in the southern United States. South. J. Appl. For. 2007, 31, 5–11. [Google Scholar]
- Miller, H.G. Forest fertilization: Some guiding concepts. Forestry 1981, 54, 157–167. [Google Scholar] [CrossRef]
- Chapin, F.S.; Vitousek, P.M.; Van Cleve, K. The nature of nutrient limitation in plant-communities. Am. Nat. 1986, 127, 48–58. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea—How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Linder, S. Responses to water and nutrients in coniferous ecosystems. In Potentials and Limitations of Ecosystems Analysis; Schulze, E.D., Wolfer, H.Z., Eds.; Springer-Verlag: New York, NY, USA, 1987; pp. 180–202. [Google Scholar]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.L.; Nelson, L.E. Nutrient accumulation and cycling in loblolly pine (Pinus taeda L.) plantation ecosystems: The first 20 years. Soil Sci. Soc. Am. Proc. 1972, 36, 143–147. [Google Scholar] [CrossRef]
- Wells, C.G.; Jorgensen, J.R. Effect of intensive harvesting on nutrient supply and sustained productivity. In Proceedings of the Impacts of Intensive Harvesting on Forest Nutrient Cycling, Syracuse, NY, USA, 1979; pp. 212–230.
- Vitousek, P.M.; Matson, P.A. Intensive harvesting and site preparation decrease soil nitrogen availability in young plantations. South. J. Appl. For. 1985, 9, 120–125. [Google Scholar]
- Fox, T.R.; Burger, J.A.; Kreh, R.E. Effects of site preparation on nitrogen dynamics in the southern Piedmont. For. Ecol. Manag. 1986, 15, 241–256. [Google Scholar] [CrossRef]
- Birk, E.M.; Vitousek, P.M. Nitrogen availability and nitrogen use efficiency in loblolly pine stands. Ecology 1986, 67, 69–79. [Google Scholar] [CrossRef]
- Pritchett, W.L.; Smith, W.H. Fertilizer response in young slash pine stands. Soil Sci. Soc. Am. J. 1972, 36, 660–663. [Google Scholar] [CrossRef]
- Martin, S.W.; Bailey, R.L.; Jokela, E.J. Growth and yield predictions for lower Coastal Plain slash pine plantations fertilized at mid-rotation. South. J. Appl. For. 1999, 23, 39–45. [Google Scholar]
- Amateis, R.L.; Liu, J.; Ducey, M.J.; Allen, H.L. Modeling response to midrotation nitrogen and phosphorus fertilization in loblolly pine plantations. South. J. Appl. For. 2000, 24, 207–212. [Google Scholar]
- Carlson, C.A.; Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.A.; Stape, J.L. Growth responses of loblolly pine in the southeast united states to midrotation applications of nitrogen, phosphorus, potassium, and micronutrients. For. Sci. 2014, 60, 157–169. [Google Scholar] [CrossRef]
- Rojas, J.C. Factors influencing responses of loblolly pine stands to fertilization. Ph.D. Dissertation, North Carolina State University, Raleigh, NC, USA, 2005. [Google Scholar]
- Mead, D.J.; Pritchett, W.L. Fertilizer movement in a slash pine ecosystem. Plant Soil 1975, 43, 451–465. [Google Scholar] [CrossRef]
- Melin, J.; Nommik, H.; Lohm, U.; Flower-Ellis, J. Fertilizer nitrogen budget in a Scots pine ecosystem attained by using root-isolated plots and 15N technique. Plant Soil 1983, 74, 249–263. [Google Scholar] [CrossRef]
- Johnson, D.W.; Todd, D.E. Nitrogen fertilization of young yellow poplar and loblolly pine plantations at differing frequencies. Soil Sci. Soc. Am. J. 1988, 52, 1468–1477. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Allen, H.L.; Dougherty, P.M.; Johnsen, K.H. Long term growth responses of loblolly pine to optimal nutrient and water resource availability. For. Ecol. Manag. 2004, 192, 3–19. [Google Scholar] [CrossRef]
