Forest Biomass and Net Primary Productivity in Southwestern China: A Meta-Analysis Focusing on Environmental Driving Factors
Abstract
:1. Introduction
2. Materials and Methods
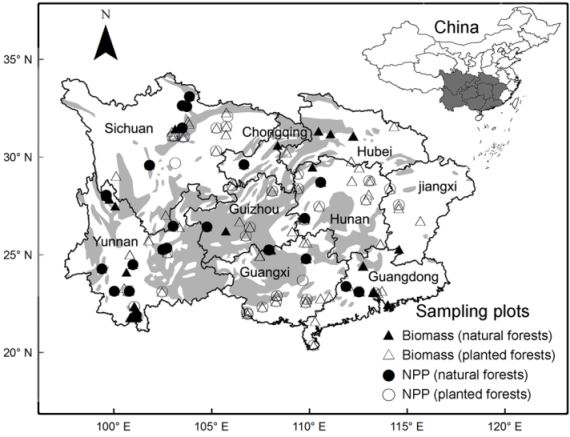
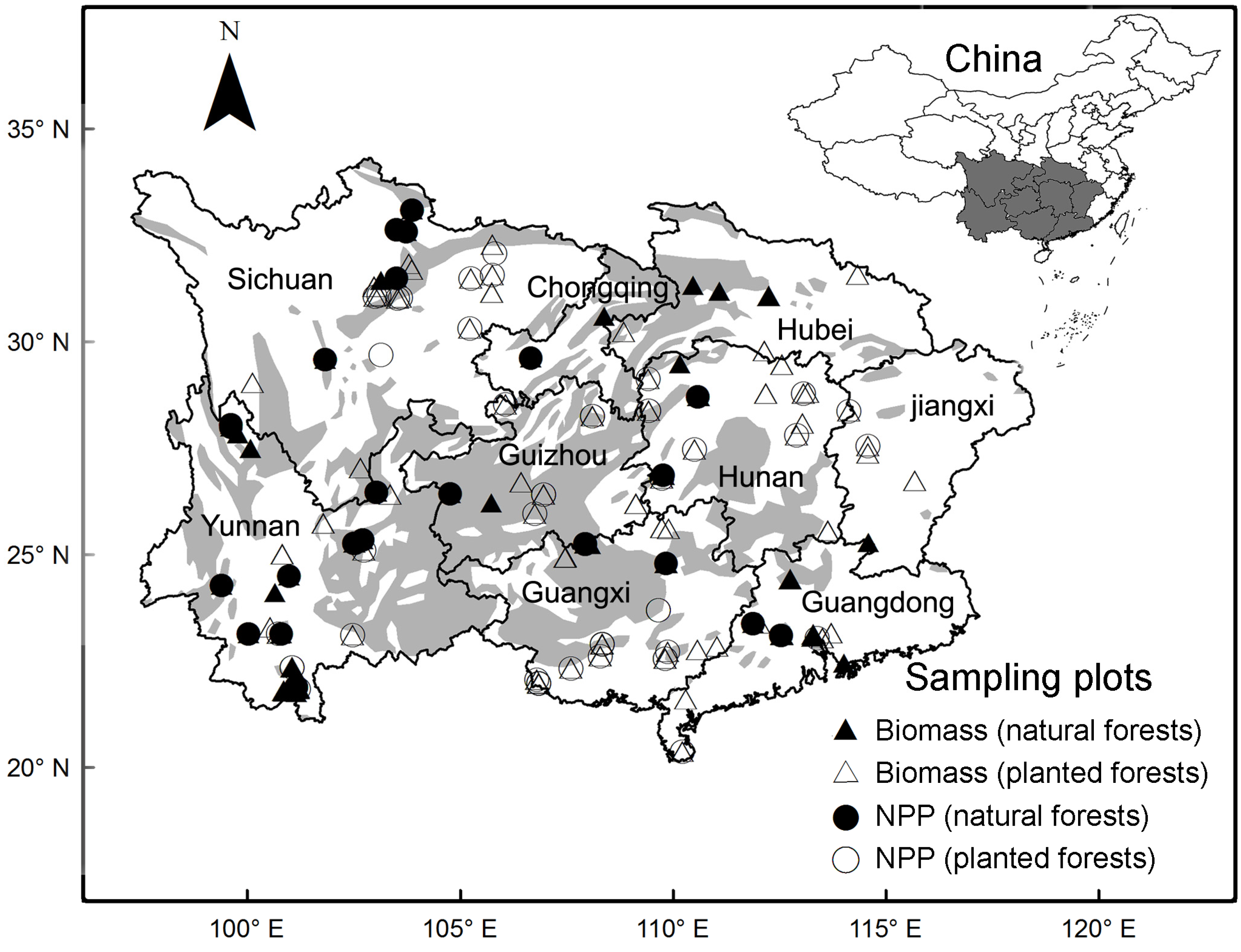
2.1. Data Collection and Treatments
2.2. Climate Factors
2.3. Biomass and NPP of Karst and Non-Karst Forests
2.4. Data Analyses
3. Results
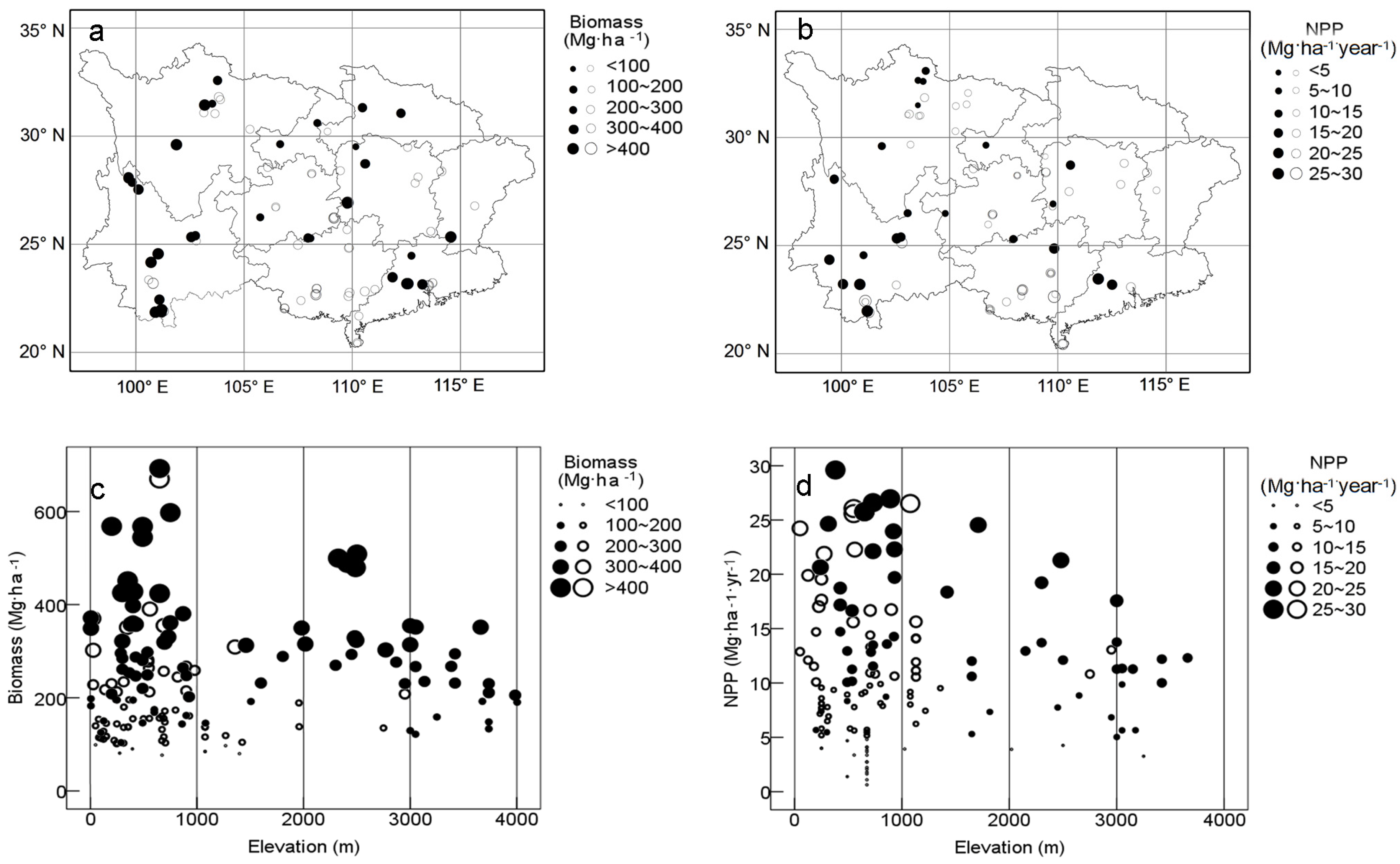
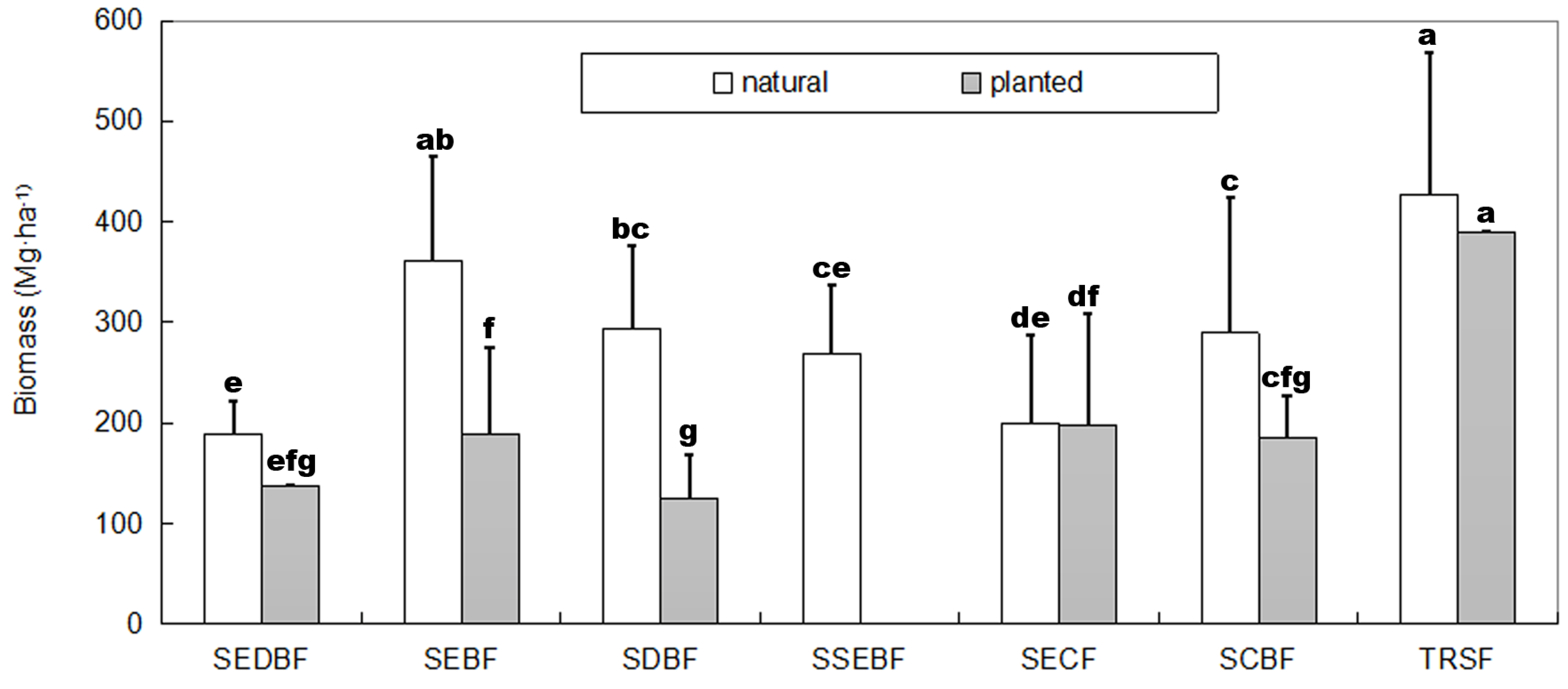
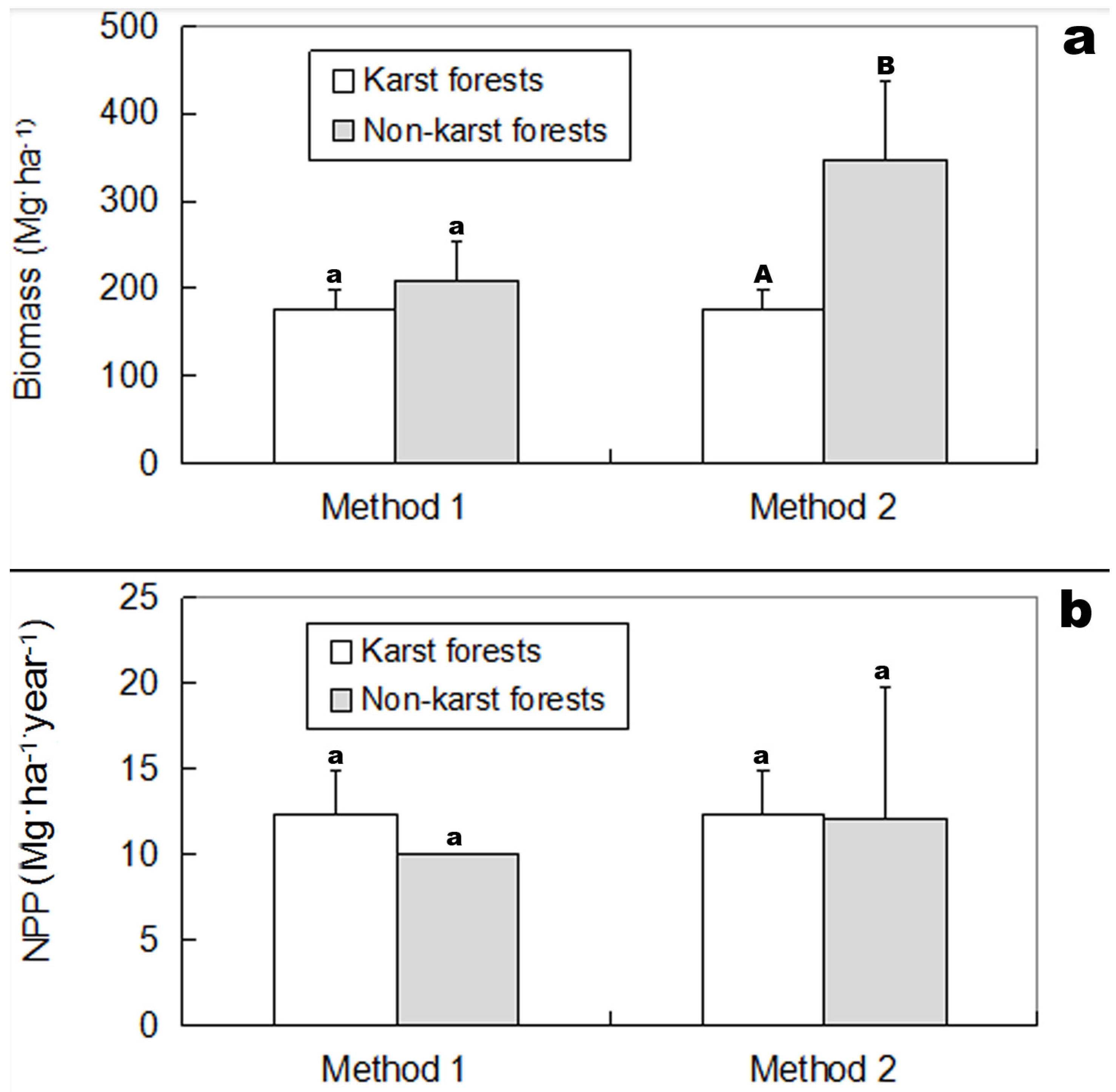
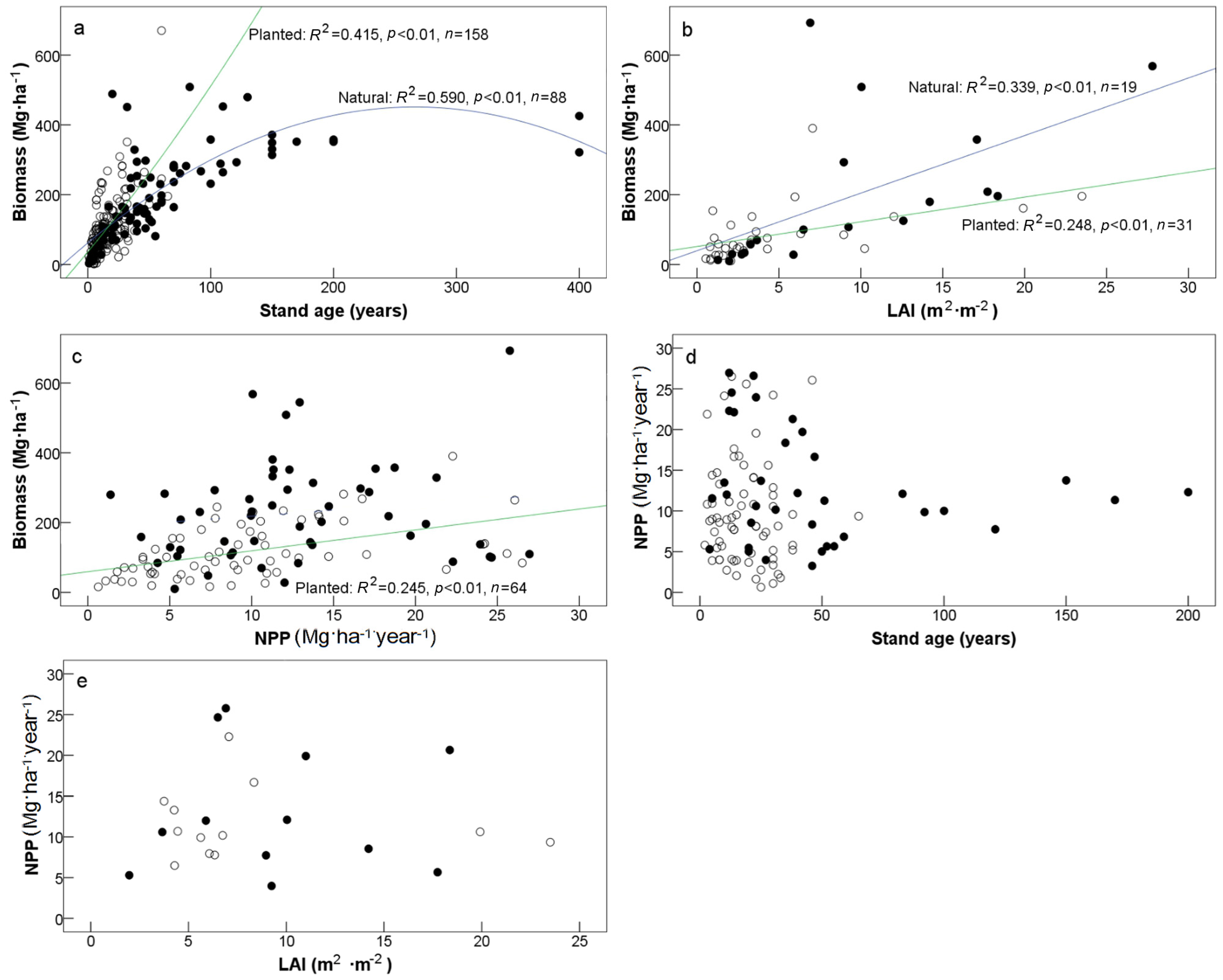
3.1. Biomass and NPP
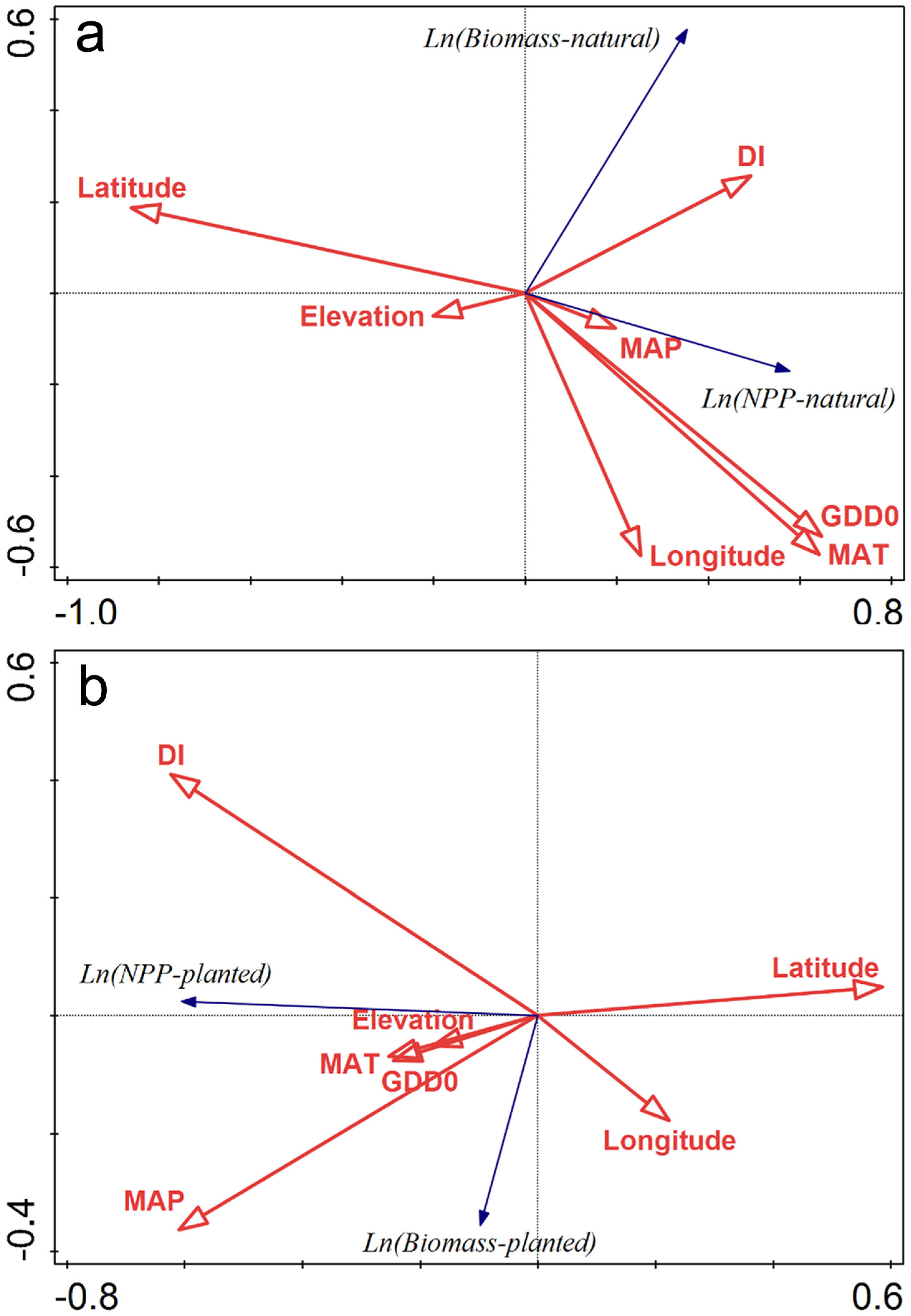
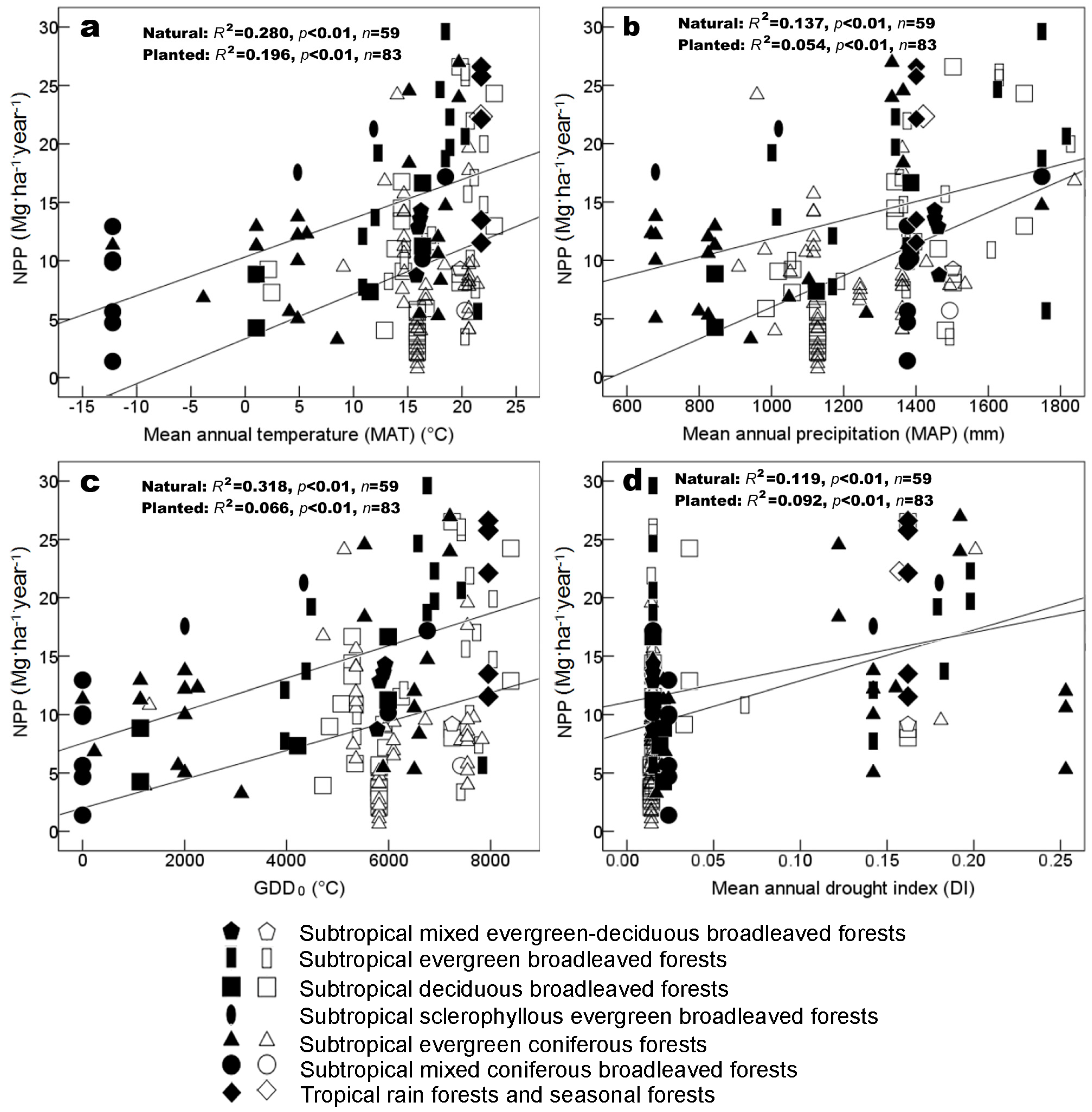
3.2. Environmental Factors Controlling Biomass and NPP
4. Discussion
4.1. Productivity vs. Forest Stand Origin
4.2. Productivity vs. Topography and Parent Materials
4.3. Productivity vs. Latitude and Climates
4.4. Inspirations for the Development of Regional to Global Productivity Databases
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, V.; Birdsey, R.; Stakanov, V.; Korotkov, T. Carbon in vegetation of Russian forests: Methods to estimate storage and geographical distribution. Water Air Soil Pollut. 1995, 82, 271–282. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, X.S.; Scurlock, J.M.O. Synthesis and analysis of biomass and net primary productivity in Chinese forests. Ann. For. Sci. 2001, 58, 351–384. [Google Scholar] [CrossRef]
- Ni, J. Net primary productivity in forests of China: Scaling-up of national inventory data and comparison with model predictions. For. Ecol. Manag. 2003, 176, 485–495. [Google Scholar] [CrossRef]
- Houghton, R.A. Aboveground forest biomass and the global carbon balance. Glob. Chang. Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- Powell, S.L.; Cohen, W.B.; Healey, S.P.; Kennedy, R.E.; Moisen, G.G.; Pierce, K.B.; Ohmann, J.L. Quantification of live aboveground forest biomass dynamics with Landsat time-series and field inventory data: A comparison of empirical modeling approaches. Remote Sens. Environ. 2010, 114, 1053–1068. [Google Scholar] [CrossRef]
