Phosphorus in Preferential Flow Pathways of Forest Soils in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Field Experiments
2.3. Chemical Analyses
2.4. Statistical Analysis
3. Results and Discussion
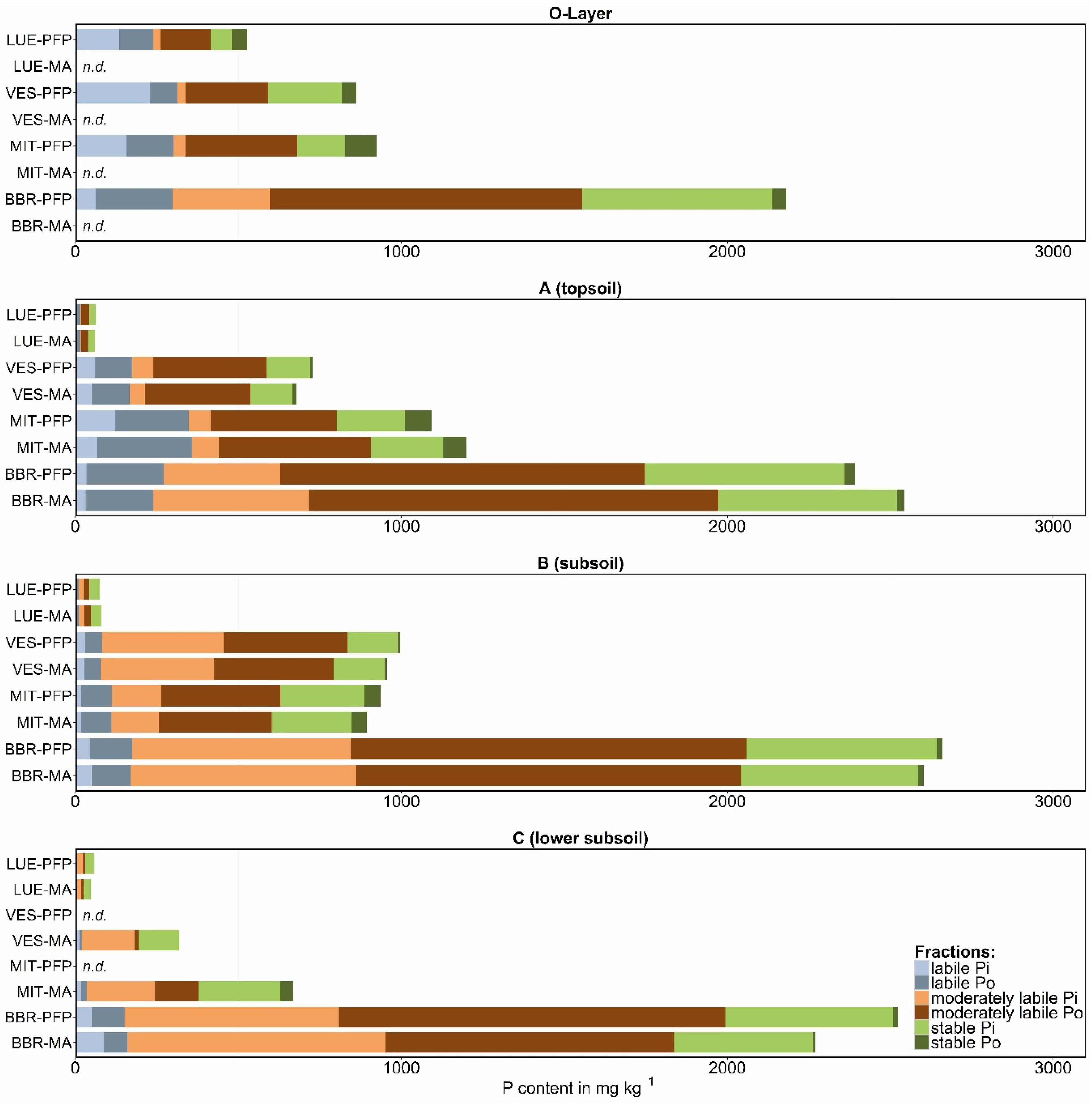
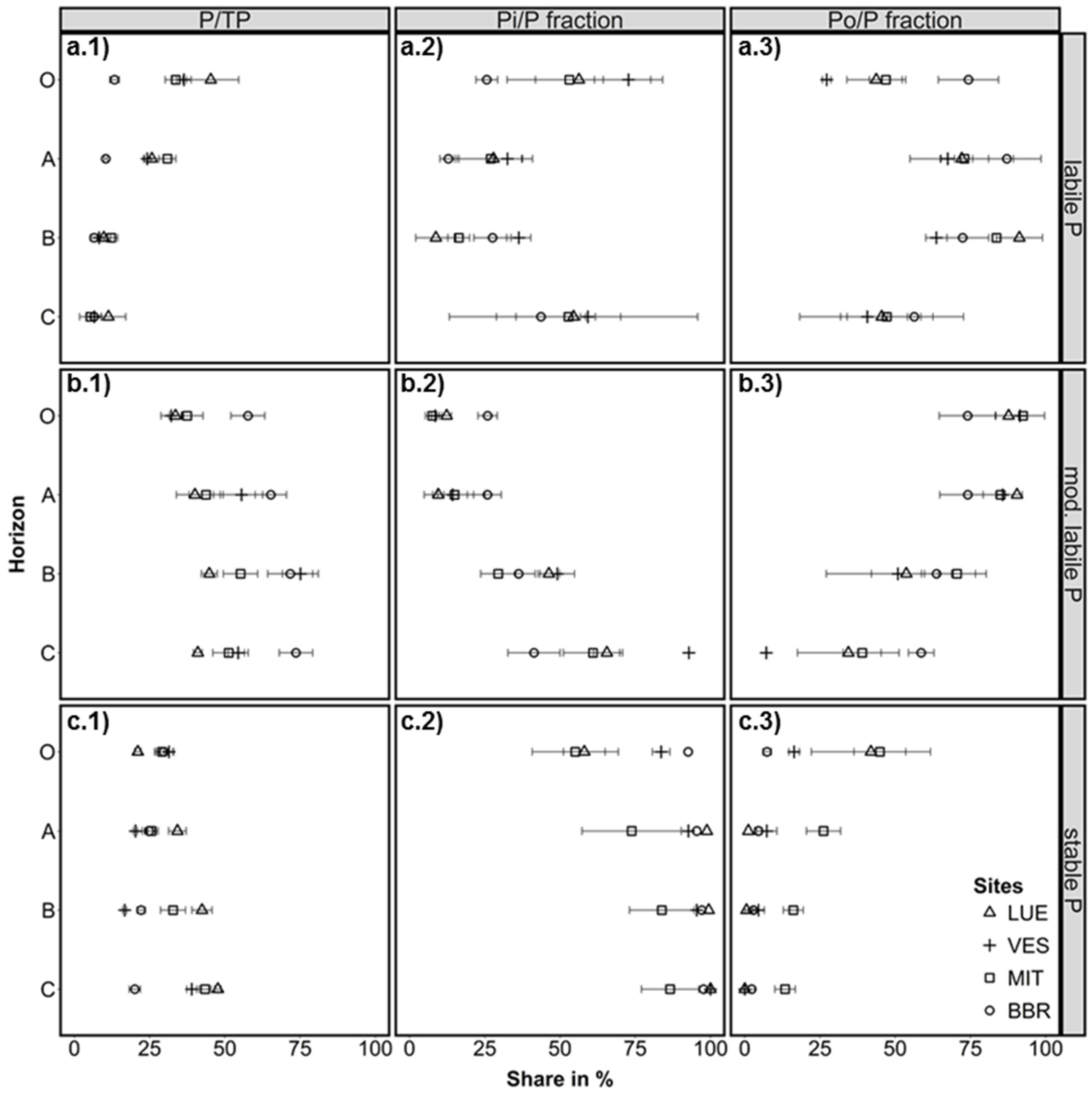
3.1. Labile P Fractions
3.2. Moderately Labile P Fractions
3.3. Stable P Fractions
3.4. Factors Affecting the Distribution of P Fractions in the Soil Profiles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bol, R.; Julich, D.; Brödlin, D.; Siemens, J.; Kaiser, K.; Dippold, M.A.; Spielvogel, S.; Zilla, T.; Mewes, D.; von Blanckenburg, F.; et al. Dissolved and colloidal phosphorus fluxes in forest ecosystems—An almost blind spot in ecosystem research. J. Plant Nutr. Soil Sci. 2016, 179, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Newman, R.H.; Tate, K.R. Soil phosphorus characterisation by 31p nuclear magnetic resonance. Commun. Soil Sci. Plant Anal. 1980, 11, 835–842. [Google Scholar] [CrossRef]
- Cade-Menun, B.; Liu, C.W. Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: A review of sample preparation and experimental parameters. Soil Sci. Soc. Am. J. 2014, 78, 19. [Google Scholar] [CrossRef]
- Prietzel, J.; Klysubun, W.; Werner, F. Speciation of phosphorus in temperate zone forest soils as assessed by combined wet-chemical fractionation and XANES spectroscopy. J. Plant Nutr. Soil Sci. 2016, 179, 168–185. [Google Scholar] [CrossRef]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Lang, F.; Bauhus, J.; Frossard, E.; George, E.; Kaiser, K.; Kaupenjohann, M.; Krüger, J.; Matzner, E.; Polle, A.; Prietzel, J.; et al. Phosphorus in forest ecosystems: New insights from an ecosystem nutrition perspective. J. Plant Nutr. Soil Sci. 2016, 179, 129–135. [Google Scholar] [CrossRef]
