1. Introduction
Seedling quality is critical to the ability of seedlings to survive extended environmental stresses and produce vigorous growth following outplanting. Rapid early growth of tree seedlings is closely related to the success of plantation establishment [
1]. It has been reported that larger seedlings have better performance than small seedlings after planting under conditions of competition for resources with weeds [
2,
3,
4], while decreasing survivals with increasing seedling sizes were observed on extremely harsh sites [
5,
6]. The morphological criteria for assessing seedling quality can be manipulated by nursery cultural practices [
7], and the impact of nursery management practices on seedling quality has been studied on various plant species [
8,
9,
10,
11]. However, most of these studies were conducted in research station settings far from actual nursery production conditions [
7].
Many advantages of container seedlings over bareroot stock have been reported [
12,
13], such as quick production, uniform size, extending the planting season, and performing well on adverse sites. Moreover, container planting offers potentially increased seed efficiency (the ratio of plantable seedlings to filled seeds), because growing conditions can be better controlled, which is very important for the tree seeds with low fertilization and germination. However, some disadvantages also exist in the production and use of container seedlings, such as nutritional imbalances and higher cost [
14]. During the container seedling production, container size, growth medium, growing density, and design characteristics of the containers are important determinants of seedling quality [
11,
13], but are varied among the tree species [
13]. Generally, the type and volume of the containers are some of the most important characteristics, because they have both major and direct impacts on seedling quality and production costs. Meanwhile, the optimum container size varies according to many different factors, including species, growing density, environmental conditions, and length of the growing season. Furthermore, one of the most serious problems in containers (especially for the seedlings with tap roots) is the tendency of root spiraling around the inside of the container when round, smooth-walled plastic containers were used [
13], which can seriously reduce seedling quality and field performance after planting. A suitable growth medium for container seedlings is critical in order to provide support and nutrients, retain water, and allow oxygen diffusion to the roots. Although there is not an ideal growth medium for all growing plants, a growth medium should incorporate physical, chemical, and biological requirements for good plant growth together with the requirements of practical plant production [
15]. Peat moss is used extensively as a substrate in container seedlings; however, in recent years, there has been increasing environmental and ecological concerns against the use of peat due to endangered bog ecosystems [
16]. Moreover, due to the increased cost and decreased availability of peat moss in many countries, numerous organic materials (such as manure compost and rice hulls ash) have been sought and studied worldwide [
14,
17,
18]. Recently, seedling growers have been looking for more local growth medium components to reduce the increased transportation costs of growing media [
14,
19].
As a highly valued and multiple-function tree species,
Cyclocarya paliurus (Batal) Iljinskaja belongs to the Juglandaceae family, and is naturally distributed in the mountainous regions of subtropical China [
20]. Many studies have demonstrated that
C. paliurus not only has a great potential for fine timber production [
21], the extracts of its leaves also possess a variety of bioactivities, such as hypoglycemic [
22,
23], anti-hyperlipidemia [
24], anti-HIV-1 [
25], antioxidant [
23,
26], and anticancer [
27]. Therefore, a huge production of leaves is required for
C. paliurus tea production and medical use. However, most of the
C. paliurus resources are distributed in natural forests, and there are not enough
C. paliurus plantations for leaf production [
28]. Currently,
C. paliurus can be only propagated from seeds, but the seeds have pronounced dormancy [
20], and about 75% seeds are “empty” due to the asynchronous flowering period [
29]. In order to improve the seed efficiency and outplanting performance of
C. paliurus, producing container seedlings is one of the most important options to quickly increase plantation resources of
C. paliurus, due to its flexibility of seedling production and field planting. However, the influence of nursery cultivation regimes on container seedling quality and the outplanting performance of
C. paliurus has received almost no attention, and no study on seedling growth and root morphology of the species has been reported until now. The objectives of this study were to determine the effects of container type and growth medium on seedling growth and root morphology of
C. paliurus during nursery culture and to select an optimum container type and growth medium for the seedling production of this species.
2. Materials and Methods
2.1. Plant Material and Experimental Layout
The seeds used in this study were collected in October 2013 from the dominant trees of the C. paliurus natural population located at the Tonggu site in Jiangxi Province of China (approximately 28°35′ N latitude, 114°22′ E longitude). To overcome the dormancy, the collected seeds were subjected to exogenous GA3 (gibberellin A3) and stratification treatments in early January 2014. After about 15 months of stratification treatment, the germinated seeds were sown into each container in 2015.
A randomized complete block design with a factorial treatment structure was conducted and carried out with three replications. Factors and levels were: container type and size with four levels (C1: non-woven container with 760 mL; C2: non-woven container with 500 mL; C3: non-woven container with 280 mL; C4: black plastic container with 500 mL, all bought from Anqing Yike Seedling Company, Anqing, China), and growth medium with three levels (yellow subsoil: perlite: fermented chicken manure: peat by volume = 2:2:1:5 (F1); 2:2:2:4 (F2); 2:2:3:3 (F3)). Yellow subsoil was the soil under the depth of 20 cm collected from a field near the experimental site, which is the most readily available soil type in the region, with an organic content of 2.4 g kg−1, available N content of 0.98 mg kg−1, available P content of 10.1 mg kg−1, available K content of 41.8 mg kg−1, and pH value of 5.5. Fermented chicken manure (chicken manure fermented with agricultural residues) was bought directly from Nanjing Woyou Biological Fertilizer Company Limited (Nanjing, China), with an organic content of 45.0%, total N content of 3.5%, P content (P2O5) of 6.4%, K content (K2O) of 1.6%, and pH value of 6.7. The levels of all of the heavy metals in the manure were within the permissible limits of Chinese agricultural standard for Microbial Organic Fertilizers (NY 884-2012). The peat was turfy peat with medium structure and the perlite was of coarse structure. After mixed according to the formula designed, the growth medium was sieved to 5 mm, and then filled in the containers by hand.
