2.1. The Model
We extended an earlier forest-level optimisation model by Nghiem [
29] to access level of species richness, and to estimate opportunity costs for different cutting practices. The previous model considered a plantation forest that consisted of 1 ×
n stands, but in this study, we improved the model so that it could include a forest with two dimensions,
n ×
n stands. Let
and
be the area and the age of stand
i,
j (
i,
j = 1 …
n) in period
t, respectively. In this study, we considered a forest with one among two alternative forest cutting practices: clear-cutting and patch-clear-cutting. Let
be a control parameter of stand
i,
j in period
t, where
or 0 denotes clear cutting or no action taken, respectively:
Let
qt be the cutting volume of timber in period
t, where the timber growth is a function of age
in a form of Gompertz model used by Woollons and Whyte [
30] as follows:
where the parameter
a0 represents a maximum asymptotic yield,
b0 and
c0 control growth rate while in trajectory towards
a0 at a certain age
.
Let
be a positive discount factor and
be the price of timber. We assumed
varied with timber age
t. For simplicity, we assumed that the planting cost, the initial value of forest, and the value of standing timber at the end time
T were constant. Let
A be an age at which forest stands generate a suitable habitat for understorey plant species to survive, hence plant species other than dominant trees only appear in the forest stands, which reach age
A and beyond. Age of forests was found to explain the species richness variation in plantation forests. Keenan et al. [
31] suggest that this could be induced in their case by the fact that the density of recruited tree seedlings also increased with plantation age. Older plantation forests established on previously forested lands are generally expected to support higher levels of diversity given the additional time to develop structural complexity [
32,
33]. Additionally, it has been shown that the succession of indigenous and adventive species in the understorey of plantations is related to temporal changes in the structure of the canopy [
3].
Forest stands can be divided into three age groups: zero (i.e., being cut), equal to or greater than A, and smaller than A. Spatially connected stands of a same age group, either zero or equal to or greater than A, form a cluster.
Let
and
denote the total area of stands and the number of clusters aged equal to or greater than
A in period
t. Then
where
is the area of stands aged equal to or greater than
A of cluster
w in period
t.
When a forest stand reaches age A, it creates a favourable habitat for a certain type of understorey plant species (e.g., an indigenous plant species).
Let
S be the number of species in a cluster
w, and the species richness that relates to the landscape area is defined by Preston [
34] with the power relationship described by:
Equation (4) was used by Roy et al. [
35] to calculate species richness for each connected available habitat in each period. They found that
z ∈ [0, 0.32] is the value of the exponent for the dynamic landscape. We used this magnitude of
z to calculate species richness for each connected available habitat at each time period. The species richness dynamic in these connected stands (or in a cluster),
, evolved similar to the model described by Roy et al. [
35]. That is, each cluster independently followed a species area relation law. This is because understorey species supported by different clusters could be slightly different in species composition. Hence, the total number of species supported by different clusters in a plantation forest is calculated as follows:
Let
represent the total area of cut stands in period
t. Then
, where
denotes the number of clusters cut in period
t and
is the area of cut stands of cluster
w in period
t.
It was assumed that a stand was replanted one year after cutting. The cutting cost of each cut cluster, per unit volume, varied with the size of the cut cluster, , and where is the slope of harvesting cost and represents the economies of scale, that is the larger the cutting area, the smaller the harvesting cost per ha.
Since at any time
t, some cut stands may form different clusters, the total cutting cost of the forest in year
t is
, which is defined as follows.
where
is cutting volume of cluster
w in period
t.
Let
NPV denote the NPV from selling timber, the objective of the optimal management was to maximise
NPV:
In Equation (10), S is the number of species that is maintained in the plantation forest over a T-year period. The optimal timber revenue, rotation age, and number of species were denoted in turn as NPV*, T*, S*.