- Blazier, M.A.; Hennessey, T.C.; Dougherty, P.; Campbell, R. Nitrogen accumulation and use by a young loblolly pine plantation in southeast Oklahoma: Effects of fertilizer formulation and date of application. South. J. Appl. For. 2006, 30, 66–78. [Google Scholar]
- Raison, R.J.; Khanna, P.K.; Connell, M.J.; Falkiner, R.A. Effects of water availability and fertilization on nitrogen cycling in a stand of Pinus radiate. For. Ecol. Manag. 1990, 30, 31–43. [Google Scholar] [CrossRef]
- Kiser, L.C.; Fox, T.R. Soil accumulation of nitrogen and phosphorus following annual fertilization of loblolly pine and sweetgum on sandy sites. Soil Sci. Soc. Am. J. 2012, 76, 2278–2288. [Google Scholar] [CrossRef]
- Villanueva, A.T. Impacts of Fertilization on Soil Properties in Loblolly Pine Plantations in the Southeastern United States. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2015. [Google Scholar]
- Fisher, R.F.; Binkley, D. Ecology and Management of Forest Soils; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Flint, C.M. Leaching of Nitrogen from the Rooting Zone of Douglas-Fir Forests Following Urea Fertilization and Potential Impacts on the Water Quality of Hood Canal. Master’s Thesis, University of Washington, Seattle, WA, USA, 2007. [Google Scholar]
- Knowles, R.; Blackburn, T.H. Nitrogen Isotope Techniques; Academic Press, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Nadelhoffer, K.J.; Fry, B. Nitrogen isotope studies in forest ecosystems. In Stable Isotopes in Ecology and Environmental Science; Lajtha, K., Michener, R.H., Eds.; Blackwell Scientific Publications: New York, NY, USA, 1994; pp. 22–44. [Google Scholar]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; TuSource, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Hauck, R.D.; Bystrom, M. 15N-A Selected Bibliography for Agricultural Scientists; The Iowa State University Press: Ames, IA, USA, 1971; p. 206. [Google Scholar]
- Hauck, R.D. Nitrogen source requirements in different soil-plant systems. In Forest Fertilization: Theory and Practice; TVA National Fertilizer Development Center: Muscle Shoals, AL, USA, 1968; pp. 47–57. [Google Scholar]
- Currie, W.S.; Nadelhoffer, K.J. Dynamic redistribution of isotopically labeled cohorts of nitrogen inputs in two temperate forests. Ecosystems 1999, 2, 4–18. [Google Scholar] [CrossRef]
- Dinkelmeyer, H.; Lehmann, J.; Renck, A.; Trujillo, L.; Pereira da Silva, J., Jr.; Gebauer, G.; Kaiser, K. Nitrogen uptake from 15N-enriched fertilizer by four tree crops in an Amazonian agroforest. Agrofor. Syst. 2003, 57, 213–224. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Colman, B.P.; Currie, W.S.; Magill, A.; Aber, J.D. Decadal-scale fates of 15 N tracers added to oak and pine stands under ambient and elevated N inputs at the Harvard Forest (USA). For. Ecol. Manag. 2004, 196, 89–107. [Google Scholar] [CrossRef]
- Tietema, A.; Emmett, B.A.; Gundersen, P.; Kjonaas, O.J.; Koopmans, C.J. The fate of 15N-labelled nitrogen deposition in coniferous forest ecosystems. For. Ecol. Manag. 1998, 101, 19–27. [Google Scholar] [CrossRef]
- Templer, P.H.; Mack, M.C.; Chapin, F.S., III; Christenson, L.M.; Compton, J.E.; Crook, H.D.; Currie, W.S.; Curtis, C.J.; Dail, D.B.; D’Antonio, C.M.; et al. Sinks for nitrogen inputs in terrestrial ecosystems: A meta-analysis of 15N tracer field studies. Ecology 2012, 93, 1816–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.X.; Preston, C.M.; McCullough, K.; Weetman, G.; Barker, J. Effect of understory competition on distribution and recovery of 15N applied to a western red cedar—Western hemlock clear-cut site. Can. J. For. Res. 1996, 26, 313–321. [Google Scholar] [CrossRef]
