Carbon Sequestration and Sedimentation in Mangrove Swamps Influenced by Hydrogeomorphic Conditions and Urbanization in Southwest Florida
Abstract
:1. Introduction
1.1. Mangrove Hydrogeomorphology
1.2. Goal and Objectives
2. Materials and Methods
2.1. Study Site
2.2. Mangrove Community Structure
2.3. Soil Sampling and Preparation
2.4. Sedimentation Rates
2.5. Soil Carbon Analysis
2.6. Carbon Sequestration
2.7. Statistical Analysis
3. Results
3.1. Mangrove Forest Community Structure
3.2. Soil Bulk Density
3.3. Soil Carbon Profile
3.4. Soil Carbon Pool
3.5. Sedimentation Rates
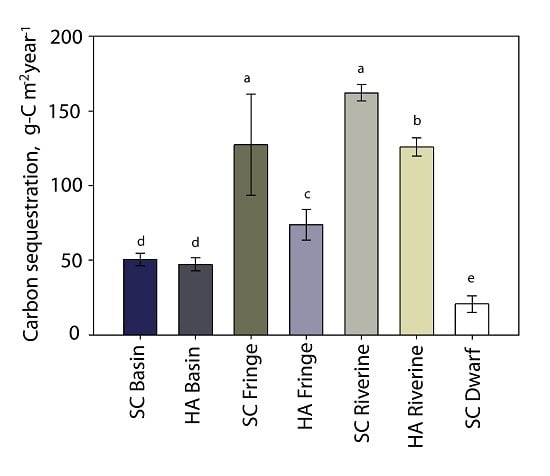
3.6. Carbon Sequestration
4. Discussion
4.1. Carbon Dynamics in Mangrove Wetlands
4.2. Mangrove Sedimentation Comparisons
4.3. Radiometric Dating
4.4. Carbon Sequestration Rate Comparisons
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, U.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 311–342. [Google Scholar]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. Lond. B 2008, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.J.; Rodríguez, J.F.; Saco, P.M. Surface evolution and carbon sequestration in disturbed and undisturbed wetland soils of the Hunter estuary, southeast Australia. Estuar. Coast. Shelf Sci. 2009, 84, 75–83. [Google Scholar] [CrossRef]
- Gastusso, J.P.; Frankignoulle, M.; Wollast, R. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu. Rev. Ecol. Sci. 1998, 29, 405–434. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G.; Zhang, L.; Anderson, C.J. Wetland Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 19–86, 163–190. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 23, 154–159. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.P.; Losada, I.J.; Gattuso, J.P.; Hinkel, J.; Khattabi, A.; McInnes, K.L.; Saito, Y.; Sallenger, A. Coastal systems and low-lying areas. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects; Cambridge University Press: Cambridge, UK, 2014; pp. 361–409. [Google Scholar]
- Breithaupt, J.L.; Smoak, J.M.; Smith, T.J., III; Sanders, C.J.; Hoare, A. Organic carbon burial rates in mangrove sediments: Strengthening the global budget. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetlands. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A. Estimating global “Blue Carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Bjork, M.; Duarte, C.M.; Lovelock, C.E.; Schlesiner, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Keuskamp, J.A.; Schmitt, H.; Laanbroek, H.J.; Verhoeven, J.T.A.; Hefting, M.M. Nutrient amendment does not increase mineralisation of sequestered carbon during incubation of a nitrogen limited mangrove soil. Soil Biol. Biochem. 2013, 57, 822–829. [Google Scholar] [CrossRef]
- Berner, R.A. Burial of organic carbon and pyrite sulfur in the modern ocean: Its geochemical and environmental significance. Am. J. Sci. 1982, 282, 451–473. [Google Scholar] [CrossRef]
- Jennerjahn, T.C.; Ittekkot, V. Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwiss 2002, 89, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Pil, M.W.; Boeger, M.R.T.; Muschner, V.C.; Pie, M.R.; Ostrensky, A.; Boeger, W.A. Postglacial north-south expansion of populations of Rhizophora mangle (Rhizophoraceae) along the Brazilian coast revealed by microsatellite analysis. Am. J. Bot. 2011, 98, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, K.C.; Kellner, J.R.; Forde, A.J.; Gruner, D.S.; Parker, J.D.; Rodriguez, W.; Feller, I.C. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc. Natl. Acad. Sci. USA 2014, 111, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Saintilan, N.; Wilson, N.C.; Rogers, K.L.; Rajkaran, A.; Krauss, K.W. Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob. Chang. Biol. 2014, 20, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Mitsch, W.J.; Nahlik, A.M.; Wolski, P.; Bernal, B.; Zhang, L.; Ramberg, L. Tropical wetlands: Seasonal hydrologic pulsing, carbon sequesteration, and methane emissions. Wetl. Ecol. Manag. 2010, 18, 573–586. [Google Scholar] [CrossRef]