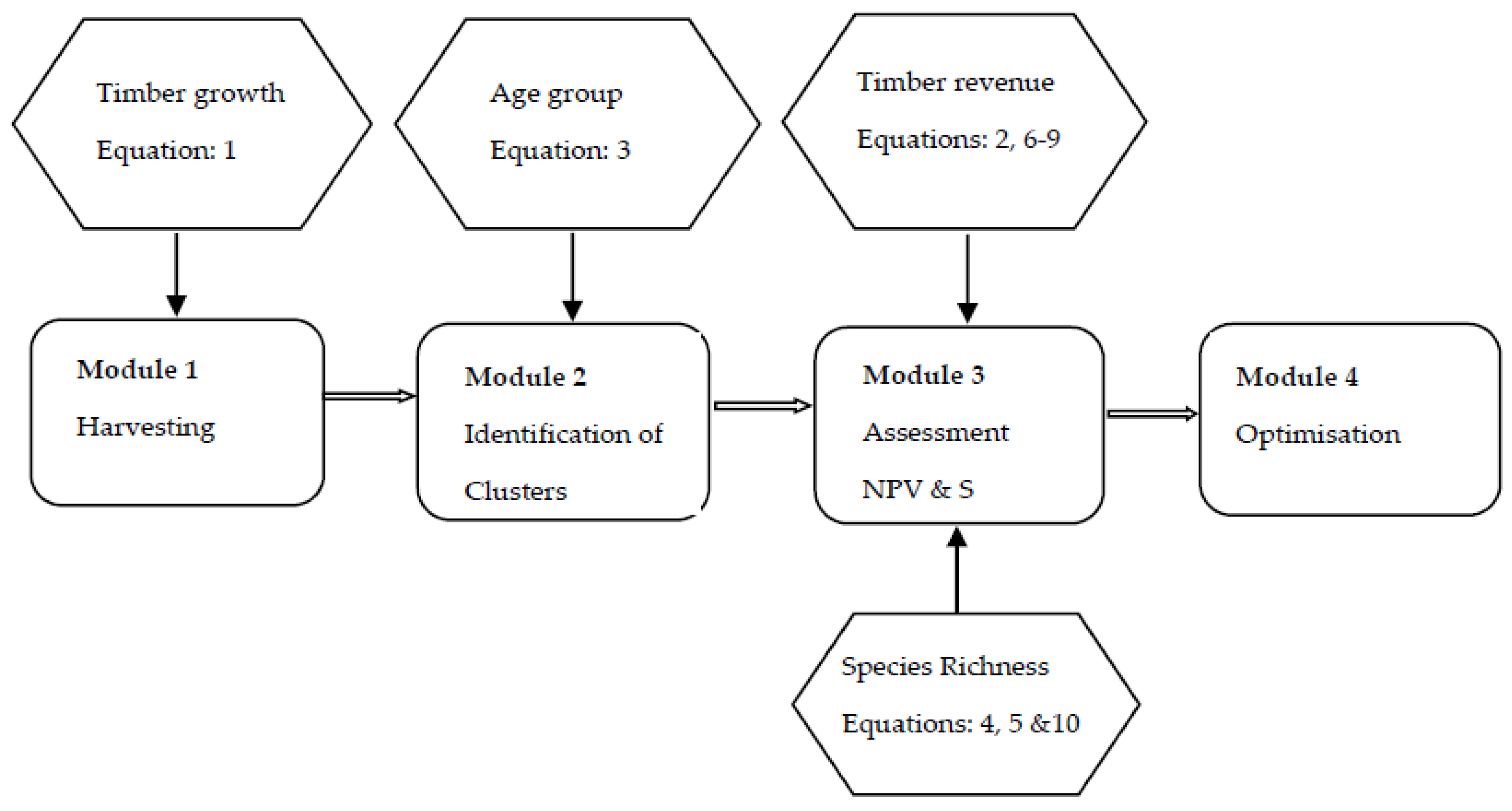
Figure 1 presents the model structure with four modules to decide cutting area and volume, identify clusters, assess profitability and species richness, and find an optimal strategy.
Module 1: Involved calculating timber growth and a decision to cut or keep a stand in period t. The decisions to cut a stand vary with alternative management strategies. We let t run from 0 to T, and calculated timber volume based on Equations (1), (2), and (8).
Module 2: Involved identifying clusters of clear-cutting area and of habitat area. We ran through stand 1 to stand n × n, ascertained whether (i.e., cut) or (i.e., habitat for plant species), and checked whether its adjacent stands are cut or aged older than A. If satisfied, clusters of clear cutting or habitat were recorded.
Module 3: Involved calculating the NPV using Equations (6)–(9) and counting the number of species by applying Equations (5) and (10).
Module 4: Involved all scenarios with different rotation ages being compared based on the objective variable. Then, the scenario that achieved the highest value of the NPV was chosen as the optimal management strategy in terms of timber extraction. The associated NPV and rotation length were referred to as NPV* and T*.