- Bubb, K.A.; Xu, Z.H.; Simpson, J.A.; Saffigna, P.G. Growth response to fertilization and recovery of l5N-labelled fertilizer by young hoop pine plantations of subtropical Australia. Nutr. Cycling Agroecosystems 1999, 54, 81–92. [Google Scholar] [CrossRef]
- Werner, A.T. Nitrogen Release, Tree Uptake, and Ecosystem Retention in a Mid-Rotation Loblolly Pine Plantation Following Fertilization with 15N-Enriched Enhanced Efficiency Fertilizers. Master’s Thesis, Virginia Tech, Blacksburg, VA, USA, 2013. [Google Scholar]
- Zhang, X.; Mauzerall, D.L.; Davidson, E.A.; Kanter, D.R.; Cai, R. The economic and environmental consequences of implementing nitrogen-efficient technologies and management practices in agriculture. J. Environ. Qual. 2015, 44, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.L.; Kissel, D.E.; Vaio, N.; Craig, J.R.; Rema, J.A.; Morris, L.A. Loblolly pine needles retain urea fertilizer that can be lost as ammonia. Soil Sci. Soc. Am. J. 2005, 69, 1525–1531. [Google Scholar] [CrossRef]
- Zerpa, J.L.; Fox, T.R. Controls on volatile NH3 losses from loblolly pine plantations fertilized with urea in the southeast USA. Soil Sci. Soc. Am. J. 2011, 75, 257–266. [Google Scholar] [CrossRef]
- Elliot, J.R.; Fox, T.R. Ammonia volatilization following fertilization with urea or ureaform in a thinned loblolly pine plantation. Soil Sci. Soc. Am. J. 2014, 78, 1469–1473. [Google Scholar] [CrossRef]
- Raymond, J.E.; Fox, T.R.; Strahm, B.; Zerpa, J. Ammonia Volatilization following Nitrogen Fertilization with Enhanced Efficiency Fertilizers and Urea in Loblolly Pine Plantations of the Southern United States. For. Ecol. Manag. 2016, 376, 247–255. [Google Scholar] [CrossRef]
- Engel, R.; Jones, C.; Wallander, R. Ammonia volatilization from urea and mitigation by by NBPT following surface application to cold soils. Soil Sci. Soc. Am. J. 2011, 75, 2348–2357. [Google Scholar] [CrossRef]
- Shrestha, R.; Strahm, B.D.; Sucre, E.B. Nitrous oxide fluxes in fertilized Pinus taeda plantations across a gradient of soil drainage classes. J. Environ. Qual. 2014, 43, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Binkley, D.; Carter, R.; Allen, H.L. Nitrogen fertilization practices in forestry. In Nitrogen Fertilization and the Environment; Bacon, P., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 421–441. [Google Scholar]
- Aust, W.M.; Blinn, C.R. Forestry best management practices for timber harvesting and site preparation in the eastern United States: An overview of water quality and productivity research during past 20 years. Water Air Soil Poll. Focus 2004, 4, 5–36. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The Nitrogen Cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of Fertilizer Production and Use to Improve Nutrient Efficiency and Minimize Environmental Impacts. In Advances in Agronomy 102; Elsevier Inc.: Newark, NJ, USA, 2009; pp. 267–322. [Google Scholar]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An option for Enhancing Nutrient Use Efficiency in Agriculture; IFA, International Fertilizer Industry Association: Paris, France, 2010. [Google Scholar]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [PubMed]
- Fertilizer Institute. Available online: https://www.tfi.org/introduction-fertilizer/nutrient-science/enhanced-efficiency-fertilizers (accessed on 11 October 2015).
