Spatial Patterns of Irradiance and Advanced Reproduction along a Canopy Disturbance Severity Gradient in an Upland Hardwood Stand
Abstract
:1. Introduction
2. Study Area and Methods
2.1. Study Area
2.2. Field Methods
2.3. Analytical Methods
3. Results
3.1. Structure and Composition
3.2. Quercus Advanced Reproduction and Competition
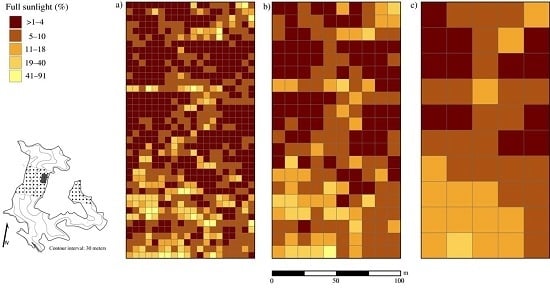
3.3. Disturbance and Patterns of Irradiance
3.4. Spatial Patterns of Advanced Reproduction
4. Discussion
4.1. Quercus Advanced Reproduction and Competition
4.2. Disturbance and Patterns of Irradiance
4.3. Spatial Patterns of Advanced Reproduction
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hogan, C. Oak, 2012. Available online: http://www.eoearth.org/view/article/161730 (accessed on 15 March 2015).
- Dyer, J.M. Revisiting the deciduous forests of eastern North America. Bioscience 2006, 56, 341–352. [Google Scholar] [CrossRef]
- Johnson, P.S.; Shifley, S.R.; Rogers, R. The Ecology and Silviculture of Oaks, 2nd ed.; CABI Publishing: New York, NY, USA, 2009. [Google Scholar]
- Smith, W.B.; Miles, P.D.; Perry, C.H.; Pugh, S.A. Forest Resources of the United States, 2007; General Technical Report WO-78; USDA Forest Service: Washington, DC, USA, 2009.
- Delcourt, H.R. Late Quaternary vegetation history of the Eastern Highland Rim and adjacent Cumberland Plateau of Tennessee. Ecol. Monogr. 1979, 49, 255–280. [Google Scholar] [CrossRef]
- Delcourt, H.R.; Delcourt, P.A. Eastern deciduous forests. In North American Terrestrial Vegetation, 2nd ed.; Barbour, M.G., Billings, D.W., Eds.; Cambridge University Press: Cambridge, UK, 2000; Chapter 10. [Google Scholar]
- Abrams, M.D. The postglacial history of oak forests in eastern North America. In Oak Forest Systems Ecology and Management for Wildlife; McShea, W.J., Healy, W.M., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2002; pp. 34–45. [Google Scholar]
- Fei, S.; Steiner, K.C. Evidence for increasing red maple abundance in the eastern United States. For. Sci. 2007, 53, 473–477. [Google Scholar]
- Nowacki, G.J.; Abrams, M.D. The demise of fire and “mesophication” of forest in the eastern United States. Bioscience 2008, 58, 123–138. [Google Scholar] [CrossRef]
- Fei, S.; Kong, N.; Steiner, K.C.; Moser, W.K.; Steiner, E.B. Change in oak abundance in the eastern United States from 1980 to 2008. For. Ecol. Manag. 2011, 255, 2297–2305. [Google Scholar] [CrossRef]
- Watt, A.S. On the natural cause of failure of regeneration in British oak woods. J. Ecol. 1919, 17, 173–203. [Google Scholar] [CrossRef]
- Shaw, M.W. Factors affecting the natural regeneration of sessile oak (Quercus petraea) in north Wales. J. Ecol. 1968, 56, 565–583. [Google Scholar] [CrossRef]
- Li, Q.; Ma, K. Factors affecting establishment of Quercus liaotungensis Koidz. under mature mixed oak forest overstory and in shrubland. For. Ecol. Manag. 2003, 176, 133–146. [Google Scholar] [CrossRef]
- Esteso-Martinez, J.; Camarero, J.J.; Gil-Pelegrin, E. Competitive effects of herbs on Quercus faginea seedlings inferred from vulnerability curves and spatial-pattern analyses in a Mediterranean stand (Iberian System, northeast Spain). Ecoscience 2006, 13, 378–387. [Google Scholar] [CrossRef]
- Hanberry, B.B. Changing eastern broadleaf, southern mixed, and northern mixed forest ecosystems of the eastern United States. For. Ecol. Manag. 2013, 306, 171–178. [Google Scholar] [CrossRef]
- Abrams, M.D. Fire and the development of oak forests. Bioscience 1992, 42, 346–353. [Google Scholar] [CrossRef]
- Abrams, M.D. Prescribing fire in eastern oak forests: Is time running out? North. J. Appl. For. 2005, 22, 190–196. [Google Scholar]
