Major Changes in Growth Rate and Growth Variability of Beech (Fagus sylvatica L.) Related to Soil Alteration and Climate Change in Belgium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tree Selection and Ring-Width Series
2.2. Low-Frequency Signal and High-Frequency Variability of Beech Ring-Width
2.3. Statistical Methodology, Model Formulation and Evaluation
2.4. Distinction of Size- and Time-Dependent Effects
3. Results
3.1. Modeling Steps and Model Selection
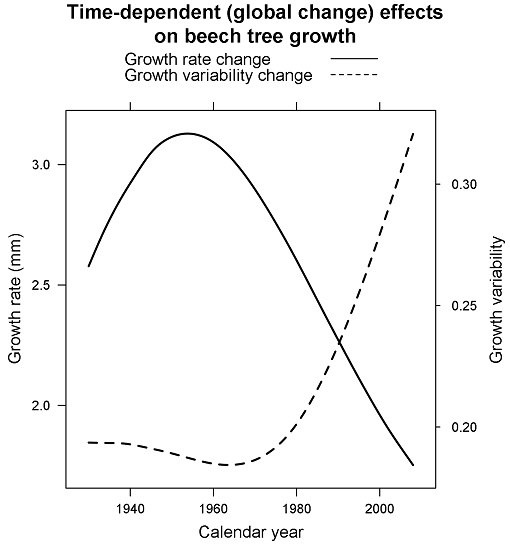
3.2. Size- and Time-Dependent Changes over Time
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Lloret, F.; Breshears, D.D. Drought-induced forest decline: Causes, scope and implications. Biol. Lett. 2012, 8, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Waldner, P.; Marchetto, A.; Thimonier, A.; Schmitt, M.; Rogora, M.; Granke, O.; Mues, V.; Hansen, K.; Pihl Karlsson, G.; Žlindra, D.; et al. Detection of temporal trends in atmospheric deposition of inorganic nitrogen and sulphate to forests in Europe. Atmos. Environ. 2014, 95, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Guillemot, J.; Delpierre, N.; Vallet, P.; François, C.; Martin-StPaul, N.K.; Soudani, K.; Nicolas, M.; Badeau, V.; Dufrêne, E. Assessing the effects of management on forest growth across France: Insights from a new functional–structural model. Ann. Bot. 2014, 114, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Trouvé, R.; Bontemps, J.D.; Collet, C.; Seynave, I.; Lebourgeois, F. Growth partitioning in forest stands is affected by stand density and summer drought in sessile Oak and Douglas-fir. For. Ecol. Manag. 2014, 334, 358–368. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Bradford, J.B.; Fraver, S.; Palik, B.J. Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol. Appl. 2013, 23, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Lebourgeois, F.; Eberlé, P.; Mérian, P.; Seynave, I. Social status-mediated tree-ring responses to climate of Abies alba and Fagus sylvatica shift in importance with increasing stand basal area. For. Ecol. Manag. 2014, 328, 209–218. [Google Scholar] [CrossRef]
- Cambi, M.; Certini, G.; Neri, F.; Marchi, E. The impact of heavy traffic on forest soils: A review. For. Ecol. Manag. 2015, 338, 124–138. [Google Scholar] [CrossRef]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010; p. 368. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976; p. 567. [Google Scholar]
- Fritts, H.C.; Swetnam, T.W. Dendroecology: A tool for evaluating variations in past and present forest environments. Adv. Ecol. Res. 1989, 19, 111–188. [Google Scholar]
- Weiskittel, A.R.; Hann, D.W.; Kershaw, J.A.; Vanclay, J.K. Forest Growth and Yield Modeling; Wiley-Blackwell: Hoboken, NJ, USA, 2011; p. 430. [Google Scholar]
- Bowman, D.M.J.S.; Brienen, R.J.W.; Gloor, E.; Phillips, O.L.; Prior, L.D. Detecting trends in tree growth: Not so simple. Trends Plant Sci. 2013, 18, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.R.; Kairiukstis, L.A. Methods of dendrochronology: Applications in the environmental sciences; Kluwer Academic Publishers: Boston, MA, USA, 1990; p. 394. [Google Scholar]
- Rozas, V.; DeSoto, L.; Olano, J.M. Sex-specific, age-dependent sensitivity of tree-ring growth to climate in the dioecious tree Juniperus thurifera. New Phytol. 2009, 182, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Genet, H.; Bréda, N.; Dufrêne, E. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiol. 2009, 30, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Copenheaver, C.A.; Crawford, C.J.; Fearer, T.M. Age-specific responses to climate identified in the growth of Quercus alba. Trees-Struct. Funct. 2011, 25, 647–653. [Google Scholar] [CrossRef]
- Mérian, P.; Lebourgeois, F. Size-mediated climate-growth relationships in temperate forests: A multi-species analysis. For. Ecol. Manag. 2011, 261, 1382–1391. [Google Scholar] [CrossRef]
- Rozas, V. Individual-based approach as a useful tool to disentangle the relative importance of tree age, size and inter-tree competition in dendroclimatic studies. For. Biogeosci. For. 2015, 8, 187–194. [Google Scholar] [CrossRef]