- AAPFCO. Association of American Plant Food Control Officials. 2011. Available online: http://www.aapfco.org/index.html (accessed on 1 October 2015).
- Shaviv, A. Controlled Release Fertilizers. IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt; International Fertilizer Industry Association: Paris, France, 2005. [Google Scholar]
- Hauck, R.D. Slow release and bio-inhibitor-amended nitrogen fertilizers. In Fertilizer Technology and Use, 3rd ed.; Engelstad, O.P., Ed.; SSSA: Madison, WI, USA, 1985; pp. 293–322. [Google Scholar]
- Goertz, H.M. Controlled Release Technology. In Kirk-Othmer Encyclopedia of Chemical Technology; Controlled Release Technology (Agricultural): New York, NY, USA, 1993; pp. 251–274. [Google Scholar]
- Gowariker, V.; Krishnamurthy, V.N.; Gowariker, S.; Dhanorkar, M.; Paranjape, K. The Fertilizer Encyclopedia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bremner, J.M.; Douglas, L.A. Inhibition of urease activity in soils. Soil Biol. Biochem. 1971, 3, 297–307. [Google Scholar] [CrossRef]
- Bremner, J.M.; Chai, H.S. Evaluation of N-butyl phosphorothioic triamide for retardation of urea hydrolysis in soil. Commun. Soil Sci. Plant Anal. 1986, 17, 337–351. [Google Scholar] [CrossRef]
- Högberg, P. 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Powlson, D.S.; Barraclough, D. Mineralization and assimilation in soil-plant systems. In Nitrogen Isotope Techniques; Academic Press, Inc.: New York, NY, USA, 1993; pp. 209–242. [Google Scholar]
- Nadelhoffer, K.J.; Downs, M.R.; Fry, B.; Aber, J.D.; Magill, A.H.; Melillo, J.M. The Fate of 15N-Labelled Nitrate Additions to a Northern Hardwood Forest in Eastern Maine, USA. Oecologia 1995, 103, 292–301. [Google Scholar] [CrossRef]
- Kissel, D.; Cabrera, M.; Vaio, N.; Craig, J.; Rema, J.; Morris, L. Rainfall timing and ammonia loss from urea in a loblolly pine plantation. Soil Sci. Soc. Am. J. 2004, 68, 1744–1750. [Google Scholar] [CrossRef]
- Kissel, D.; Cabrera, M.L.; Vaio, N.; Craig, J.R.; Rema, J.A.; Morris, L.A. Forest floor composition and ammonia loss from urea in a loblolly pine plantation. Soil Sci. Soc. Am. J. 2009, 73, 630–637. [Google Scholar] [CrossRef]
