Resource Limitations Influence Growth and Vigor of Idaho Fescue, a Common Understory Species in Pacific Northwest Ponderosa Pine Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Protocol
2.2. Greenhouse Protocol and Experimental Design
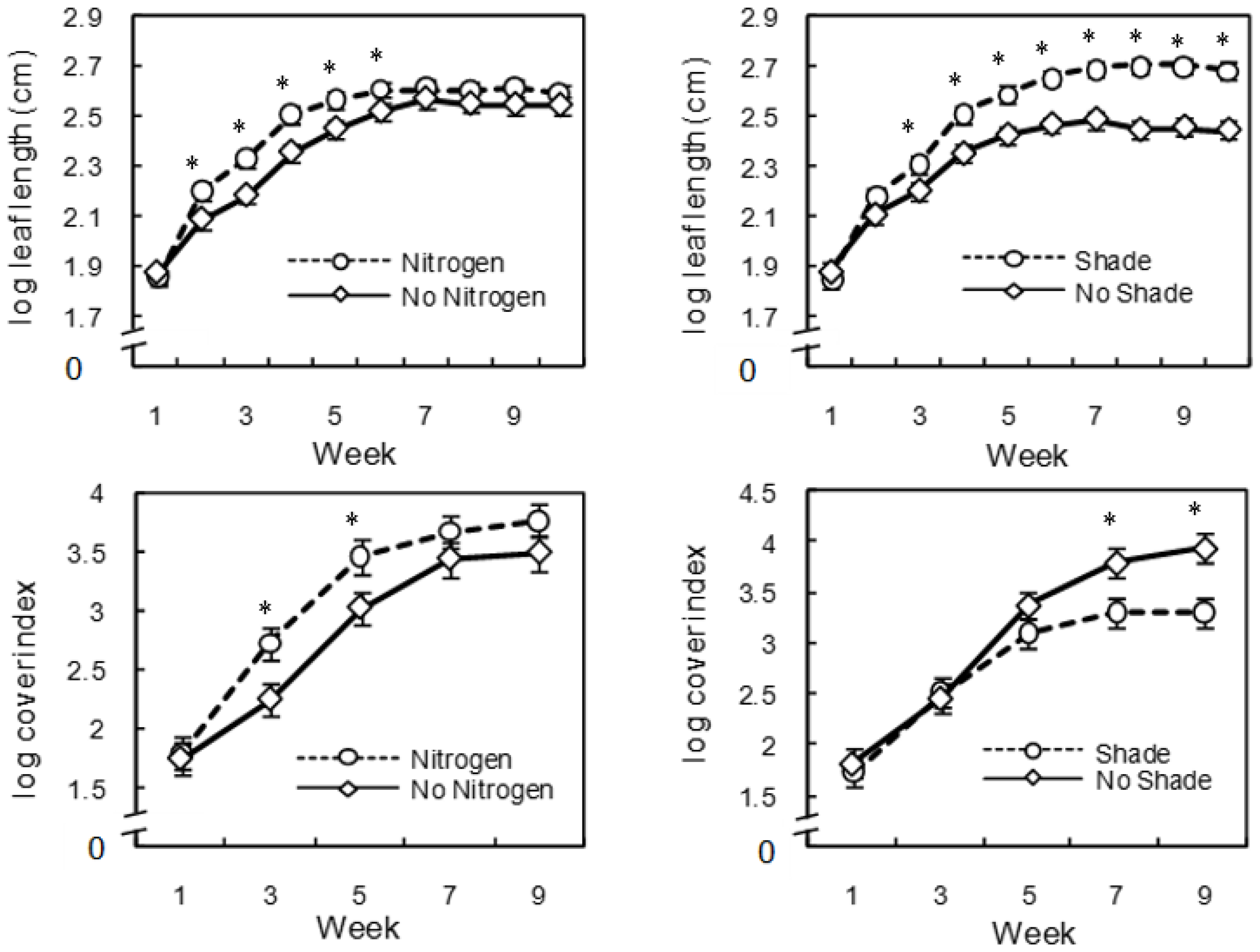
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Covington, W.; Moore, M. Southwestern ponderosa forest structure: Changes since Euro-American settlement. Forestry 1994, 92, 39–47. [Google Scholar]
- Johnson, C.G. Forest Health in the Blue Mountains: A Plant Ecologist’s Perspective on Ecosystem Processes and Biological Diversity; Pacific Northwest Research Station PNW-GRR-339; USDA Forest Service: Portland, OR, USA, 1994; p. 23.