- Prietzel, J.; Kaiser, K. De-eutrophication of a nitrogen-saturated Scots pine forest by prescribed litter-raking. J. Plant Nutr. Soil Sci. 2005, 168, 461–471. [Google Scholar] [CrossRef]
- Neary, D.G.; Ice, G.G.; Jackson, C.R. Linkages between forest soils and water quality and quantity. For. Ecol. Manag. 2009, 258, 2269–2281. [Google Scholar] [CrossRef]
- Van Miegrot, H.; Johnson, D.W. Feedbacks and synergism among biogeochemistry, basic ecology, and forest soil science. Forest Ecol. Manag. 2009, 258, 2214–2223. [Google Scholar] [CrossRef]
- Santos, R.M.B.; Sanches Fernandes, L.F.; Pereira, M.G.; Cortes, R.M.V.; Pacheco, F.A.L. A framework model for investigating the export of phosphorus to surface waters in forested watersheds: Implications to management. Sci. Total Environ. 2015, 536, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Tromp-van Meerveld, I.; McDonnell, J.J. The role of lateral pipe flow in hillslope runoff response: An intercomparison of non-linear hillslope response. J. Hydrol. 2005, 311, 117–133. [Google Scholar] [CrossRef]
- Bogner, C.; Borken, W.; Huwe, B. Impact of preferential flow on soil chemistry of a podzol. Geoderma 2012, 175, 37–46. [Google Scholar] [CrossRef]
- Van der Heijden, G.; Legout, A.; Pollier, B.; Bréchet, C.; Ranger, J.; Dambrine, E. Tracing and modeling preferential flow in a forest soil—Potential impact on nutrient leaching. Geoderma 2013, 195–196, 12–22. [Google Scholar] [CrossRef]
- Van Verseveld, W.J.; McDonnell, J.J.; Lajtha, K. The role of hillslope hydrology in controlling nutrient loss. J. Hydrol. 2009, 367, 177–187. [Google Scholar] [CrossRef]
- Bundt, M.; Jäggi, M.; Blaser, P.; Siegwolf, R.; Hagedorn, F. Carbon and nitrogen dynamics in preferential flow paths and matrix of a forest soil. Soil Sci. Soc. Am. J. 2001, 65, 1529–1538. [Google Scholar] [CrossRef]