- Lynch, J.C.; Meriwether, J.R.; McKee, B.A.; Vera-Herrera, F.; Twilley, R.R. Recent accretion in mangrove ecosystems based on 137Cs and 210Pb. Estuaries 1989, 12, 284–299. [Google Scholar] [CrossRef]
- Bloom, A.A.; Palmer, P.I.; Fraser, A.; Reay, D.S.; Frankenberg, C. Large-scale controls of methanogenesis inferred from methane and gravity spaceborne data. Science 2010, 327, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Odum, E.P. The status of three ecosystem-level hypotheses regarding salt marsh estuaries: Tidal subsidy, outwelling, and detritus-based food chains. In Estuarine Perspectives; Kennedy, V.S., Ed.; Academic Press: New York, NY, USA, 1980; Volume 1, pp. 485–495. [Google Scholar]
- Bouillon, S.; Connolly, R.M.; Lee, S.Y. Organic matter exchange and cycling in mangrove ecosystems: Recent insights from stable isotope studies. J. Sea Res. 2008, 59, 44–58. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Twilley, R.W.; Chen, R.H.; Hargis, T. Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut. 1992, 64, 265–288. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon payments for mangrove conservation: Ecosystem constraints and uncertainties of sequestration potential. Environ. Sci. Policy 2011, 14, 462–470. [Google Scholar] [CrossRef]
- Lewis, R.R. Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 2005, 24, 403–418. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Heider, C.; Cole, T.G.; Dwire, K.A.; Donato, D.C. Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands 2011, 31, 343–352. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Annu. Rev. Ecol. Evol. Syst. 1979, 5, 39–64. [Google Scholar] [CrossRef]
- Thom, B.G. Mangrove ecology: A geomorphological perspective. In Mangrove Ecosystem in Australia: Structure, Function, and Management; Clough, B.F., Ed.; Australian National University Press: Canberra, Australia, 1982; pp. 3–18. [Google Scholar]
- Twilley, R.W.; Lugo, A.E.; Patterson-Zucca, C. Litter production and turnover in basin mangrove forests in Southwest Florida. Ecology 1986, 67, 670–683. [Google Scholar] [CrossRef]
- Schmid, J.R.; Worley, K.; Addison, D.S.; Zimmerman, A.R.; Van Eaton, A. Naples Bay Past and Present: A Chronology of Disturbance to an Estuary; Naples Bay Initiative: Naples, FL, USA, 2005. [Google Scholar]
- Isacksson, M.; Erlandsson, B.; Mattsson, S. A 10-year study of the 137Cs distribution in soil and comparison of Cs soil inventory with precipitation-determined deposition. J. Environ. Radioact. 2001, 55, 47–59. [Google Scholar] [CrossRef]
- Stark, K.; Wallberg, P.; Nylen, T. Post-depositional redistribution and gradual accumulation of 137Cs in a riparian wetland ecosystem in Sweden. J. Environ. Radioact. 2006, 87, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Donato, D.C.; Kauffman, J.B.; Mackenzie, R.A.; Ainsworth, A.; Pfleeger, A.Z. Whole-island carbon stocks in the tropical Pacific: Implications for mangrove conservation and upland restoration. J. Environ. Manag. 2012, 97, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.G.; Oldfield, F. The calculation of Lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena 1978, 5, 1–8. [Google Scholar] [CrossRef]
- Richie, J.C.; McHenry, J.R. Application of radioactive fallout Cesium-137 for measuring soil erosion and sediment accumulation rates and patterns: A review. J. Environ. Qual. 1990, 19, 215–233. [Google Scholar] [CrossRef]