- Cramer, W.; Moore, B., III; Sahagian, D. Data needs for modelling global biospheric carbon fluxes: Lessons from a comparison of models. IGBP Newsl. 1996, 27, 13–15. [Google Scholar]
- Scurlock, J.M.O.; Cramer, W.; Olson, R.J.; Parton, W.J.; Prince, S.D. Terrestrial NPP: Towards a consistent data set for global model evaluation. Ecol. Appl. 1999, 9, 913–919. [Google Scholar] [CrossRef]
- Keeling, H.C.; Phillips, O.L. The global relationship between forest productivity and biomass. Glob. Ecol Biogeogr. 2007, 16, 618–631. [Google Scholar] [CrossRef]
- Luyssaert, S.; Inglima, I.; Jung, M.; Richardson, A.D.; Reichstein, M.; Papale, D.; Piao, S.L.; Schulze, E.D.; Wingate, L.; Matteucci, G.; et al. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob. Chang. Biol. 2007, 13, 2509–2537. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Randerson, J.T.; Abramowita, G.; Bacour, C.; Blyth, E.; Carvalhais, N.; Ciais, P.; Dalmonech, D.; Fisher, J.B.; Fisher, R.; et al. A framework for benchmarking land models. Biogeosciences 2012, 9, 3857–3874. [Google Scholar] [CrossRef]
- Kelley, D.I.; Prentice, I.C.; Harrison, S.P.; Wang, H.; Simard, M.; Fisher, J.B.; Willis, K.O. A comprehensive benchmarking system for evaluating global vegetation models. Biogeosciences 2013, 10, 3313–3340. [Google Scholar] [CrossRef]
- Piao, S.L.; Sitch, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Wang, X.H.; Ahlström, A.; Anav, A.; Canadell, J.G.; Cong, N.; et al. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob. Chang. Biol. 2013, 19, 2117–2132. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Chen, A.P.; Peng, C.H.; Zhao, S.Q.; Ci, L.J. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.L.; Fang, J.Y.