- Flury, M.; Flühler, H. Brilliant Blue FCF as a dye tracer for solute transport studies—A toxicological overview. J. Environ. Qual. 1994, 23, 1108–1112. [Google Scholar] [CrossRef]
- Weiler, M.; Flühler, H. Inferring flow types from dye patterns in macroporous soils. Geoderma 2004, 120, 137–153. [Google Scholar] [CrossRef]
- Hagedorn, F.; Bundt, M. The age of preferential flow paths. Geoderma 2002, 108, 119–132. [Google Scholar] [CrossRef]
- Backnäs, S.; Laine-Kaulio, H.; Kløve, B. Phosphorus forms and related soil chemistry in preferential flowpaths and the soil matrix of a forested podzolic till soil profile. Geoderma 2012, 189, 50–64. [Google Scholar] [CrossRef]
- Jarvis, N.J. A review of non-equilibrium water flow and solute transport in soil macropores: principles, controlling factors and consequences for water quality. Eur. J. Soil Sci. 2007, 58, 523–546. [Google Scholar] [CrossRef]
- Jensen, M.B. Subsurface transport of phosphorus in relation to its mobilization and immobilization in structured soil (steady state flow experiments). Acta Agric. Scand. Sect. B-Soil Plant Sci. 1998, 48, 11–17. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of Available P by Sequential Extraction. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; Canadian Society of Soil Science: Boca Raton, FL, USA, 2008; Chapter 25; pp. 293–306. [Google Scholar]
- Sibbesen, E. A simple ion-exchange resin procedure for extracting plant-available elements from soil. Plant Soil 1977, 46, 665–669. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- O’Halloran, I.P.; Cade-Menun, B.J. Total and Organic Phosphorus. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; Canadian Society of Soil Science: Boca Raton, FL, USA, 2008; pp. 265–291. [Google Scholar]