We applied this model according to clear-cutting and patch-clear-cutting strategies in order to analyse the trade-off between the economies of cutting scale and species richness.
In the clear-cutting strategy, a forest manager seeks to maximise the NPV from selling timber and ignores the impacts of this decision on the enhancement of plant species richness. To take advantage of the economies of scale (i.e., the larger the cutting area, the smaller the harvesting cost per ha) the forest manager cuts all the stands after T* years. The model of this strategy includes Equations (1)–(9). A stand is harvested () when it reaches year T* (Module 1). The optimal management strategy is denoted by , , and .
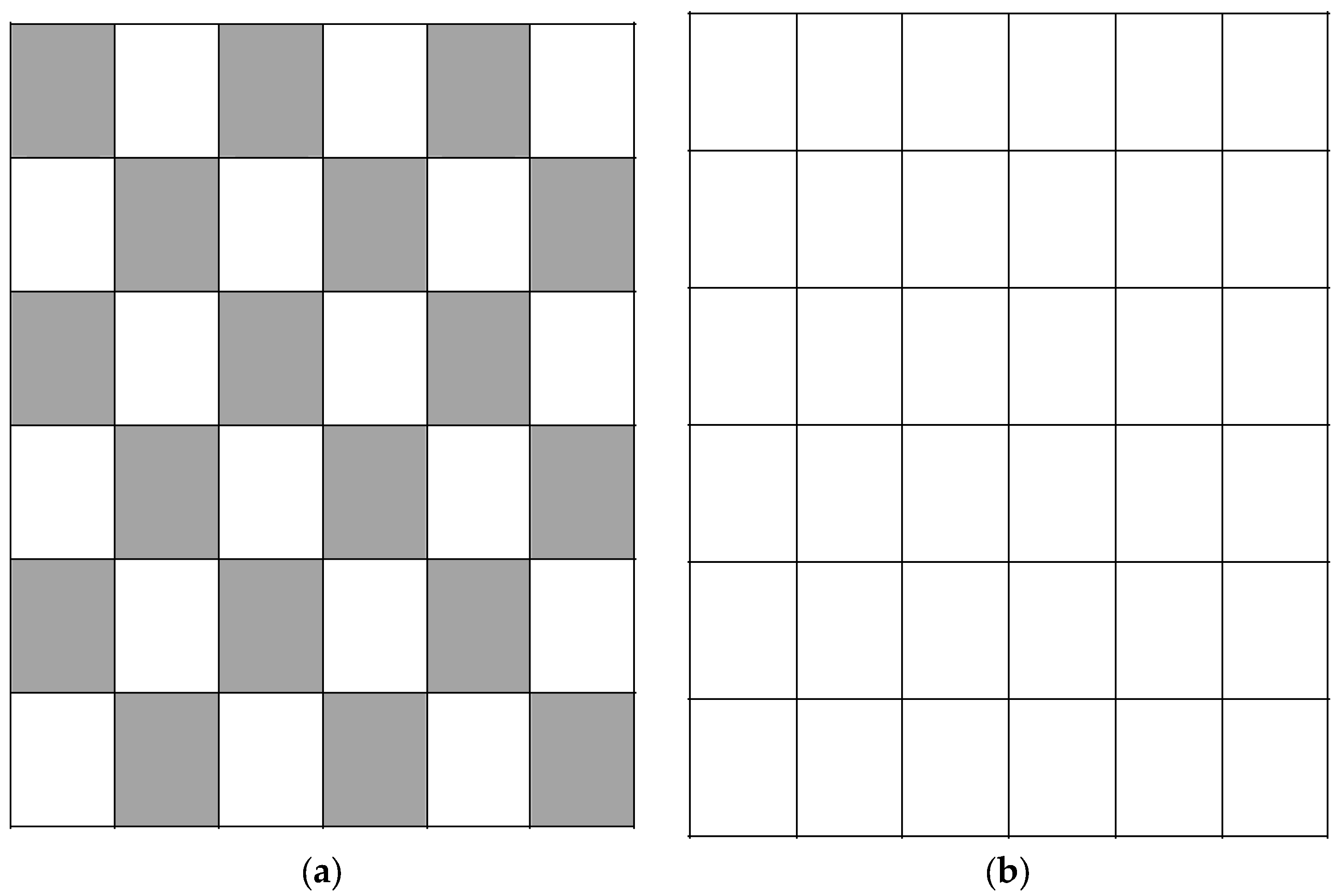
In the patch-clear-cutting strategy, the forest manager’s objective is to maximise the NPV from timber production but also to promote biodiversity by applying a small clear-cut size. In this strategy, the manager cuts a stand when it reaches
T* years without cutting adjacent stands occurring at the same time (see
Figure 2a,b). This strategy allows clear-cutting areas to be as fragmented as possible in order to increase landscape heterogeneity. This would help to promote conservation values through variation in the range of age classes of stands and the spatial juxtaposition of stands of different types and ages [
36]. The model of this strategy includes Equations (1)–(10). In Module 1, a stand can be cut
when it reaches year
T* or beyond and
and
and
and
. The optimal management strategy is denoted by
,
, and
.