- McEwan, R.W.; Dyer, J.M.; Pederson, N. Multiple interacting ecosystem drivers: Toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography 2011, 34, 224–256. [Google Scholar] [CrossRef]
- McShea, W.J.; Healy, W.M.; Devers, P.; Fearer, T.; Koch, F.H.; Stauffer, D.; Waldon, J. Forestry matters: Decline of oaks will impact wildlife in hardwood forests. J. Wildl. Manag. 2007, 71, 1717–1728. [Google Scholar] [CrossRef]
- Alexander, D.C.; Arthur, M.A. Implications of a predicted shift from upland oaks to red maple on forest hydrology and nutrient availability. Can. J. For. Res. 2010, 40, 716–726. [Google Scholar] [CrossRef]
- Dey, D.C. Sustaining oak forests in eastern North America: Regeneration and recruitment, the pillars of sustainability. For. Sci. 2014, 60, 926–942. [Google Scholar] [CrossRef]
- Lorimer, C.G. Development of the red maple understory in northeastern oak forests. For. Sci. 1984, 30, 3–22. [Google Scholar]
- Abrams, M.D.; Nowacki, G.J. Historical variation in fire, oak recruitment and post-logging accelerated succession in central Pennsylvania. Bull. Torrey Bot. Club 1992, 119, 19–25. [Google Scholar] [CrossRef]
- McGee, C.E.; Loftis, D.L. Oak regeneration: A summary. In Oak Regeneration: Serious Problems, Practical Recommendations; General Technical Report SE-84; Loftis, D.L., McGee, C.E., Eds.; USDA Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1993; pp. 316–319. [Google Scholar]
- Lorimer, C.G. Causes of the oak regeneration problem. In Oak Regeneration: Serious Problems, Practical Recommendations; General Technical Report SE-84; Loftis, D.L., McGee, C.E., Eds.; USDA Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1993; pp. 14–39. [Google Scholar]
- Smith, D.W. Oak regeneration: The scope of the problem. In Oak Regeneration: Serious Problems, Practical Recommendations; General Technical Report SE-84; Loftis, D.L., McGee, C.E., Eds.; USDA Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1993; pp. 40–52. [Google Scholar]
- Crow, T.R. Reproductive mode and mechanisms for self-replacement of northern red oak (Quercus rubra)—A review. For. Sci. 1988, 34, 19–40. [Google Scholar]
- Abrams, M.D.; Downs, J.A. Successional replacement of old-growth white oak by mixed mesophytic hardwoods in southwest Pennsylvania. Can. J. For. Res. 1990, 20, 1864–1870. [Google Scholar] [CrossRef]
- Nowacki, G.J.; Abrams, M.D.; Lorimer, C.G. Composition, structure, and historical development of northern red oak stands along an edaphic gradient in north-central Wisconsin. For. Sci. 1990, 36, 276–292. [Google Scholar]
- Abrams, M.D. Where has all the white oak gone? Bioscience 2003, 53, 927–939. [Google Scholar] [CrossRef]
- Abrams, M.D. The red maple paradox. Bioscience 1998, 45, 355–363. [Google Scholar] [CrossRef]
- Fei, S.; Steiner, K.C. Rapid capture of growing space by red maple. Can. J. For. Res. 2009, 39, 1444–1452. [Google Scholar] [CrossRef]
- Hart, J.L.; Clark, S.L.; Torreano, S.J.; Buchanan, M.L. Composition, structure, and dendroecology of an old-growth Quercus forest on the tablelands of the Cumberland Plateau, USA. For. Ecol. Manag. 2012, 266, 11–24. [Google Scholar] [CrossRef]
- Johnson, P.S. Thinking about oak forests as responsive ecosystems. In Upland Oak Ecology Symposium: History Current Conditions, and Sustainability; Gen. Tech. Rep. SRS-73; Spetich, M.A., Ed.; U.S.D.A. Forest Service, Southern Research Station: Asheville, NC, USA, 2004; pp. 13–18. [Google Scholar]
- Gottschalk, K.W. Management strategies for successful regeneration: Oak-hickory. In Proceedings of the Forestry Issues Conference, University Park, PA, USA, 15–16 March 1983; pp. 190–213.