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.X. Patterns of Net Primary Productivity for Chinese Major Forest Types and Their Mathematical Models; The Commission for Integrated Survey of Natural Resources, Chinese Academy of Sciences: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Feng, Z.W.; Wang, X.K.; Wu, G. Biomass and Primary Productivity of Forest Ecosystems in China; Science Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Li, G.F.; Ren, H. Biomass and net primary productivity of the forests in different climatic zones of China. Trop. Geogr. 2004, 24, 306–310. (In Chinese) [Google Scholar] [CrossRef]
- Luo, Y.J.; Wang, X.K.; Zhang, X.Q.; Lu, F. Biomass and Its Allocation of Forest Ecosystems in China; China Forestry Publishing House: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Xiang, W.H.; Liu, S.H.; Deng, X.W.; Shen, A.H.; Lei, X.D.; Tian, D.L.; Zhao, M.F.; Peng, C.H. General allometric equations and biomass allocation of Pinus massoniana trees on a regional scale in southern China. Ecol. Res. 2011, 26, 697–711. (In Chinese) [Google Scholar] [CrossRef]
- Liu, W.W. Application of Allometric Equations and Biomass Expansion Factor for Estimating Cunninghamia lanceolata Forest Biomass on Large Scale. Master’s Thesis, Central South University of Forestry & Technology, Changsha, China, 2009. [Google Scholar]
- Editorial Committee for Vegetation of China (ECVC). Vegetation of China; Science Press: Beijing, China, 1980. (In Chinese) [Google Scholar]
- Editorial Committee of Vegetation Map of the People’s Republic of China (ECVMC); Chinese Academy of Sciences. Vegetation Map of the People’s Republic of China (1:1 000 000); Geology Press: Xi’an, China, 2007. (In Chinese) [Google Scholar]
- Jiang, Z.C.; Lian, Y.Q.; Qin, X.Q. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Yu, W.L.; Dong, D.; Ni, J. Comparisons of biomass and net primary productivity of karst and non-karst forests in mountainous areas, Southwestern China. J. Subtrop. Resour. Environ. 2010, 5, 25–30. (In Chinese) [Google Scholar]