- Mencuccini, M.; Martínez-Vilalta, J.; Vanderklein, D.; Hamid, H.A.; Korakaki, E.; Lee, S.; Michiels, B. Size-mediated ageing reduces vigour in trees. Ecol. Lett. 2005, 8, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Geßler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees-Struct. Funct. 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Bontemps, J.D.; Hervé, J.C.; Dhôte, J.F. Dominant radial and height growth reveal comparable historical variations for common beech in north-eastern France. For. Ecol. Manag. 2010, 259, 1455–1463. [Google Scholar] [CrossRef]
- Charru, M.; Seynave, I.; Morneau, F.; Bontemps, J.D. Recent changes in forest productivity: An analysis of national forest inventory data for common beech (Fagus sylvatica L.) in north-eastern France. For. Ecol. Manag. 2010, 260, 864–874. [Google Scholar] [CrossRef]
- Kint, V.; Aertsen, W.; Campioli, M.; Vansteenkiste, D.; Delcloo, A.; Muys, B. Radial growth change of temperate tree species in response to altered regional climate and air quality in the period 1901–2008. Clim. Chang. 2012, 115, 343–363. [Google Scholar] [CrossRef]
- Aertsen, W.; Janssen, E.; Kint, V.; Bontemps, J.D.; van Orshoven, J.; Muys, B. Long-term growth changes of common beech (Fagus sylvatica L.) are less pronounced on highly productive sites. For. Ecol. Manag. 2014, 312, 252–259. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Friedrichs, D.A.; Trouet, V.; Büntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Löffler, J. Species-specific climate sensitivity of tree growth in Central-West Germany. Trees-Struct. Funct. 2009, 23, 729–739. [Google Scholar] [CrossRef]
- Scharnweber, T.; Manthey, M.; Criegee, C.; Bauwe, A.; Schröder, C.; Wilmking, M. Drought matters—Declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For. Ecol. Manag. 2011, 262, 947–961. [Google Scholar] [CrossRef]
- Weber, P.; Bugmann, H.; Pluess, A.R.; Walthert, L.; Rigling, A. Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees-Struct. Funct. 2013, 27, 171–181. [Google Scholar] [CrossRef]
- Castagneri, D.; Nola, P.; Motta, R.; Carrer, M. Summer climate variability over the last 250 years differently affected tree species radial growth in a mesic Fagus-Abies-Picea old-growth forest. For. Ecol. Manag. 2014, 320, 21–29. [Google Scholar] [CrossRef]
- Latte, N.; Lebourgeois, F.; Claessens, H. Increased tree-growth synchronization of beech (Fagus sylvatica L.) in response to climate change in northwestern Europe. Dendrochronologia 2015, 33, 69–77. [Google Scholar] [CrossRef]
- Latte, N.; Kint, V.; Drouet, T.; Penninckx, V.; Lebourgeois, F.; Vanwijnsberghe, S.; Claessens, H. Dendroécologie du Hêtre en Forêt de Soignes. Les cernes des arbres nous renseignent sur les changements récents et futurs. Forêt Nat. 2015, 137, 24–37. [Google Scholar]
- Jump, A.S.; Hunt, J.M.; Pen̈uelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Di Filippo, A.; Biondi, F.; Maugeri, M.; Schirone, B.; Piovesan, G. Bioclimate and growth history affect beech lifespan in the Italian Alps and Apennines. Glob. Chang. Biol. 2012, 18, 960–972. [Google Scholar] [CrossRef]
- Bolte, A.; Hilbrig, L.; Grundmann, B.; Kampf, F.; Brunet, J.; Roloff, A. Climate change impacts on stand structure and competitive interactions in a southern Swedish spruce-beech forest. Eur. J. For. Res. 2010, 129, 261–276. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Bréda, N.; Ulrich, E.; Granier, A. Climate-tree-growth relationships of European beech (Fagus sylvatica L.) in the French Permanent Plot Network (RENECOFOR). Trees-Struct. Funct. 2005, 19, 385–401. [Google Scholar] [CrossRef]
- Demarée, G.R.; Lachaert, P.J.; Verhoeve, T.; Thoen, E. The long-term daily central Belgium temperature (CBT) series (1767–1998) and early instrumental meteorological observations in Belgium. Clim. Chang. 2002, 53, 269–293. [Google Scholar] [CrossRef]
- IRM. Vigilance Climatique. Institut Royal Météorologique de Belgique, 2015. Available online: http://www.meteo.be/resources/20150508vigilance-oogklimaat/vigilance_climatique_IRM_2015_WEB_FR_BAT.pdf (accessed on 15 April 2016).