- Dey, D.C. The ecological basis for oak silviculture in eastern North America. In Oak Forest Ecosystems; McShea, W.J., Healy, W.M., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2002; pp. 46–60. [Google Scholar]
- Rogers, R. Spatial Pattern and Growth in a Missouri Oak-Hickory Stand. Ph.D. Dissertation, University of Missouri, Columbia, MO, USA, 1983. [Google Scholar]
- O’Hara, K.L.; Nagel, L.M. The stand: Revisiting a central concept in forestry. J. For. 2013, 111, 335–340. [Google Scholar]
- Puettman, K.J.; Coates, D.K.; Messier, C. A Critique of Silviculture: Managing for Complexity; Island Press: Washington, DC, USA, 2009. [Google Scholar]
- Churchill, D.C.; Larson, A.J.; Dalhgreen, M.C.; Franklin, J.F.; Hessburg, P.F.; Lutz, J.A. Restoring forest resilience: From reference spatial patterns to silvicultural prescriptions and monitoring. For. Ecol. Manag. 2013, 291, 442–457. [Google Scholar] [CrossRef]
- Runkle, J.R. Disturbance regimes in temperate forests. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 17–33. [Google Scholar]
- White, P.S.; Pickett, S.T.A. Natural disturbance and patch dynamics: An introduction. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 3–13. [Google Scholar]
- Canham, C.D.; Papaik, M.J.; Latty, E.F. Interspecific variation in susceptibility to windthrow as a function of tree size and storm severity for northern temperate tree species. Can. J. For. Res. 2001, 31, 1–10. [Google Scholar] [CrossRef]
- Hanson, J.J.; Lorimer, C.G. Forest structure and light regimes following moderate wind storms: Implications for multi-cohort management. Ecol. Appl. 2007, 17, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.L.; Kupfer, J.A. Sapling richness and composition in canopy gaps of a southern Appalachian mixed Quercus forest. J. Torrey Bot. Soc. 2011, 138, 207–219. [Google Scholar] [CrossRef]
- Wiens, J.A. Spatial scaling in ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Fenneman, N.M. Physiography of Eastern United States; McGraw-Hill Book Company: New York, NY, USA, 1938. [Google Scholar]
- Smalley, G.W. Classification and Evaluation for Forest Sites on the Southern Cumberland Plateau; General Technical Report SO-23; USDA, Forest Service, Southern Forest Experiment Station: New Orleans, LA, USA, 1979.
- Szabo, M.W.; Osborne, E.W.; Copeland, C.W., Jr.; Neathery, T.L. Geologic Map of Alabama, Special Map 220 Scale 1:250,000; Geological Survey of Alabama: Tuscaloosa, AL, USA, 1988. [Google Scholar]
- USDA SCS (United Stated Department of Agriculture, Soil Conservation Service). Soil Survey: Lawrence County, AL; Series 1949, No. 10; Government Printing Office: Washington, DC, USA, 1959.
- Thornthwaite, C.W. An approach toward rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- PRISM Climate Group. Northwest Alliance for Computational Science and Engineering. Available online: http://www.prism.oregonstate.edu/ (accessed on 3 March 2011).