- Crews, T.E.; Brookes, P.C. Changes in soil phosphorus forms through time in perennial versus annual agroecosystems. Agric. Ecosyst. Environ. 2014, 184, 168–181. [Google Scholar] [CrossRef]
- Negassa, W.; Leinweber, P. How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: A review. J. Plant Nutr. Soil Sci. 2009, 172, 305–325. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Augstburger, S.; Frossard, E. Dominance of either physicochemical or biological phosphorus cycling processes in temperate forest soils of contrasting phosphate availability. Soil Biol. Biochem. 2016, 101, 85–95. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B.; Cole, C.V. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853. [Google Scholar] [CrossRef]
- Renneson, M.; Dufey, J.; Legrain, X.; Genot, V.; Bock, L.; Colinet, G. Relationships between the P status of surface and deep horizons of agricultural soils under various cropping systems and for different soil types: A case study in Belgium. Soil Use Manag. 2013, 29, 103–113. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Watanabe, Y. Combined applications of chemical fractionation, solution 31P-NMR and P K-edge XANES to determine phosphorus speciation in soils formed on serpentine landscapes. Geoderma 2014, 230, 143–150. [Google Scholar] [CrossRef]
- Frizano, J.; Johnson, A.H.; Vann, D.R.; Scatena, F.N. Soil phosphorus fractionation during forest development on landslide scars in the Luquillo Mountains, Puerto Rico. Biotropica 2002, 34, 17–26. [Google Scholar] [CrossRef]
- Thomas, S.M.; Johnson, A.H.; Frizano, J.; Vann, D.R.; Zarin, D.J.; Joshi, A. Phosphorus fractions in montane forest soils of the Cordillera de Piuchué, Chile: Biogeochemical implications. Plant Soil 1999, 211, 139–148. [Google Scholar] [CrossRef]