- Burke, I.C.; Lauenroth, W.K. Ecosystem ecology at regional scales. Ecology 2002, 83, 305–306. [Google Scholar] [CrossRef]
- Blanes, M.C.; Emmett, B.A.; Viñegla, B.; Carreira, J.A. Alleviation of P limitation makes tree roots competitive for N against microbes in a N-saturated conifer forest: A test through P fertilization and 15N labelling. Soil Biol. Biochem. 2012, 48, 51–59. [Google Scholar] [CrossRef]
- Preston, C.M. The availability of residual fertilizer nitrogen immobilized as clay-fixed ammonium and organic N. Can. J. Soil Sci. 1982, 62, 479–486. [Google Scholar] [CrossRef]
- Foster, N.W.; Beauchamp, E.G.; Corke, C.T. Reactions of 15N-labelled urea with Jack pine forest floor materials. Soil Biol. Biochem. 1985, 17, 699–703. [Google Scholar] [CrossRef]
- Foster, N.W.; Beauchamp, E.G.; Corke, C.T. Immobilization of 15N-labelled urea in a Jack pine forest floor. Soil Sci. Soc. Am. J. 1985, 49, 448–452. [Google Scholar] [CrossRef]
- Hulm, S.C.; Killham, K. Response over two growing seasons of a Sitka spruce stand to l5N-urea fertilizer. Plant Soil. 1990, 124, 65–72. [Google Scholar] [CrossRef]
- NÔmmik, H.; Larsson, K. Effects of nitrogen source and placement on fertilizer 15N enrichment in Pinus sylvestris foliage. Scand. J. For. Res. 1992, 7, 155–163. [Google Scholar] [CrossRef]
- Preston, C.M.; Mead, D.J. Growth response and recovery of 15N-fertilizer one and eight growing season after application to lodgepole pine in British Columbia. For. Ecol. Manag. 1994, 65, 219–229. [Google Scholar] [CrossRef]
- Swanston, C.W.; Preston, C.M. Availability of residual fertilizer 15N from forest floor and mineral soil to Douglas-fir seedlings ten years after fertilization. Plant Soil 2014, 381, 381–394. [Google Scholar] [CrossRef]
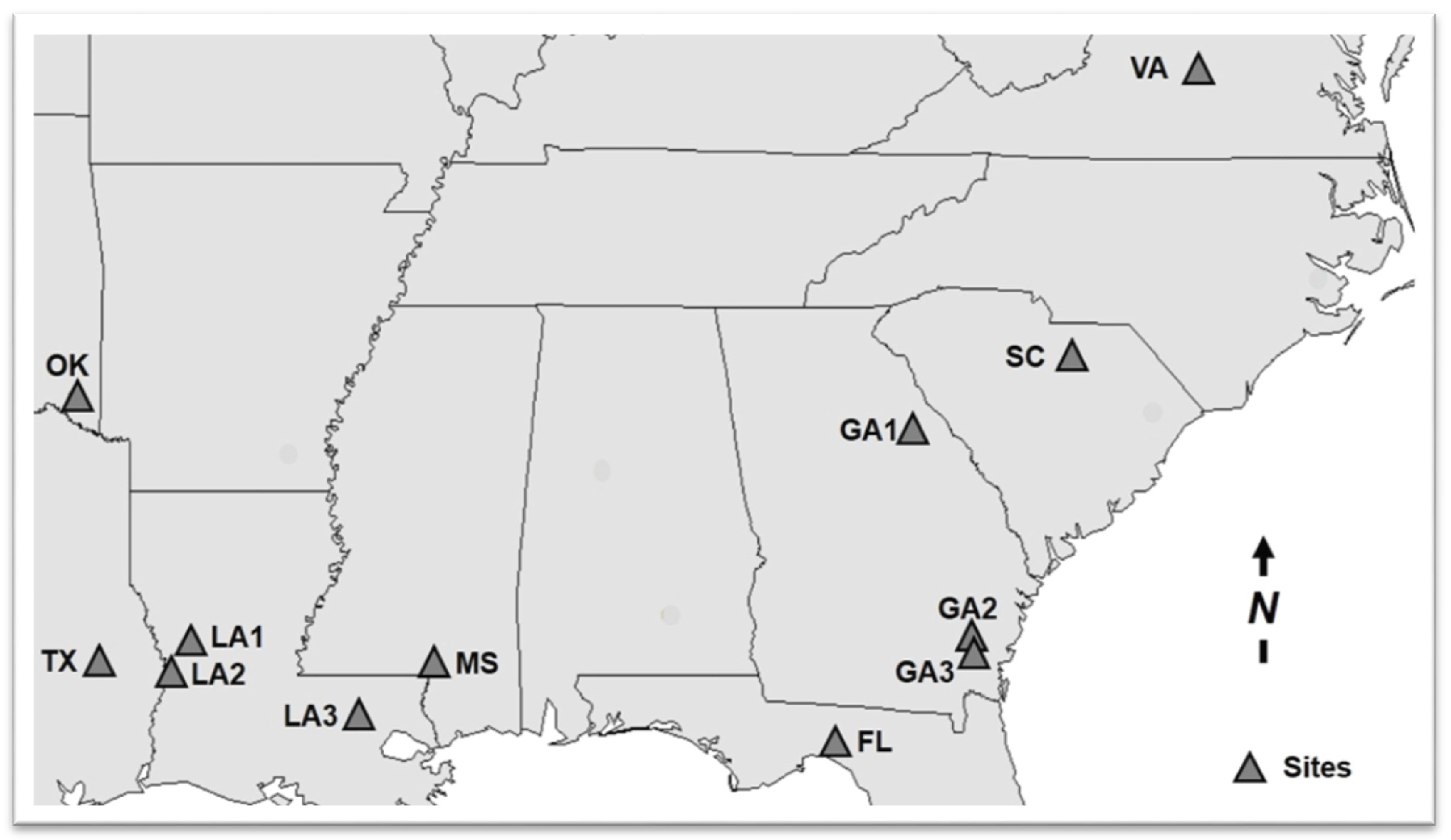
| State | Latitude | Longitude | Alt (m) | MAP (cm) | MAT (°C) | Physiographic Region | Soil Taxonomic Class | Drainage Class | Trees plot−1 | Trees ha−1 | Ht (m) | DBH (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VA | 37.445331 | 78.662917 | 60 | 109 | 13 | Piedmont | fine, mixed, subactive, mesic Typic Hapludults | Well | 8 | 880 | 9.1 | 15.1 |