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Greenwood, D.L.; Weisberg, P.J. Density-dependent tree mortality in pinyon-juniper woodlands. For. Ecol. Manag. 2008, 255, 2129–2137. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Chang. Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Latte, N.; Lebourgeois, F.; Claessens, H. Growth partitioning within beech trees (Fagus sylvatica L.) varies in response to summer heat waves and related droughts. Trees-Struct. Funct. 2016, 30, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Campioli, M.; Vincke, C.; Jonard, M.; Kint, V.; Demarée, G.; Ponette, Q. Current status and predicted impact of climate change on forest production and biogeochemistry in the temperate oceanic European zone: Review and prospects for Belgium as a case study. J. For. Res. 2012, 17, 1–18. [Google Scholar] [CrossRef]
- Mérian, P.; Bert, D.; Lebourgeois, F. An approach for quantifying and correcting sample size-related bias in population estimates of climate-tree growth relationships. For. Sci. 2013, 59, 444–452. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- The R Core Team. R: A language and Environment for Statistical Computing. Available online: http://r-project.org/ (accessed on 5 August 2016).
- Wuertz, D.; Chalabi, Y.; Miklovic, M.; Boudt, C.; Chausse, P. fGarch: Rmetrics—Autoregressive Conditional Heteroskedastic Modelling. Available online: http://CRAN.R-project.org/package=fGarch (accessed on 5 August 2016).
- Bunn, A.G.; Jansma, E.; Korpela, M.; Westfall, R.D.; Baldwin, J. Using simulations and data to evaluate mean sensitivity (ζ) as a useful statistic in dendrochronology. Dendrochronologia 2013, 31, 250–254. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed Effects Models in S and S-PLUS; SpringerVerlag: New York, NY, USA, 2000; p. 528. [Google Scholar]
- Wykoff, W.R. A basal area increment model for individual conifers in the northern Rocky Mountains. For. Sci. 1990, 36, 1077–1104. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. Available online: http://CRAN.R-project.org/package=nlme (accessed on 5 August 2016).