- Carr, C.A.; Krueger, W.C. Understory vegetation and ponderosa pine abundance in eastern Oregon. Rangel. Ecol. Manag. 2011, 64, 533–542. [Google Scholar] [CrossRef]
- Clary, W.P.; Kruse, W.H.; Larson, F.R. Cattle grazing and wood production with different basal areas of ponderosa pine. J. Range Manag. 1975, 434–437. [Google Scholar] [CrossRef]
- Moore, M.M.; Covington, W.W.; Fule, P.Z. Reference conditions and ecological restoration: A southwestern ponderosa pine perspective. Ecol. Appl. 1999, 9, 1266–1277. [Google Scholar] [CrossRef]
- Naumburg, E.; DeWald, L.E. Relationships between Pinus ponderosa forest structure, light characteristics, and understory graminoid species presence and abundance. Forest Ecol. Manag. 1999, 124, 205–215. [Google Scholar] [CrossRef]
- Uresk, D.W.; Severson, K.E. Response of understory species to changes in ponderosa pine stocking levels in the black hills. Great Basin Nat. 1998, 58, 312–327. [Google Scholar]
- Wienk, C.L.; Sieg, C.H.; McPherson, G.R. Evaluating the role of cutting treatments, fire and soil seed banks in an experimental framework in ponderosa pine forests of the black hills, South Dakota. Forest Ecol. Manag. 2004, 192, 375–393. [Google Scholar] [CrossRef]
- Moore, M.M.; Casey, C.A.; Bakker, J.D.; Springer, J.D.; Fule, P.Z.; Covington, W.W.; Laughlin, D.C. Herbaceous vegetation responses (1992–2004) to restoration treatments in a ponderosa pine forest. Rangel. Ecol. Manag. 2006, 59, 135–144. [Google Scholar] [CrossRef]
- Naumburg, E.; Dewald, L.E.; Kolb, T.E. Shade responses of five grasses native to southwestern US Pinus ponderosa forests. Can. J. Bot. 2001, 79, 1001–1009. [Google Scholar]
- Riegel, G.M.; Miller, R.F.; Krueger, W.C. Understory vegetation response to increasing water and nitrogen levels in a pinus-ponderosa forest in northeastern Oregon. Northwest Sci. 1991, 65, 10–15. [Google Scholar]
- Riegel, G.M.; Miller, R.F.; Krueger, W.C. Competition for resources between understory vegetation and overstory pinus-ponderosa in northeastern Oregon. Ecol. Appl. 1992, 2, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Riegel, G.M.; Miller, R.F.; Krueger, W.C. The effects of aboveground and belowground competition on understory species composition in a pinus-ponderosa forest. Forest Sci. 1995, 41, 864–889. [Google Scholar]
- Johnson, C.; Clausnitzer, R. Plant Associations of the Blue and Ochoco Mountains; USDA Forest Service R6-ERW-TP-036–92; USDA Forest Service: Portland, OR, USA, 1992.
- Western Regional Climate Center(WRCC). Climatalogical Summary for Seneca, Oregon. Available online: http://www.wrcc.dri.edu/cgi-bin/cliMONtpre.pl?or7675 (accessed on 10 May 2010).