| SC | 34.450000 | 80.505383 | 29 | 107 | 16 | Sandhills | thermic coated Typic Quartzipsamments | Excessively | 15 | 1560 | 12.5 | 16.1 |
| GA1 | 33.625317 | 82.801183 | 35 | 118 | 16 | Piedmont | fine kaolinitic thermic Rhodic Kandiudults | Well | 19 | 1800 | 7.2 | 8.6 |
| GA2 | 31.339978 | 81.857283 | 1 | 114 | 18 | Atlantic Coastal Plain | sandy siliceous thermic Aeric Alaquods | Poorly | 15 | 1460 | 14.7 | 16.5 |
| GA3 | 31.299333 | 81.847217 | 1 | 114 | 18 | Atlantic Coastal Plain | loamy, siliceous, subactive, thermic Arenic Paleaquults | Somewhat Poorly | 13 | 1340 | 13.6 | 15.1 |
| FL | 30.205267 | 83.866817 | 0.6 | 142 | 20 | Eastern Gulf Coastal Plain | loamy siliceous superactive thermic Aquic Arenic Hapludalfs | Somewhat Poorly | 16 | 1580 | 10.2 | 13.4 |
| MS | 31.066717 | 89.602467 | 26 | 152 | 19 | Western Gulf Coastal Plain | coarse loamy siliceous subactive thermic Typic Paleudults | Well | 21 | 2160 | 12.5 | 13.8 |
| LA1 | 31.337017 | 93.182783 | 28 | 147 | 19 | Western Gulf Coastal Plain | fine smectitic thermic Albaquic Hapludalfs | Moderately Well | 7 | 720 | 14.9 | 19.3 |
| LA2 | 31.013333 | 93.422600 | 28 | 147 | 19 | Western Gulf Coastal Plain | fine loamy siliceous subactive thermic Plinthic Paleudults | Well | 6 | 780 | 14.8 | 17.5 |
| LA3 | 30.560533 | 90.727650 | 0.9 | 160 | 19 | Western Gulf Coastal Plain | fine silty mixed active thermic Typic Glossaqualfs | Poorly | 23 | 2380 | 12.0 | 13.3 |
| OK | 34.029333 | 94.825017 | 42 | 136 | 16 | Western Gulf Coastal Plain | fine silty mixed active thermic Aquic Paleudalfs | Moderately well | 15 | 1580 | 3.0 | 4.0 |
| TX | 31.13255 | 94.462533 | 32 | 127 | 20 | Western Gulf Coastal Plain | fine loamy siliceous semiacitve thermic Oxyaquic Glossudalfs | Moderately well | 13 | 1360 | 13.9 | 11.2 |
| Ecosystem Component | Treatment | Fertilizer N Recovery (% of Applied N) | δ15N values (‰) | N Concentration (g·kg−1) |
|---|---|---|---|---|
| Foliage | Control | 0.0 a | −2.4 (0.5) a | 12.5 (0.4) a |