- McDowell, R.W.; Stewart, I. The phosphorus composition of contrasting soils in pastoral, native and forest management in Otago, New Zealand: Sequential extraction and 31P NMR. Geoderma 2006, 130, 176–189. [Google Scholar] [CrossRef]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257, 29–39. [Google Scholar] [CrossRef]
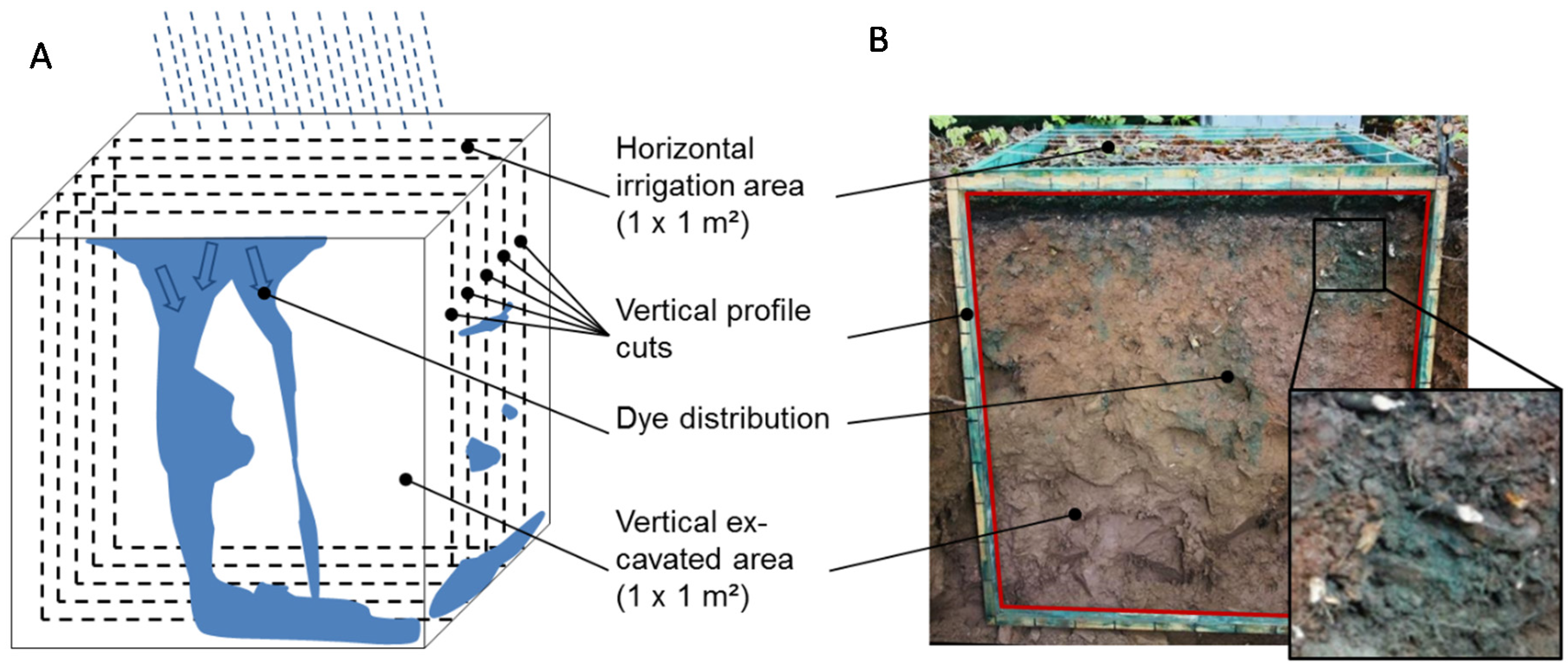
| LUE | VES | MIT | BBR | |
|---|---|---|---|---|
| Location | 52°50′32′′ N 10°16′06′′ E | 50°36′26′′ N 10°46′20′′ E | 48°58′35′′ N 12°52′49′′ E | 50°21′38′′ N 9°55′71′′ E |
| Elevation (m a.s.l.) | 115 | 810 | 1023 | 809 |
| MAT (°C) | 8.0 | 5.5 | 4.5 | 5.8 |
| MAP (mm) | 779 | 1200 | 1229 | 1031 |
| Parent material | Pleistocene sands | Trachyandesite | Gneiss | Basalt |
| Soil type (WRB *) | Dystric skeletic Cambisol | Hyperdystric skeletic folic Cambisol | Hyperdystric skeletic chromic Cambisol | Hyperdystric folic Cambisol |
| Study Site/ | Depth | Texture | pH | C | N | Alox | Aldi | Feox | Fedi | Pox | TP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | (cm) | (CaCl2) | (%) | (%) | (g·kg−1) | ||||||
| Lüss (LUE) | |||||||||||
| O | 9 to 0 | 2.8 | 36.63 | 1.59 | 0.73 | 0.79 | 0.87 | 1.30 | 0.15 | 0.49 | |
| A | 0 to −12 | Loamy sand | 3.0 | 1.69 | 0.07 | 0.18 | 0.37 | 0.29 | 2.17 | <0.04 | 0.06 |
| B | −12 to −52 | Loamy sand | 3.7 | 0.90 | 0.04 | 1.04 | 1.00 | 1.40 | 3.21 | <0.04 | 0.08 |
| C | −52 to −100 | Sand | 4.3 | 0.12 | 0.01 | 0.50 | 0.41 | 0.15 | 1.01 | <0.04 | 0.04 |
| Vessertal (VES) | |||||||||||
| O | 4 to 0 | 3.4 | 32.17 | 1.72 | 4.11 | 1.20 | 1.28 | 1.75 | 0.30 | 0.86 | |
| A | 0 to −3 | Sandy loam | 3.0 | 7.49 | 0.45 | 2.75 | 1.68 | 3.14 | 7.46 | 0.53 | 0.70 |
| B | −3 to −65 | Sandy loam | 4.1 | 3.03 | 0.20 | 9.44 | 7.40 | 5.13 | 9.23 | 0.83 | 0.98 |