- Wu, P.; Ding, F.J.; Chen, J. Study on the biomass and productivity of forest in Southwest China. Hubei Agric. Sci. 2012, 51, 1513–1518, 1527. (In Chinese) [Google Scholar]
- Hutchinson, M.; Hancock, P.A. Spatial interpolation of large climate data sets using bivariate thin plate smoothing splines. Environ. Model. Softw. 2006, 21, 1684–1694. [Google Scholar]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The shuttle radar topography mission. Rev. Geophys. 2007, 45. [Google Scholar] [CrossRef]
- Prentice, I.C.; Sykes, M.T.; Cramer, W. A simulation model for the transient effects of climate change on forest landscapes. Ecol. Model. 1993, 65, 51–70. [Google Scholar] [CrossRef]
- Gallego-Sala, A.V.; Clark, J.M.; House, J.I.; Orr, H.G.; Prentice, I.C.; Smith, P.; Farewell, T.; Chapman, S.J. Bioclimatic envelope model of climate change impacts on blanket peatland distribution in Great Britain. Clim. Res. 2011, 45, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Coomes, D.A.; Flores, O.; Holdaway, R.; Jucker, T.; Lines, E.R.; Vanderwel, M.C. Wood production response to climate change will depend critically on forest composition and structure. Glob. Chang. Biol. 2014, 20, 3632–3645. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Bartlett, M.; Wang, Y.; He, F.; Weiner, J.; Chave, J.; Sack, L. Does climate directly influence NPP globally? Glob. Chang. Biol. 2016, 22, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.C. A comparative evaluation of the man-made forest and natural forest. World For. Res. 1991, 3, 50–56. (In Chinese) [Google Scholar]
- Xing, S.H.; Ji, W.Y.; Guo, N.; Cui, G.F.; Wang, M.; Xue, Q.; Jiang, X.M. Comparison of community character between the natural spruce forest and the plantation in Miyaluo in west Sichuan. J. Mt. Sci. 2010, 28, 218–225. (In Chinese) [Google Scholar]