- Craft, C.B.; Richardson, C.J. Peat accretion and N, P, and organic C accumulation in nutrient-enriched and unenriched Everglades peatlands. Ecol. Appl. 1993, 3, 446–458. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. Comparing carbon sequestration in temperate freshwater wetland communities. Glob. Chang. Biol. 2012, 18, 1636–1647. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. Carbon sequestration in two created riverine wetlands in the Midwestern United States. J. Environ. Qual. 2013, 42, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Villa, J.A.; Mitsch, W.J. Carbon sequestration in different wetland plant communities in Southwest Florida. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 17–28. [Google Scholar] [CrossRef]
- Odum, E.P. Tidal marshes as outwelling/pulsing systems. In International Symposium: Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 3–7. [Google Scholar]
- Furukawa, K.; Wolanski, E.; Mueller, H. Currents and sediment transport in mangrove forests. Estuar. Coast. Shelf Sci. 1997, 44, 301–310. [Google Scholar] [CrossRef]
- Adame, M.F.; Lovelock, C.E. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 2011, 663, 23–50. [Google Scholar] [CrossRef]
- McKee, K.L.; Faulkner, P.L. Restoration of biogeochemical function in mangrove forests. Restor. Ecol. 2000, 8, 247–259. [Google Scholar] [CrossRef]
- Koch, M.S.; Snedaker, S.C. Factors influencing Rhizophora mangle (red mangrove) seedling development in Everglades carbonate soils. Aquat. Bot. 1997, 59, 87–98. [Google Scholar] [CrossRef]
- Harris, R.J.; Milbrandt, E.C.; Everham, E.M.; Bovard, B.D. The effects of reduced tidal flushing on mangrove structure and function across a disturbance gradient. Estuar. Coasts 2010, 33, 1176–1185. [Google Scholar] [CrossRef]
- Carter, M.R.; Burns, L.A.; Cavinger, T.R.; Dugger, K.R.; Fore, P.L.; Hicks, D.B.; Revells, H.L.; Schmidt, T.W. Ecosystems Analysis of the Big Cypress Swamp and Estuaries; Environmental Protection Agency: Athens, GA, USA, 1973. [Google Scholar]
- Cabezas, A.; MacDonnell, C; Lasso, A.; Bidalek, F.; Mitsch, W.J. Methane emission from mangroves in SW Florida. Environ. Technol. 2016. in review. [Google Scholar]
- National Oceanic and Atmospheric Administration. Sea-Level Trends. Available online: http://tidesandcurrents.noaa.gov/sltrends/sltrends.html (accessed on 3 September 2014).
- The Royal Society and the National Academy of Sciences. Climate Change: Evidence and Causes. An Overview from the Royal Society and the US National Academy of Sciences. 2014. Available online: https://royalsociety.org/topics-policy/projects/climate-evidence-causes/?utm_source=social_media&utm_medium=hootsuite&utm_campaign=standard (accessed on 18 October 2014).
- Watson, C.S.; White, N.J.; Church, J.A.; King, M.A.; Burgette, R.J.; Legresy, B. Unbated global mean sea-level rise over the satellite altimeter era. Nat. Clim. Chang. 2015. [Google Scholar] [CrossRef]
- Callaway, J.C.; DeLaune, R.D.; Patrick, W.H., Jr. Sediment accretion rates from four coastal wetlands along the Gulf of Mexico. J. Coast. Res. 1997, 13, 181–191. [Google Scholar]
- Breithaupt, J.L.; Smoak, J.M.; Smith, T.J., III; Sanders, C.J. Temporal variability of carbon and nutrient burial, sediment accretion, and mass accumulation over the past century in a carbonate platform mangrove forests of the Florida Everglades. J. Geophys. Res. Biogeosci. 2014, 119, 2032–2048. [Google Scholar] [CrossRef]
- Craft, C.B. Dynamics of nitrogen and phosphorus retention during wetland ecosystem succession. Wetl. Ecol. Manag. 1997, 4, 177–187. [Google Scholar] [CrossRef]
- Robbins, J.A. Geochemical and geophysical applications of radioactive lead. Biogeochem. Lead Environ. 1978, 2, 285–393. [Google Scholar]
- Lugo, A.E.; Brown, S.L.; Dodson, R.; Smith, T.S.; Shugart, H.H. The Holdridge life zones of the coterminous United States in relation to ecosystem mapping. J. Biogeogr. 1999, 26, 1025–1038. [Google Scholar] [CrossRef]
- Sanders, C.J.; Smoak, J.M.; Naidu, A.S.; Araripe, D.R.; Sanders, L.M.; Patchineelam, S.R. Mangrove forest sedimentation and its reference to sea level rise, Cananeia, Brazil. Environ. Earth Sci. 2010, 60, 1291–1301. [Google Scholar] [CrossRef]