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press Ltd.: San Diego, CA, USA, 1995. [Google Scholar]
- Environmental Systems Research Institute. Arcmap 9.1; Environmental Systems Research Institute, Inc.: Redlands, CA, USA, 2005. [Google Scholar]
- Louhaichi, M.; Johnson, D.E.; Borman, M.M. Manual for Assessing Depredation on Grain and Seed Yields, Ver 1.1; Department of Rangeland Resources, Oregon State University: Corvallis, OR, USA, 2003. [Google Scholar]
- SAS Institute. Sas for Windows Version 9.1; SAS Institute, Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Littell, R.C.; Stroup, W.W.; Milliken, G.A.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models; SAS Institute: Cary, NC, USA, 2006. [Google Scholar]
- Allard, G.; Nelson, C.J.; Pallardy, S.G. Shade effects on growth of tall fescue: 1. Leaf anatomy and dry-matter partitioning. Crop Sci. 1991, 31, 163–167. [Google Scholar] [CrossRef]
- Boardman, N. Comparative photosynthesis of sun and shade plants. Ann. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Moir, W.H. Influence of ponderosa pine on herbaceous vegetation. Ecology 1966, 47, 1045–1048. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F., III; Pons, L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998; p. 540. [Google Scholar]
- Coyne, P.; Trlica, M.; Owensby, C. Carbon and nitrogen dynamics in range plants. In Wildland Plants: Physiological Ecology and Developmental Morphology; Society for Range Management: Denver, CO, USA, 1995; pp. 59–167. [Google Scholar]
- Carr, C.A. An Evaluation of Understory Vegetation Dynamics, Ecosystem Resilience and State and Transition Ecological Theory in an Eastern Oregon Ponderosa Pine Forest. Ph.D Dissertation, PhOregon State University, Corvallis, OR, USA, 2007. [Google Scholar]
- Murchie, E.H.; Horton, P. Acclimation of photosynthesis to irradiance and spectral quality in British plant species: Chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ. 1997, 20, 438–448. [Google Scholar] [CrossRef]
- Bjorkman, O. Some viewpoints on photosynthetic response and adaptation to environmental stress. In Photosynthesis; Briggs, W.R., Ed.; Alan R. Liss: New York, NY, USA, 1989. [Google Scholar]
- Smith, H. Light quality, photoperception, and plant strategy. Ann. Rev. Plant Phsyiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, 6th ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 2007; p. 499. [Google Scholar]
- Kaye, J.P.; Hart, S.C. Ecological restoration alters nitrogen transformations in a ponderosa pine bunchgrass ecosystem. Ecol. Appl. 1998, 8, 1052–1060. [Google Scholar] [CrossRef]
- Kaye, J.P.; Hart, S.C.; Fule, P.Z.; Covington, W.W.; Moore, M.M.; Kaye, M.W. Initial carbon, nitrogen, and phosphorus fluxes following ponderosa pine restoration treatments. Ecol. Appl. 2005, 15, 1581–1593. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Roberts, M.R. The Herbaceous Layer in Forests of Eastern North America; Oxford Universtiy Press: New York, NY, USA, 2003. [Google Scholar]