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature 2007, 447, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Bontemps, J.D.; Hervé, J.C.; Leban, J.M.; Dhôte, J.F. Nitrogen footprint in a long-term observation of forest growth over the twentieth century. Trees-Struct. Funct. 2011, 25, 237–251. [Google Scholar] [CrossRef]
- Braun, S.; Thomas, V.F.D.; Quiring, R.; Flückiger, W. Does nitrogen deposition increase forest production? The role of phosphorus. Environ. Pollut. 2010, 158, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, W.; Chen, H.; Mo, J. Impacts of nitrogen deposition on soil nitrogen cycle in forest ecosystems: A review. Acta Ecol. Sin. 2015, 35, 35–43. [Google Scholar] [CrossRef]
- De Vries, W.; Reinds, G.J.; Posch, M.; Sanz, M.J.; Krause, G.H.M.; Calatayud, V.; Renaud, J.P.; Dupouey, J.L.; Sterba, H.; Vel, E.M.; et al. Intensive Monitoring of Forest Ecosystems in Europe: Technical Report; UnEce: Brussels, Belgium, 2003. [Google Scholar]
- European Environment Agency (EEA). Air Pollution in Europe 1990–2004: EEA Report No 2/2007; European Environment Agency: Copenhagen, Denmark, 2007. [Google Scholar]
- Le Goff, N.; Ottorin, J.M. Effects of thinning on beech growth. Interaction with climatic factors. Rev. Forest. Fr. 1999, 51, 355–364. [Google Scholar]
- Van der Maaten, E. Thinning prolongs growth duration of European beech (Fagus sylvatica L.) across a valley in southwestern Germany. For. Ecol. Manag. 2013, 306, 135–141. [Google Scholar] [CrossRef]
- Diaconu, D.; Kahle, H.P.; Spiecker, H. Tree- and stand-level thinning effects on growth of European Beech (Fagus sylvatica L.) on a Northeast- and a Southwest-facing slope in Southwest Germany. Forests 2015, 6, 3256–3277. [Google Scholar] [CrossRef]
- Penninckx, V.; Meerts, P.; Herbauts, J.; Gruber, W. Ring width and element concentrations in beech (Fagus sylvatica L.) from a periurban forest in central Belgium. For. Ecol. Manag. 1999, 113, 23–33. [Google Scholar] [CrossRef]
- Lévesque, M.; Walthert, L.; Weber, P. Soil nutrients influence growth response of temperate tree species to drought. J. Ecol. 2016, 104, 377–387. [Google Scholar] [CrossRef]
- Gillner, S.; Rüger, N.; Roloff, A.; Berger, U. Low relative growth rates predict future mortality of common beech (Fagus sylvatica L.). For. Ecol. Manag. 2013, 302, 372–378. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Engardt, M.; Langner, J. Simulations of future sulphur and nitrogen deposition over Europe using meteorological data from three regional climate projections. Available online: http://dx.doi.org/10.3402/tellusb.v65i0.20348 (accessed on 5 August 2016).
- IPCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPPC: Geneva, Switzerland, 2014. [Google Scholar]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. For. Res. 2016, 1–13. [Google Scholar] [CrossRef]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in central European mixed forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Metz, J.; Annighöfer, P.; Schall, P.; Zimmermann, J.; Kahl, T.; Schulze, E.-D.; Ammer, C. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Chang. Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef] [PubMed]







| Ring-Width Low Frequency (RWLF) Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Parameters | AIC | p-value | rRMSE (%) | Mean Error (±Std. dev.) | ||||
| Fixed effects | Random effects | ||||||||
| Overall | Ecoregion | Ecoregion | Forest | Tree | |||||
| size 1 | , s, s | / | / | / | / | 51332 | / | 42.21 | 0.000 ± 1.052 |
| size 2 | , s, s | / | / | / | 51138 | <0.001 | 41.97 | 0.000 ± 1.047 | |
| size 3 | , s, s | / | / | 46253 | <0.001 | 36.35 | 0.001 ± 0.906 | ||