- Brunskill, G.J.; Zagorskis, I.; Pfitzner, J. Carbon burial rates in sediments and a carbon mass balance for the Herbert River region of the Great Barrier Reef continental shelf, North Queensland, Australia. Estuar. Coast. Shelf Sci. 2002, 54, 677–700. [Google Scholar] [CrossRef]







| SC | HA | |||||
|---|---|---|---|---|---|---|
| Basin | Fringe | Riverine | Basin | Fringe | Riverine | |
| Tree density (stems m−2) | ||||||
| Rhizophora mangle | 1.2 | 0.39 | 0.51 | 0.18 | 0.46 | 0.77 |
| Avicennia germinans | 0.04 | 0.01 | 0 | 0 | 0 | 0 |
| Laguncularia racemosa | 0.08 | 0.03 | 0.01 | 0.02 | 0.18 | 0.21 |
| Mean DBH (cm) ± SE. | ||||||
| Rhizophora mangle | 3.6 ± 0.6 | 5.5 ± 0.7 | 5.9 ± 0.8 | 7.4 ± 0.9 | 3.2 ± 0.5 | 3.3 ± 0.2 |
| Avicennia germinans | 10.7 ** | 24.4 ** | - * | - * | - * | - * |
| Laguncularia racemosa | 4.5 ± 0.2 | 18.2 ± 1.1 | 25 ** | 8.1 ± 0.8 | 1.4 ± 0.2 | 7.0 ± 0.4 |
| plot size (m) | 5 × 5 | 10 × 10 | 10 × 10 | 10 × 10 | 5 × 10 | 5 × 7.5 |
| Hydrogeomorphic Setting | |||||||
|---|---|---|---|---|---|---|---|
| Basin | Fringe | Riverine | Dwarf | ||||
| Tidal Creek | SC | HA | SC | HA | SC | HA | SC |
| bulk density (g·cm−3) | 0.13 ± 0.009 | 0.78 ± 0.09 | 0.23 ± 0.03 | 0.25 ± 0.004 | 0.22 ± 0.02 | 0.13 ± 0.005 | 0.10 ± 0.003 |
| accretion rate (210Pb) (mm·year−1) | 1.68 | 1.58 | 2.28 | 2.23 | 5.43 | 3.04 | 1.41 |
| accretion rate (137Cs) (mm·year−1) | - | 7.34 | 3.26 | 3.67 | 3.67 | 2.85 | - |
| carbon concentration (g-C kg−1) | 238 ± 3 | 40 ± 7 | 232 ± 38 | 131 ± 18 | 143 ± 21 | 324 ± 4 | 91 ± 5 |
| carbon pool (kg-C m−2) | 2.5 ± 0.2 | 2.3 ± 0.2 | 6.3 ± 1.7 | 3.7 ± 0.5 | 8.1 ± 0.3 | 6.2 ± 0.3 | 1.0 ± 0.3 |
| carbon sequestration rate (g-C m−2·year−1) | 50 ± 4 | 47 ± 4 | 127 ± 33 | 74 ± 10 | 162 ± 5 | 126 ± 6 | 21 ± 5 |
| Location | Hydrogeomorphic Environment | Sequestration Rate (g-C m−2·year−1) | References |
|---|---|---|---|
| Susan’s Creek (reference tidal creek) | Riverine | 162 | This study |
| Fringe | 127 | ||
| Basin | 50 | ||
| Dwarf | 21 | ||
| Hamilton Avenue Creek (disturbed tidal creek) | Riverine | 125 | This study |
| Fringe | 73 | ||
| Basin | 47 | ||
| Global Average | 174 ± 23 | [9] | |
| Global Average | 163 | [11] | |
| Global Average | 226 ± 39 | [15] | |
| Everglades, Southeast Florida | 123 ± 19 | [58] | |
| Rookery Bay, Southwest Florida | Fringe | 69 | [24] |
| Rookery Bay, Southwest Florida | Fringe | 99 | [24] |
| Henderson Creek, Southwest Florida | 20 | Cahoon and Lynch unpublished | |
| Henderson Creek, Southwest Florida | 39 | Cahoon and Lynch unpublished | |
| Florida Keys | 209 | [57] | |
| Florida Keys | 67 | [57] | |
| Shark River, Southeast Florida | 151 | [58] | |
| Terminos Lagoon, Mexico | Riverine | 137 | [24] |
| Tamandare, Brazil | 949 | [62] | |
| Trat, Thailand | Riverine | 600 | [63] |
| Hinchinbrook Channel, Australia | 26 | [63] | |
| Hinchinbrook Channel, Australia | 336 | [63] | |
| Irian Jaya, Indonesia | 412 | [63] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchio, D.A.; Savarese, M.; Bovard, B.; Mitsch, W.J. Carbon Sequestration and Sedimentation in Mangrove Swamps Influenced by Hydrogeomorphic Conditions and Urbanization in Southwest Florida. Forests 2016, 7, 116. https://doi.org/10.3390/f7060116
Marchio DA, Savarese M, Bovard B, Mitsch WJ. Carbon Sequestration and Sedimentation in Mangrove Swamps Influenced by Hydrogeomorphic Conditions and Urbanization in Southwest Florida. Forests. 2016; 7(6):116. https://doi.org/10.3390/f7060116
Chicago/Turabian StyleMarchio, Daniel A., Michael Savarese, Brian Bovard, and William J. Mitsch. 2016. "Carbon Sequestration and Sedimentation in Mangrove Swamps Influenced by Hydrogeomorphic Conditions and Urbanization in Southwest Florida" Forests 7, no. 6: 116. https://doi.org/10.3390/f7060116