- Nuefeld, H.S.; Young, D.R. Ecophysiology of the herbaceous layer in temperate deciduous forests. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F.S., Roberts, M.R., Eds.; Oxford Universtiy Press: New York, NY, USA, 2003; pp. 38–90. [Google Scholar]
- Pearcy, R.W. Photosynthetic gas exchange responses of Australian tropical forests trees in canopy, gap, and understory micro-environments. Funct. Ecol. 1987, 1, 169–178. [Google Scholar] [CrossRef]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Rees, M. Delayed germination of seeds—A look at the effects of adult longevity, the timing of reproduction, and population age/stage structure. Am. Nat. 1994, 144, 43–64. [Google Scholar] [CrossRef]
- Korb, J.E.; Springer, J.D.; Powers, S.R.; Moore, M.M. Soil seed banks in Pinus ponderosa forests in Arizona: Clues to site history and restoration potential. Appl. Veg. Sci. 2005, 8, 103–112. [Google Scholar] [CrossRef]
- Page, H.N.; Bork, E.W. Effect of planting season, bunchgrass species, and neighbor control on the success of transplants for grassland restoration. Restor. Ecol. 2005, 13, 651–658. [Google Scholar] [CrossRef]
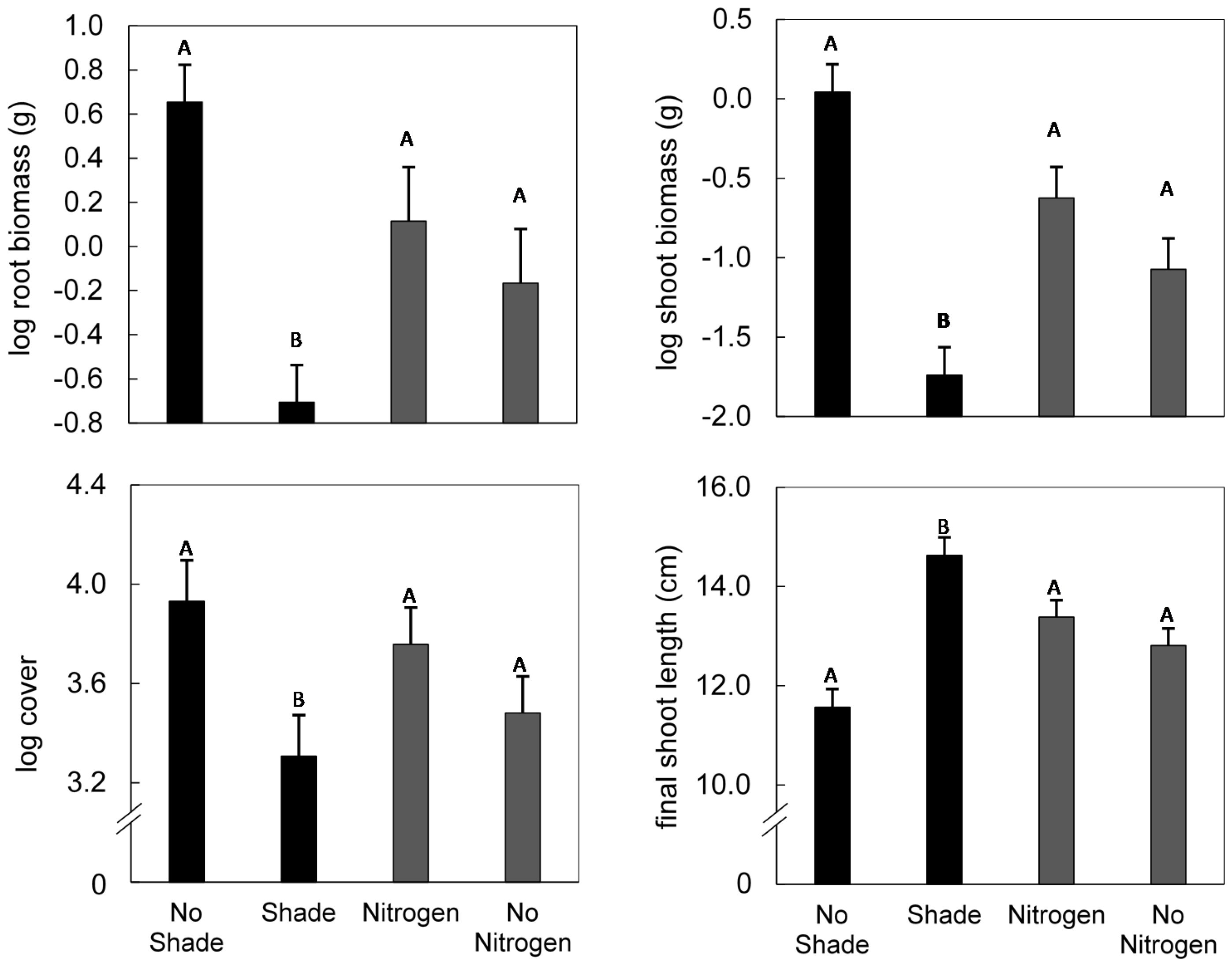
| Shade | Nitrogen | Shade * Nitrogen | ||||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |
| Shoot Weight | 56.61 | 0.0017 | 2.40 | 0.1602 | 1.10 | 0.3244 |
| Root Weight | 433.46 | 0.0027 | 0.47 | 0.5129 | 0.10 | 0.7567 |
| Final Length | 50.19 | 0.0021 | 2.57 | 0.1478 | 0.03 | 0.8596 |
| Final Cover | 9.35 | 0.0378 | 3.85 | 0.0853 | 0.02 | 0.8932 |
| Shade | Nitrogen | Time | Shade *Time | Nitrogen * Time | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | F | p-Value | F | p-Value | |
| Length | 29.19 | 0.0001 | 7.50 | 0.0169 | 295.16 | <0.0001 | 10.36 | <0.0001 | 3.50 | 0.0006 |
| Cover | 3.57 | 0.1309 | 5.61 | 0.0422 | 183.57 | <0.0001 | 5.63 | 0.0009 | 4.13 | 0.0059 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carr, C.A.; Krueger, W.C. Resource Limitations Influence Growth and Vigor of Idaho Fescue, a Common Understory Species in Pacific Northwest Ponderosa Pine Forests. Forests 2017, 8, 6. https://doi.org/10.3390/f8010006
Carr CA, Krueger WC. Resource Limitations Influence Growth and Vigor of Idaho Fescue, a Common Understory Species in Pacific Northwest Ponderosa Pine Forests. Forests. 2017; 8(1):6. https://doi.org/10.3390/f8010006
Chicago/Turabian StyleCarr, Craig A., and William C. Krueger. 2017. "Resource Limitations Influence Growth and Vigor of Idaho Fescue, a Common Understory Species in Pacific Northwest Ponderosa Pine Forests" Forests 8, no. 1: 6. https://doi.org/10.3390/f8010006