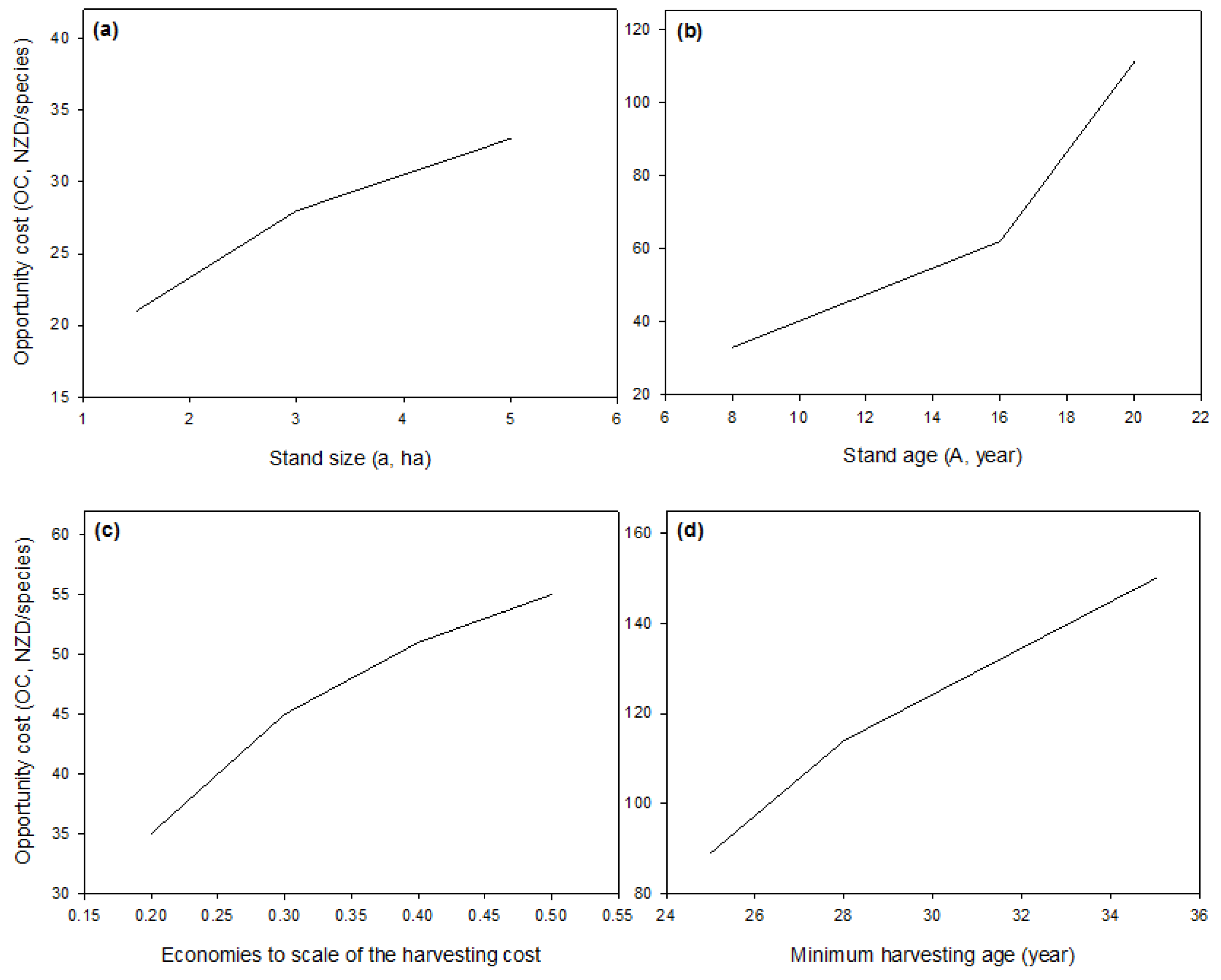
The opportunity cost (OC) of increasing biodiversity via using a patch-clear-cutting strategy (over a clear-cutting strategy) is defined as the difference in the NPV between the two approaches. Therefore, it is the amount of money foregone when the patch-clear-cutting strategy is applied and a certain level of additional biodiversity is achieved as follows:
This model (i.e., Equations (1)–(11)) was used to estimate the opportunity cost of plant diversity in New Zealand Pinus radiata plantation forests in the next section.