- Burt, C.C.; Stroud, M. Extreme Weather: A Guide and Record Book; W.W. Norton & Company: New York, NY, USA, 2007. [Google Scholar]
- Braun, E.L. Eastern Deciduous Forests of North America; Blakiston: Philadelphia, PA, USA, 1950. [Google Scholar]
- Hinkle, C.R. Forest communities of the Cumberland Plateau of Tennessee. J. Tenn. Acad. Sci. 1989, 64, 123–129. [Google Scholar]
- Clatterbuck, W.K.; Smalley, G.W.; Turner, J.A.; Travis, A. Natural History and Land Use History of Cumberland Plateau Forests in Tennessee; Special Report 06-01; National Council for Air and Stream Improvement, Inc.: Research Triangle Park, NC, USA, 2006; p. 37. [Google Scholar]
- Zhang, L.; Oswald, B.P.; Green, T.H. Relationships between overstory species and community classification of the Sipsey Wilderness, Alabama. For. Ecol. Manag. 1999, 114, 377–383. [Google Scholar] [CrossRef]
- Parker, R.P.; Hart, J.L. Patterns of riparian and in-stream large woody debris across a chronosequence of southern Appalachian hardwood stands. Nat. Areas J. 2014, 34, 65–78. [Google Scholar] [CrossRef]
- Hardin, D.E.; Lewis, K.P. Vegetation analysis of Bee Branch Gorge, a hemlock beech community of the Warrior River Basin of Alabama. Castanea 1980, 45, 248–256. [Google Scholar]
- Richards, J.D.; Hart, J.L. Canopy gap dynamics and development patterns in secondary Quercus stands on the Cumberland Plateau, Alabama, USA. For. Ecol. Manag. 2011, 262, 2229–2239. [Google Scholar] [CrossRef]
- National Weather Service (NWS). Birmingham, AL Weather Forecast Office, 2011. Available online: http://www.srh.noaa.gov/bmx/?n=event_04202011 (accessed on 1 April 2015).
- Cowden, M.C.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Effects of intermediate-scale wind disturbance on composition, structure, and succession in Quercus stands: Implications for natural disturbance-based silviculture. For. Ecol. Manag. 2014, 330, 240–251. [Google Scholar] [CrossRef]
- White, S.D.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Altered structural development and accelerated succession from intermediate-scale wind disturbance in Quercus stands on the Cumberland Plateau, USA. For. Ecol. Manag. 2015, 336, 52–64. [Google Scholar] [CrossRef]
- NCDC (National Climate Data Center). Tornado Climatology, NOAA, 2013. Available online: http://www.ncdc.noaa.gov/oa/climate/severeweather/tornadoes.html (accessed on 1 March 2015).
- Clinton, B.D.; Vose, J.M.; Swank, W.T. Site preparation burning to improve southern Appalachian pine-hardwood stands: Vegetation composition and diversity of 13-year-old stands. Can. J. For. Res. 1993, 23, 2271–2277. [Google Scholar] [CrossRef]
- Fraver, S.; Wagner, R.G.; Day, M. Dynamics of coarse woody debris following gap harvesting in the Acadian forest of central Maine, U.S.A. Can. J. For. Res. 2002, 32, 2094–2105. [Google Scholar] [CrossRef]
- Sander, I.L. Size of Oak Reproduction: Key to Growth Following Harvest Cutting; USDA Forest Service Res. Pap. NC-97; North Central Experiment Station: St. Paul, MN, USA, 1972; p. 6. [Google Scholar]
- Zenner, E.K.; Heggenstaller, D.J.; Brose, P.H.; Peck, J.E.; Steiner, K.C. Reconstructing the competitive dynamics of mixed-oak neighborhoods. Can. J. For. Res. 2012, 42, 1714–1723. [Google Scholar] [CrossRef]