- Ghimire, C.P.; Bruijnzeel, L.A.; Lubczynski, M.W.; Bonell, M. Rainfall interception by natural and planted forests in the middle mountains of central Nepal. J. Hydrol. 2012, 475, 270–280. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Dobbertin, M.; Fernández-Cancio, Á.; Vilà-Cabrera, A.; Manzanedo, R.D.; Zavala, M.A.; Navarro-Cerrillo, R.M. Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. natural rear-edge Pinus nigra forests. For. Ecol. Manag. 2013, 310, 956–967. [Google Scholar] [CrossRef]
- Woziwoda, B.; Kopeć, D. Afforestation or natural succession? Looking for the best way to manage abandoned cut-over peatlands for biodiversity conservation. Ecol. Eng. 2014, 63, 143–152. [Google Scholar] [CrossRef]
- Scurlock, J.M.O.; Olson, R.J. Terrestrial net primary productivity—A brief history and a new worldwide database. Environ. Rev. 2002, 10, 91–109. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, G.S. Estimation of biomass and net primary productivity of major planted forests in China based on forest inventory data. For. Ecol. Manag. 2005, 207, 295–313. [Google Scholar] [CrossRef]
- Schulze, E.D.; Wirth, C.; Heimann, M. Managing forests after Kyoto. Science 2000, 289, 204–205. [Google Scholar] [CrossRef]
- Gillman, L.N.; Wright, S.D.; Cusens, J.; McBride, P.D.; Malhi, Y.; Whittaker, R.J. Latitude, productivity and species richness. Glob. Ecol. Biogeogr. 2015, 24, 107–117. [Google Scholar] [CrossRef]
- Lieth, H.F.H.; Whittaker, R.H. Primary Productivity of the Biosphere; Springer-Verlag: New York, NY, USA, 1975. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the World’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Kindermann, G.E.; McCallum, I.; Fritz, S.; Obersteiner, M. A global forest growing stock, biomass and carbon map based on FAO statistics. Silva Fenn. 2008, 42, 387–396. [Google Scholar] [CrossRef]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Reichstein, M.; Margolis, H.A.; Cescatti, A.; Richardson, A.D.; Arain, M.A.; Arneth, A.; Bernhofer, C.; Bonal, D.; Chen, J.Q.; et al. Global patterns of land-atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. J. Geophys. Res.-Biogeosci. 2011, 116, G00J07. [Google Scholar] [CrossRef]