| CUF | 10.7 (1.1) bc | 118.3 (11.2) b | 14.1 (0.8) ab | |
| NBPT | 14.8 (1.8) c | 126.1 (8.5) b | 13.9 (0.6) ab | |
| PCU | 8.1 (1.1) b | 101.6 (9.8) b | 13.2 (0.5) ab | |
| Urea | 11.0 (1.7) bc | 124.5 (11.2) b | 14.2 (0.5) b | |
| Fine Branches | Control | 0.0 a | −2.0 (0.6) a | 5.1 (0.3) a |
| CUF | 3.2 (0.4) b | 110.2 (10.2) b | 7.3 (0.6) b | |
| NBPT | 4.1 (0.5) c | 111.8 (7.4) b | 6.7 (0.3) b | |
| PCU | 2.6 (0.5) b | 98.7 (10.5) b | 6.1 (0.5) ab | |
| Urea | 2.9 (0.5) b | 108.4 (9.67) b | 6.2 (0.4) ab | |
| Coarse Branches | Control | 0.0 a | −1.8 (0.4) a | 2.8 (0.2) a |
| CUF | 2.9 (0.4) bc | 72.6 (9.3) b | 3.7 (0.3) ab | |
| NBPT | 3.0 (0.6) c | 78.9 (8.2) b | 3.6 (0.4) ab | |
| PCU | 2.6 (0.4) b | 73.7 (8.6) b | 3.3 (0.3) ab | |
| Urea | 3.2 (1.0) bc | 69.3 (6.1) b | 3.9 (0.4) b | |
| Bark | Control | 0.0 a | −2.1 (0.4) a | 2.1 (0.2) a |
| CUF | 0.6 (0.2) b | 22.1 (2.1) b | 2.5 (0.2) ab | |
| NBPT | 0.8 (0.2) b | 22.5 (2.1) b | 2.9 (0.1) b | |
| PCU | 0.5 (0.1) b | 20.5 (2.4) b | 2.4 (0.1) a | |
| Urea | 0.4 (0.1) ab | 20.8 (2.1) b | 2.5 (0.2) ab | |
| Current year Growth Ring (CGR)- year of fertilization | Control | 0.0 a | −1.9 (0.4) a | 1.8 (0.1) a |
| CUF | 2.2 (0.4) bc | 80.0 (8.0) b | 2.4 (0.5) bc | |
| NBPT | 3.1 (0.6) c | 88.1 (5.8) b | 2.7 (0.1) c | |
| PCU | 1.7 (0.3) b | 71.9 (7.6) b | 2.3 (0.1) b | |
| Urea | 1.9 (0.3) b | 78.9 (7.5) b | 2.3 (0.1) b | |
| Previous year growth rings (PGR)—growth prior to fertilization | Control | 0.0 a | −1.9 (0.4) a | 1.4 (0.1) a |
| CUF | 4.4 (0.8) bc | 40.2 (3.9) b | 1.6 (0.1) a | |
| NBPT | 5.1 (0.7) c | 40.3 (1.3) b | 1.6 (0.1) a | |
| PCU | 3.7 (0.4) bc | 37.1 (3.9) b | 1.6 (0.1) a | |
| Urea | 2.9 (0.5) b | 34.8 (5.0) b | 1.4 (0.1) a | |
| Fine roots (<2 mm) | Control | 0.0 a | −0.3 (0.9) a | 9.4 (0.9) a |
| CUF | 19.2 (1.8) c | 36.1 (3.1) b | 10.5 (0.6) a | |
| NBPT | 16.2 (1.5) c | 33.2 (3.8) b | 10.4 (0.4) a | |
| PCU | 16.4 (1.8) c | 36.0 (3.5) b | 10.3 (0.5) a | |
| Urea | 10.8 (1.4) b | 31.2 (5.1) b | 9.7 (0.5) a | |
| Coarse roots (>2 mm) | Control | 0.0 a | −0.1 (0.37) a | 6.0 (0.6) a |
| CUF | 3.8 (1.4) b | 18.3 (1.6) b | 6.2 (0.5) a | |