- Clatterbuck, W.K.; Hodges, J.D. Development of cherrybark oak and sweetgum in a mixed, even-aged bottomland stand in central Mississippi, USA. Can. J. For. Res. 1988, 18, 12–18. [Google Scholar] [CrossRef]
- Lockhart, B.R.; Ezell, A.W.; Hodges, J.D.; Clatterbuck, W.K. Using natural stand development patterns in artificial mixtures: A case study with cherrybark oak and sweetgum in east-central Mississippi, USA. For. Ecol. Manag. 2006, 222, 202–210. [Google Scholar] [CrossRef]
- Krajicek, J.E.; Brinkman, K.A.; Gingrich, S.F. Crown competition-a measure of density. For. Sci. 1961, 7, 35–42. [Google Scholar]
- Runkle, J.R. Patterns of disturbance in some old-growth mesic forests of eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Hegyi, F. A simulation model for managing jack-pine stands. In Growth Models for Tree and Stand Simulation; Fries, J., Ed.; Royal College of Forestry: Stockholm, Sweden, 1974; pp. 74–90. [Google Scholar]
- Lorimer, C.G. Tests of age-independent competition indices for individual trees in natural hardwood stands. For. Ecol. Manag. 1983, 6, 343–360. [Google Scholar] [CrossRef]
- Holmes, M.J.; Reed, D.D. Competition indices for mixed species northern hardwoods. For. Sci. 1991, 37, 1338–l349. [Google Scholar]
- Cole, W.G.; Lorimer, C.G. Predicting tree growth from crown variables in managed Northern hardwood stands. For. Ecol. Manag. 1994, 67, 159–175. [Google Scholar] [CrossRef]
- Weber, P.; Bugmann, H.; Fonti, P.; Rigling, A. Using a retrospective dynamic competition index to reconstruct forest succession. For. Ecol. Manag. 2008, 254, 96–106. [Google Scholar] [CrossRef]
- Fu, W.J.; Jiang, P.K.; Zhou, G.M.; Zhao, K.L. Using Moran’s I and GIS to study the spatial pattern of forest litter carbon density in a subtropical region of southeastern China. Biogeosciences 2014, 11, 2401–2409. [Google Scholar] [CrossRef]
- Crain, B.J.; Tremblay, R.L. Do richness and rarity hotspots really matter for orchid conservation in light of anticipated habitat loss? Divers. Distrib. 2014, 20, 652–662. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Peterson, C.J. Consistent influence of tree diameter and species on damage in nine eastern North American tornado blowdowns. For. Ecol. Manag. 2007, 250, 96–108. [Google Scholar] [CrossRef]
- Schweitzer, C.J.; Dey, D.C. Forest structure, composition, and tree diversity response to a gradient of regeneration harvests in the mid-Cumberland Plateau escarpment region, USA. For. Ecol. Manag. 2011, 262, 1729–1741. [Google Scholar] [CrossRef]
- Carvell, K.L.; Tryon, E.H. The effect of environmental factors on the abundance of oak regeneration beneath mature oak stands. For. Sci. 1961, 7, 98–105. [Google Scholar]
- Dey, D.C.; Guyette, R.P. Anthropogenic fire history and red oak forests in south-central Ontario. For. Chronicle 2000, 76, 339–347. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Guo, L. An evaluation of spatial autocorrelation and heterogeneity in the residuals of six regression models. For. Sci. 2009, 55, 533–548. [Google Scholar]
- Zenner, E.K.; Sagheb-Talebi, K.; Akhavan, R.; Peck, J.E. Integration of small-scale canopy dynamics smoothes live-tree structural complexity across development stages in old-growth Oriental beech (Fagus orientalis Lipsky) forests at the multi-gap scale. For. Ecol. Manag. 2015, 335, 26–36. [Google Scholar] [CrossRef]