- Falster, D.S.; Duursma, R.A.; Ishihara, M.I.; Barneche, D.R.; FitzJohn, R.G.; Vårhammar, A.; Aiba, M.; Ando, M.; Anten, N.; Aspinwall, M.J.; et al. BAAD: A biomass and allometry database for woody plants. Ecology 2015, 96, 1445. [Google Scholar] [CrossRef]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J. Measuring net primary production in forests: Concepts and field methods. Ecol. Appl. 2001, 11, 356–370. [Google Scholar] [CrossRef]
- Fang, J.Y.; Yu, G.R.; Ren, X.B.; Liu, G.H.; Zhao, X.Q. Carbon sequestration in China’s terrestrial ecosystems under climate change: Progress on ecosystem carbon sequestration from the CAS Strategic Priority Research Program. Bull. Chin. Acad. Sci. 2015, 30, 848–857. [Google Scholar]
- Ni, J.; Luo, D.H.; Xia, J.; Zhang, Z.H.; Hu, G. Vegetation in karst terrain of southwestern China allocates more biomass to roots. Solid Earth 2015, 6, 799–810. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Wei, L.M.; Chen, Z.R.; Zhang, C.G. A preliminary study on biomass components of karst forest in Maolan of Guizhou province, China. Acta Phytoecol. Sin. 1995, 19, 358–367. (In Chinese) [Google Scholar]
- Liu, C.C.; Wei, Y.F.; Liu, Y.G.; Guo, K. Biomass of canopy and shrub layers of karst forest in Puding, Guizhou, China. Chin. J. Plant Ecol. 2009, 33, 698–705. (In Chinese) [Google Scholar]
| Forest Type | Abbreviation | Geographical Range | Elevation (m) | Dominant Taxa |
|---|---|---|---|---|
| subtropical evergreen broadleaved forest | SEBF | 99–118° E, 23–32° N | 5–2800 | Castanopsis, Cyclobalanopsis, Lithocarpus, Machilus, Schima, Theaceae |
| subtropical sclerophyllous evergreen broadleaved forest | SSEBF | 97–103° E, 25–32° N | 2000–4300 | Quercus |
| subtropical mixed evergreen-deciduous broadleaved forest | SEDBF, including northern zonal SEDBF and karst azonal SEDBF | 97–118° E, 25–34° N | 500–2000 | Carpinus, Castanopsis, Cyclobalanopsis, Cinnamomum, Itea, Lithocarpus, Platycarya |