| C | −65 to −100 | Loamy sand | 4.1 | 0.37 | 0.03 | 4.64 | 2.83 | 0.59 | 5.55 | 0.16 | 0.32 |
| Mitterfels (MIT) | |||||||||||
| O | 4 to 0 | 3.5 | 44.12 | 2.19 | 0.95 | 0.87 | 1.28 | 1.45 | 0.34 | 0.88 | |
| A | 0 to −4 | Silt loam | 3.0 | 35.50 | 2.05 | 3.32 | 3.83 | 3.58 | 4.97 | 0.47 | 1.14 |
| B | −4 to −80 | Silt loam | 3.9 | 5.45 | 0.34 | 6.26 | 5.62 | 8.05 | 12.17 | 0.53 | 0.91 |
| C | −80 to −100 | Loamy sand | 4.3 | 1.09 | 0.09 | 5.73 | 4.63 | 3.61 | 10.89 | 0.35 | 0.66 |
| Bad Brückenau (BBR) | |||||||||||
| O | 3 to 0 | 4.6 | 27.33 | 1.68 | 5.53 | 5.51 | 15.27 | 21.85 | 1.29 | 2.24 | |
| A | 0 to −7 | Silty clay | 4.2 | 17.57 | 1.28 | 7.77 | 8.30 | 21.15 | 31.52 | 1.51 | 2.45 |
| B | −7 to −30 | Silt clay loam | 4.3 | 7.45 | 0.54 | 10.58 | 10.83 | 24.46 | 35.41 | 1.84 | 2.62 |
| C | −30 to −65 | Loam | 4.5 | 4.60 | 0.34 | 10.70 | 9.57 | 18.87 | 25.92 | 1.63 | 2.39 |
| C/Po | LUE | VES | MIT | BBR | ||||
|---|---|---|---|---|---|---|---|---|
| Horizon | PFP | Ma | PFP | Ma | PFP | Ma | PFP | Ma |
| O | 1143 | 840 | 910 | 223 | ||||
| A | 512 | 494 | 155 | 174 | 593 | 371 | 139 | 97 |
| B | 356 | 274 | 89 | 66 | 101 | 93 | 58 | 51 |
| C | 204 | 88 | 170 | 59 | 42 | 43 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julich, D.; Julich, S.; Feger, K.-H. Phosphorus in Preferential Flow Pathways of Forest Soils in Germany. Forests 2017, 8, 19. https://doi.org/10.3390/f8010019
Julich D, Julich S, Feger K-H. Phosphorus in Preferential Flow Pathways of Forest Soils in Germany. Forests. 2017; 8(1):19. https://doi.org/10.3390/f8010019
Chicago/Turabian StyleJulich, Dorit, Stefan Julich, and Karl-Heinz Feger. 2017. "Phosphorus in Preferential Flow Pathways of Forest Soils in Germany" Forests 8, no. 1: 19. https://doi.org/10.3390/f8010019