2.2. A Case Study of Pinus radiata in New Zealand
New Zealand is one of 25 global biodiversity hotspots [
37] and yet plantation forests are common (at 7% and 22% of land surface and of total forest area, respectively [
38]). As a result, the New Zealand forestry sector contributes 3.4% to annual GDP [
39] and is the third largest export industry in New Zealand [
38]. In 2007, approximately 19.3 million m
3 of round wood were harvested, of which, 19.0 million m
3 came from the clear felling of 43,000 hectares of plantation forests [
40].
In New Zealand, exotic forests are planted for production purposes only [
41]. However, plantation forests are further incentivised because of the potential of such forests to sequester carbon emissions from other sectors [
42]. Forestry was the first sector to enter New Zealand’s Emission Trading Scheme (ETS), effective from 1 January 2008. This allows new forest plantations to earn carbon credits through the Kyoto Protocol. Plantation forests could also be promoted as supplying other ecosystem services, such as the maintenance of clean water, erosion control, and habitat provision [
43]. Currently, exotic trees are the main crops in New Zealand, of which, 89% is
Pinus radiata [
40].
Pinus radiata was introduced into New Zealand in the 1840s and grows faster here than in any other country, with the typical rotation length being around 28 years [
38].
Since clear cutting is popular and preserving biodiversity is becoming important in New Zealand plantations, this study applies the model described in the previous section to find the opportunity cost of conserving understorey plants in P. radiata forests. In the baseline scenario, we did not put any restriction on stand age that creates a favourable habitat for any particular species. However, in sensitivity analyses, we varied this stand age according to species re-colonisation or habitats for indigenous plant species.
For the
P. radiata growth function, we used age and total standing volume from yield tables for Central North Island [
44] since this area is the major plantation area in New Zealand [
45]. The growth data are detailed in
Appendix A.
The harvesting cost was obtained from an established database [
46,
47] with some adjustments, as detailed in
Appendix B.
where
c is the clear-cutting cost (expressed in NZD m
−3) and y is the cutting area or the stand size (ha).
In Equation (12), β = 42.9 > 0 and η = −0.118. The cost function implies a decreasing marginal cost with the cutting area. Since , the first derivative , hence (12) is a decreasing function. Therefore, the larger the cutting size, the less expensive the cutting cost m−3.
We assumed timber prices varied by tree ages. Since there are no data for the timber prices which vary with the tree ages, we estimated a weighted timber price using the ratio of pulp and logs at each tree age calculated from an official yield table [
44]. This ratio for each tree age was then used to weigh pulp prices and log prices to create the timber prices associated with the tree ages. We used an average pulp price of 51 NZD m
−3 and an average log price of 135 NZD m
−3 in 2011 [
48]. These estimated timber prices are detailed in
Appendix A.
Table 1 shows the parameters used in our baseline model. We chose
z = 0.3 in our baseline scenario since the species–area relationship for native forest remnants in New Zealand was assigned with
z = 0.4 [
49]. The quality of plantation forest habitat is lower than that of the native remnants (i.e., more disturbance), and thus can affect the dispersal of the native species. Moreover, we assumed a forest size of 400 stands to be sufficient to apply a patch-clear-cutting practice, and that the forest would exist over a 100-year time horizon, as in the literature [
18,
19]. We used a maximum rotation age for
P. radiata of 40 years, as similar to the age listed in the yield table [
44]. We also ran simulations with a maximum rotation age of 60 years but the optimal age solution was always less than 40 years, thus we used 40 years to reduce the computational time in uncertainty analyses. The discount rate of 7% (in real terms) was used as this is the rate that is mainly used in forest valuations in New Zealand [
50].