| subtropical evergreen coniferous forest | SECF | 97–118° E, 24–34 °N | 20–4000 | Abies, Cunninghamia, Cryptomeria, Cupressus, Picea, Pinus, Taiwania, Tsuga |
| subtropical mixed coniferous broadleaved forest | SCBF | 97–118° E, 23–34° N | 5–3050 | Abies, Acacia confusa, Alnus cremastogyne, Cupressus, Liquidambar formosana, Michelia macclurei, Picea, Pinus, Populus, Sassafras tzumu, Schima superba, Tsuga |
| subtropical deciduous broadleaved forest | SDBF | 97–118° E, 24–34° N | 50–2650 | Alnus, Betula, Liquidambar formosana, Populus, Quercus, Robinia pseudoacacia |
| tropical rain forest and seasonal forest | TRSF | 97–115° E, 20–23° N | 150–1500 | Baccaurea ramiflora, Dipterocarpus tonkinensis, Macaranga denticulata, Mallotus paniculatus, Parashorea chinensis, Pometia, Terminalia myriocarpa, Vatica astrotricha |
| Forest Type | SEBF | SSEBF | SEDBF | SECF | SCBF | TRSF | ||
|---|---|---|---|---|---|---|---|---|
| Biomass (Mg·ha−1) | Reference [18] | N | 292.3 ± 129.1 | / | 164.1 ± 45.2 | 158.8 ± 99.2 | / | / |
| P | 103.4 ± 86.6 | / | / | / | / | |||
| Reference [3] | 248.6 ± 111.7 | 247.9 ± 53.7 | 200.3 ± 90.4 | 147.6 ± 24.6 | 173.3 ± 82.4 | 440.0 ± 290.9 | ||
| This study | N | 361.6 ± 102.9 | 268.4 ± 68.0 | 188.8 ± 33.8 | 200.0 ± 86.8 | 289.4 ± 135.1 | 427.2 ± 141.6 | |
| P | 188.8 ± 86.1 | / | 137.1 ± 0.3 | 198.3 ± 110.2 | 185.4 ± 41.1 | 390.4 | ||
| NPP (Mg·ha−1·year−1) | Reference [18] | N | 20.3 ± 4.7 | / | 4.6 ± 2.2 | 12.7 ± 7.6 | / | / |
| P | 25.8 ± 0.3 | / | / | / | / | |||
| Reference [3] | 21.9 ± 5.3 | 11.4 ± 1.6 | 15.2 ± 3.0 | 13.5 ± 3.0 | 9.9 ± 2.7 | 27.1 ± 9.2 | ||
| This study | N | 17.6 ± 7.2 | 19.4 ± 2.6 | 11.9 ± 2.4 | 12.1 ± 6.5 | 9.0 ± 5.0 | 20.9 ± 6.7 | |
| P | 14.5 ± 6.9 | / | 9.2 | 8.3 ± 5.1 | 5.6 | 22.3 | ||
| Forest Type | Biomass Mg·ha−1 | NPP Mg·ha−1·year−1 | MAP mm | MAT °C | GDD0 °C | DI | |
|---|---|---|---|---|---|---|---|
| SEBF | N | 361.6 ± 102.9 | 17.6 ± 7.2 | 1525.3 ± 288.9 | 17.3 ± 4.0 | 6331.0 ± 1451.8 | 0.069 ± 0.073 |