| NBPT | 2.7 (0.6) ab | 19.0 (3.2) b | 6.3 (0.5) a | |
| PCU | 2.9 (0.9) b | 15.4 (2.2) b | 6.0 (0.5) a | |
| Urea | 1.7 (0.5) ab | 13.9 (3.1) b | 6.0 (0.5) a | |
| Litterfall | Control | 0.0 a | −3.0 (1.2) a | 7.4 (0.3) a |
| CUF | 1.6 (0.3) b | 48.5 (1.4) b | 8.0 (0.3) a | |
| NBPT | 1.4 (0.2) b | 55.8 (2.1) b | 8.3 (0.4) a | |
| PCU | 1.8 (0.3) b | 55.6 (1.6) b | 8.1 (0.3) a | |
| Urea | 1.7 (0.2) b | 55.1 (1.4) b | 8.2 (0.2) a | |
| Forest Floor (Organic horizon: Oi + Oe + Oa) | Control | 0.0 a | −1.9 (0.4) a | 6.6 (0.6) a |
| CUF | 3.0 (1.4) a | 62.9 (6.8) b | 8.1 (0.6) b | |
| NBPT | 2.8 (1.3) a | 59.3 (5.8) b | 8.0 (0.6) ab | |
| PCU | 3.8 (0.9) b | 91.1 (9.9) c | 8.7 (0.6) b | |
| Urea | 1.2 (0.4) a | 55.2 (6.3) b | 8.0 (0.7) ab | |
| 0–15 cm Mineral Soil | Control | 0.0 a | 3.3 (0.7) a | 0.8 (0.1) a |
| CUF | 23.1 (3.6) b | 21.8 (3.6) b | 0.6 (0.1) b | |
| NBPT | 22.4 (2.7) b | 22.3 (3.5) b | 0.6 (0.1) ab | |
| PCU | 24.2 (5.1) b | 21.0 (4.1) b | 0.7 (0.1) ab | |
| Urea | 15.9 (2.6) b | 15.9 (2.1) b | 0.6 (0.1) ab | |
| 15–30 cm Mineral Soil | Control | 0.0 a | 5.4 (0.4) a | 0.4 (0.1) a |
| CUF | 6.7 (1.1) a | 13.2 (1.5) b | 0.4 (0.0) a | |
| NBPT | 5.4 (0.9) a | 11.7 (1.0) b | 0.4 (0.1) a | |
| PCU | 10.7 (3.4) b | 16.3 (3.0) b | 0.4 (0.0) a | |
| Urea | 11.3 (3.1) b | 15.2 (2.4) b | 0.4 (0.1) a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raymond, J.E.; Fox, T.R.; Strahm, B.D. Understanding the Fate of Applied Nitrogen in Pine Plantations of the Southeastern United States Using 15N Enriched Fertilizers. Forests 2016, 7, 270. https://doi.org/10.3390/f7110270
Raymond JE, Fox TR, Strahm BD. Understanding the Fate of Applied Nitrogen in Pine Plantations of the Southeastern United States Using 15N Enriched Fertilizers. Forests. 2016; 7(11):270. https://doi.org/10.3390/f7110270
Chicago/Turabian StyleRaymond, Jay E., Thomas R. Fox, and Brian D. Strahm. 2016. "Understanding the Fate of Applied Nitrogen in Pine Plantations of the Southeastern United States Using 15N Enriched Fertilizers" Forests 7, no. 11: 270. https://doi.org/10.3390/f7110270