| size 4 | , s, s | / | 42327 | <0.001 | 31.91 | 0.001 ± 0.796 | |||
| size-time 1 | , s, s, | / | 41699 | <0.001 | 31.36 | 0.003 ± 0.782 | |||
| size-time 2 | s, s, | / | 41594 | <0.001 | 31.26 | 0.002 ± 0.779 | |||
| size-time 3 | , s, s, | / | 41478 | <0.001 | 31.16 | 0.002 ± 0.777 | |||
| size-time 4 | , s, s, | / | 41392 | <0.001 | 31.08 | 0.002 ± 0.775 | |||
| size-time 5 | , s, s, | 41194 | <0.001 | 30.90 | 0.002 ± 0.770 | ||||
| High-Frequency Variability (HFV) Models | |||||||||
| Model | Parameters | AIC | p-value | rRMSE (%) | Mean Error (±Std. dev.) | ||||
| Fixed effects | Random effects | ||||||||
| Overall | Ecoregion | Ecoregion | Forest | Tree | |||||
| size 1 | , s, s | / | / | / | / | −38724 | / | 3.20 | 0.000 ± 0.080 |
| size 2 | , s, s | / | / | / | −39149 | <0.001 | 3.16 | 0.000 ± 0.079 | |
| size 3 | , s, s | / | / | −41582 | <0.001 | 2.94 | 0.000 ± 0.073 | ||
| size 4 | , s, s | / | −43740 | <0.001 | 2.72 | 0.000 ± 0.068 | |||
| size-time 1 | , s, s, | / | −44305 | <0.001 | 2.68 | 0.000 ± 0.067 | |||
| size-time 2 | s, s, | / | −44946 | <0.001 | 2.63 | 0.000 ± 0.065 | |||
| size-time 3 | , s, s, | / | −45299 | <0.001 | 2.60 | 0.000 ± 0.065 | |||
| size-time 4 | , s, s, | / | −45436 | <0.001 | 2.59 | 0.000 ± 0.065 | |||
| size-time 5 | , s, s, | −45663 | <0.001 | 2.57 | 0.000 ± 0.064 | ||||
| “Size 4” Models | ||||||
|---|---|---|---|---|---|---|
| DF = 17400 | ||||||
| Fixed effects | Estimate | Standard error | p-value | Estimate | Standard error | p-value |
| 3.14 | 0.144 | <0.001 | 0.19 | 0.00712 | <0.001 | |
| 0.304 | 0.0063 | <0.001 | −0.121 | 0.0038 | <0.001 | |
| 15.1 | 0.1 | <0.001 | 10.8 | 0.119 | <0.001 | |
| Random effects | Ecoregion | Forest | Tree | Ecoregion | Forest | Tree |
| Std. dev. of | 0.00528 | 0.664 | 0.582 | 0.00025 | 0.0295 | 0.0274 |
| “Size-Time 5” Models | ||||||
| DF = 17300 | ||||||
| Fixed effects | Estimate | Standard error | p-value | Estimate | Standard error | p-value |
| 3.36 | 0.256 | <0.001 | 0.192 | 27.4 | <0.001 | |
| 0.305 | 0.00662 | <0.001 | −0.0975 | −21.7 | <0.001 | |
| 19.7 | 0.476 | <0.001 | 17.2 | 19.9 | <0.001 | |
| .(Intercept) | −0.753 | 0.136 | <0.001 | −0.115 | −5.44 | <0.001 |
| .Uplands | −0.641 | 0.22 | 0.00364 | 0.356 | 11.7 | <0.001 |
| .(Intercept) | 0.476 | 0.0561 | <0.001 | −0.0904 | −4.29 | <0.001 |
| .Uplands | 0.193 | 0.0908 | 0.0334 | 0.0583 | 2.22 | 0.0264 |
| .(Intercept) | −0.332 | 0.0225 | <0.001 | 0.509 | 7.88 | <0.001 |
| .Uplands | 0.157 | 0.0289 | <0.001 | −0.589 | −6.61 | <0.001 |
| .(Intercept) | −0.442 | 0.0172 | <0.001 | 0.55 | 10.3 | <0.001 |
| Uplands | 0.129 | 0.0204 | <0.001 | 0.332 | 5.04 | <0.001 |
| Random effects | Ecoregion | Forest | Tree | Ecoregion | Forest | Tree |
| Std. dev. of | 0.263 | 0.708 | 0.542 | 0.0000191 | 0.0284 | 0.0263 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latte, N.; Perin, J.; Kint, V.; Lebourgeois, F.; Claessens, H. Major Changes in Growth Rate and Growth Variability of Beech (Fagus sylvatica L.) Related to Soil Alteration and Climate Change in Belgium. Forests 2016, 7, 174. https://doi.org/10.3390/f7080174
Latte N, Perin J, Kint V, Lebourgeois F, Claessens H. Major Changes in Growth Rate and Growth Variability of Beech (Fagus sylvatica L.) Related to Soil Alteration and Climate Change in Belgium. Forests. 2016; 7(8):174. https://doi.org/10.3390/f7080174
Chicago/Turabian StyleLatte, Nicolas, Jérôme Perin, Vincent Kint, François Lebourgeois, and Hugues Claessens. 2016. "Major Changes in Growth Rate and Growth Variability of Beech (Fagus sylvatica L.) Related to Soil Alteration and Climate Change in Belgium" Forests 7, no. 8: 174. https://doi.org/10.3390/f7080174