| P | 188.8 ± 86.1 | 14.5 ± 6.9 | 1432.0 ± 185.0 | 19.6 ± 2.6 | 7148.5 ± 942.3 | 0.017 ± 0.009 | |
| SSEBF | N | 268.4 ± 68.0 | 19.4 ± 2.6 | 808.7 ± 94.5 | 8.9 ± 2.8 | 3325.4 ± 883.4 | 0.193 ± 0.045 |
| P | / | / | / | / | / | / | |
| SEDBF | N | 188.8 ± 33.8 | 11.9 ± 2.4 | 1355.7 ± 170.1 | 11.7 ± 10.6 | 4972.7 ± 2217.1 | 0.016 ± 0.003 |
| P | 137.1 ± 0.3 | 9.2 | 1502.4 ± 0.0 | 19.8 ± 0.0 | 7244.9 ± 0.0 | 0.162 ± 0.000 | |
| SECF | N | 200.0 ± 86.8 | 12.1 ± 6.5 | 1058.0 ± 375.3 | 8.8 ± 9.1 | 3685.9 ± 2646.7 | 0.085 ± 0.078 |
| P | 198.3 ± 110.2 | 8.3 ± 5.1 | 1291.4 ± 233.6 | 16.7 ± 4.1 | 6122.3 ± 1443.9 | 0.027 ± 0.044 | |
| SCBF | N | 289.4 ± 135.1 | 9.0 ± 5.0 | 1515.4 ± 195.2 | 0.6 ± 16.2 | 2899.7 ± 3673.8 | 0.020 ± 0.005 |
| P | 185.4 ± 41.1 | 5.6 | 1677.4 ± 184.8 | 20.3 ± 1.9 | 7414.8 ± 696.0 | 0.015 ± 0.000 | |
| SDBF | N | 294.5 ± 82.3 | 9.7 ± 4.7 | 1273.4 ± 256.6 | 12.8 ± 5.7 | 4795.8 ± 1852.8 | 0.017 ± 0.003 |
| P | 124.9 ± 44.0 | 9.5 ± 6.6 | 1343.1 ± 209.3 | 16.8 ± 3.0 | 6145.5 ± 1090.5 | 0.042 ± 0.055 | |
| TRSF | N | 427.2 ± 141.6 | 20.9 ± 6.7 | 1392.2 ± 63.3 | 21.6 ± 0.7 | 7903.1 ± 260.8 | 0.166 ± 0.011 |
| P | 390.4 | 22.3 | 1419.9 | 21.8 | 7941.8 | 0.157 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.B.; Yang, H.M.; Xu, Y.; Guo, Y.M.; Ni, J. Forest Biomass and Net Primary Productivity in Southwestern China: A Meta-Analysis Focusing on Environmental Driving Factors. Forests 2016, 7, 173. https://doi.org/10.3390/f7080173
Liu LB, Yang HM, Xu Y, Guo YM, Ni J. Forest Biomass and Net Primary Productivity in Southwestern China: A Meta-Analysis Focusing on Environmental Driving Factors. Forests. 2016; 7(8):173. https://doi.org/10.3390/f7080173
Chicago/Turabian StyleLiu, Li Bin, Hua Mei Yang, Yue Xu, Yin Ming Guo, and Jian Ni. 2016. "Forest Biomass and Net Primary Productivity in Southwestern China: A Meta-Analysis Focusing on Environmental Driving Factors" Forests 7, no. 8: 173. https://doi.org/10.3390/f7080173