| Species | Density (Stems ha−1) | Relative Density (%) | Dominance (m2·ha−1) | Relative Dominance (%) | Importance Value |
|---|---|---|---|---|---|
| Quercus alba | 115.5 | 16.0 | 11.5 | 56.1 | 72.1 |
| Ostrya virginiana | 280.0 | 38.8 | 1.4 | 6.6 | 45.5 |
| Fagus grandifolia | 52.5 | 7.3 | 1.4 | 6.6 | 13.9 |
| Acer saccharum | 60.0 | 8.3 | 1.0 | 5.1 | 13.4 |
| Nyssa sylvatica | 32.5 | 4.5 | 0.4 | 1.7 | 6.2 |
| Cornus florida | 32.5 | 4.5 | 0.1 | 0.7 | 5.2 |
| Carya glabra | 15.0 | 2.1 | 0.5 | 2.7 | 4.7 |
| Ulmus alata | 15.0 | 2.1 | 0.4 | 1.8 | 3.9 |
| Fraxinus americana | 9.5 | 1.3 | 0.5 | 2.4 | 3.8 |
| Carya tomentosa | 8.5 | 1.2 | 0.5 | 2.3 | 3.5 |
| Carya ovata | 7.5 | 1.0 | 0.4 | 2.0 | 3.1 |
| Quercus falcata | 3.0 | 0.4 | 0.4 | 2.1 | 2.5 |
| Liriodendron tulipifera | 3.0 | 0.4 | 0.4 | 2.1 | 2.5 |
| Ulmus rubra | 8.0 | 1.1 | 0.2 | 1.1 | 2.2 |
| Pinus taeda | 2.0 | 0.3 | 0.4 | 2.0 | 2.2 |
| Magnolia acuminata | 10.0 | 1.4 | 0.1 | 0.7 | 2.1 |
| Acer rubrum | 9.0 | 1.2 | 0.1 | 0.3 | 1.5 |
| Quercus prinus | 3.5 | 0.5 | 0.2 | 1.0 | 1.5 |
| Viburnum rufidulum | 8.5 | 1.2 | 0.0 | 0.1 | 1.3 |
| Quercus muehlenbergii | 3.5 | 0.5 | 0.2 | 0.8 | 1.3 |
| Cercis canadensis | 7.5 | 1.0 | 0.0 | 0.1 | 1.2 |
| Carpinus caroliniana | 7.0 | 1.0 | 0.0 | 0.2 | 1.1 |
| Juniperus virginiana | 4.5 | 0.6 | 0.1 | 0.4 | 1.0 |
| Magnolia macrophylla | 5.0 | 0.7 | 0.0 | 0.2 | 0.8 |
| Tilia americana | 4.5 | 0.6 | 0.0 | 0.2 | 0.8 |
| Fraxinus pennsylvanica | 3.0 | 0.4 | 0.1 | 0.3 | 0.7 |
| Oxydendrum arboreum | 2.0 | 0.3 | 0.0 | 0.1 | 0.4 |
| Quercus rubra | 1.5 | 0.2 | 0.0 | 0.2 | 0.4 |
| Prunus serotina | 2.0 | 0.3 | 0.0 | 0.1 | 0.3 |
| Ilex opaca | 1.5 | 0.2 | 0.0 | 0.1 | 0.3 |
| Frangula caroliniana | 1.0 | 0.1 | 0.0 | 0.0 | 0.2 |
| Asimina triloba | 1.0 | 0.1 | 0.0 | 0.0 | 0.1 |
| Quercus stellata | 0.5 | 0.1 | 0.0 | 0.0 | 0.1 |
| Celtis laevigata | 0.5 | 0.1 | 0.0 | 0.0 | 0.1 |
| Ligustrum sinense | 0.5 | 0.1 | 0.0 | 0.0 | 0.1 |
| Total | 721 | 100.0 | 20.6 | 100.0 | 200.0 |
| Species | n | Quercus Stem Height (m) | Competitor Stem Height (m) | Distance between Stems (m) |
|---|---|---|---|---|
| Q. rubra | 231 | 1.38 ± 0.83 | 1.78 ± 1.00 | 0.36 ± 0.23 |
| Q. muhlenbergii | 190 | 1.47 ± 0.72 | 1.77 ± 0.90 | 0.28 ± 0.27 |
| Q. alba | 111 | 1.11 ± 0.83 | 1.53 ± 1.05 | 0.29 ± 0.22 |
| Q. prinus | 13 | 0.78 ± 0.33 | 1.19 ± 0.69 | 0.46 ± 0.37 |
| Q. facalta | 8 | 0.64 ± 0.20 | 1.07 ± 0.63 | 0.43 ± 0.24 |
| Total | 553 | 1.33 ± 0.80 | 1.70 ± 0.98 | 0.33 ± 0.25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keasberry, A.M.; Hart, J.L.; Dey, D.C.; Schweitzer, C.J. Spatial Patterns of Irradiance and Advanced Reproduction along a Canopy Disturbance Severity Gradient in an Upland Hardwood Stand. Forests 2016, 7, 73. https://doi.org/10.3390/f7040073
Keasberry AM, Hart JL, Dey DC, Schweitzer CJ. Spatial Patterns of Irradiance and Advanced Reproduction along a Canopy Disturbance Severity Gradient in an Upland Hardwood Stand. Forests. 2016; 7(4):73. https://doi.org/10.3390/f7040073
Chicago/Turabian StyleKeasberry, Amanda M., Justin L. Hart, Daniel C. Dey, and Callie J. Schweitzer. 2016. "Spatial Patterns of Irradiance and Advanced Reproduction along a Canopy Disturbance Severity Gradient in an Upland Hardwood Stand" Forests 7, no. 4: 73. https://doi.org/10.3390/f7040073