The experiment was conducted in an open-sided shed with shading nets and auto-spray irrigation systems at the Baima Research Base of Nanjing Forestry University (about 31°35′ N latitude, 119°10′ E longitude). After one month of growing, the container seedlings were moved up the overhead iron-wire grid in order to incorporate air root pruning, and the growing density was 100 container seedlings m−2. There were 90 seedlings (30 seedlings for each replication) for each treatment, and a total of 1080 container seedlings were used in the experiment. During the experimental period, normal management practices were carried out, but no fertilization was applied.
2.2. Growth Medium Sampling and Measurement
After preparing the growth media, the different component materials were mixed by hand, according to the designed formula. Then, four samples of each growth medium were taken for the estimation of the physical and chemical properties prior to filling the containers. The pH values of the growing media were measured in a soil–water suspension (soil:water = 1:2.5) using an automatic titrator (SH/T0983, Changsha, China), and the bulk density of the growing media was determined by the cylindrical core method [
30]. For the measurements of total N, P and K, the growth medium materials were digested using 5 mL concentrated H
2SO
4 and 1 mL concentrated HClO
4 at 120 °C for 30 min, and then at 360 °C until the digest was clear. After digestion, the N and P concentrations in the digest were determined using a flow-injection analyzer (BRAN+LUEBBE, Hamburg, Germany), whereas the K concentration in the digest was analyzed using a Hitachi108-80 atomic adsorption spectrometer (Hitachi Ltd., Tokyo, Japan). Organic matter in the growing media was measured by oxidation with potassium dichromate [
31].
2.3. Plant Sampling and Measurement
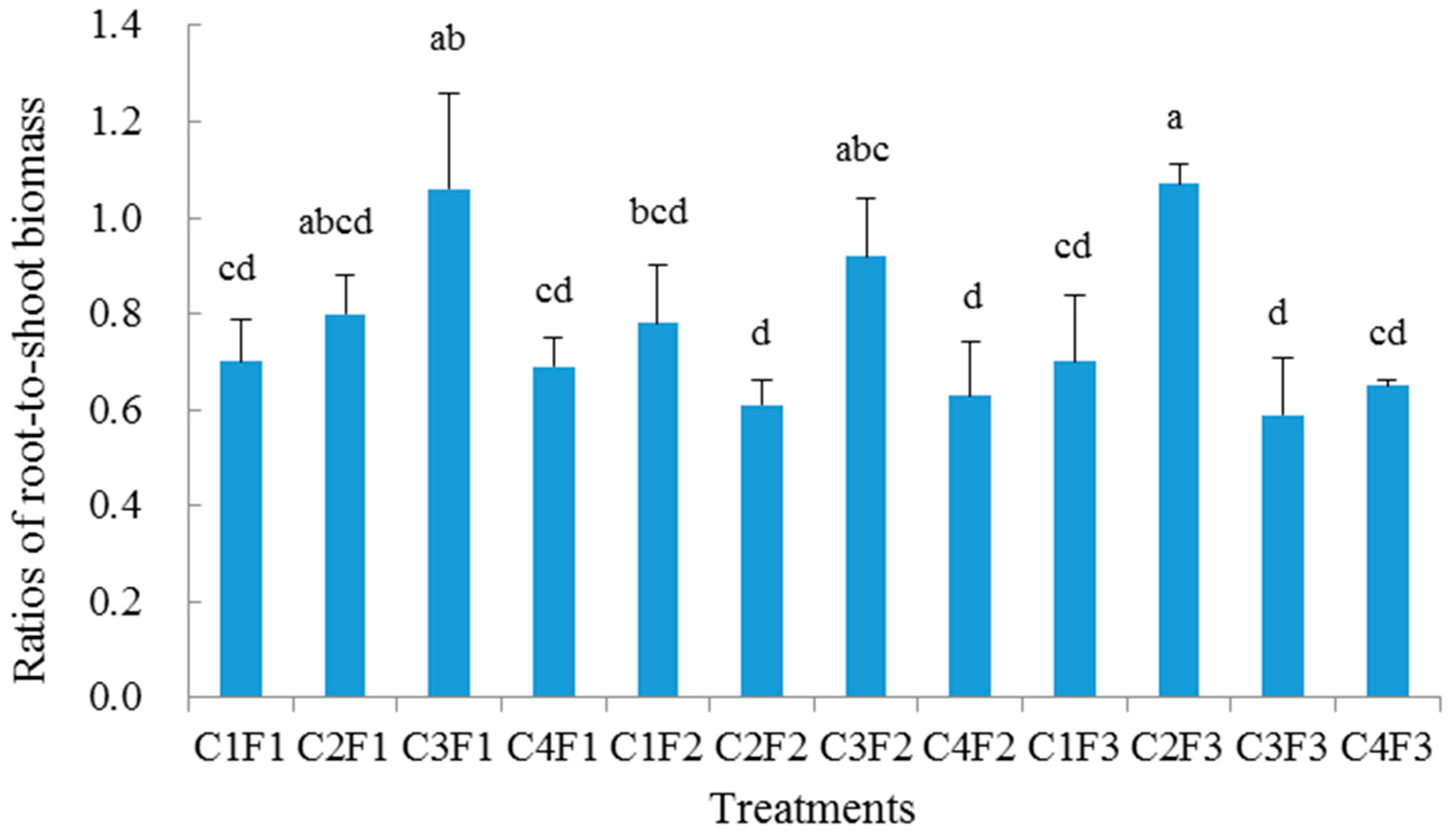
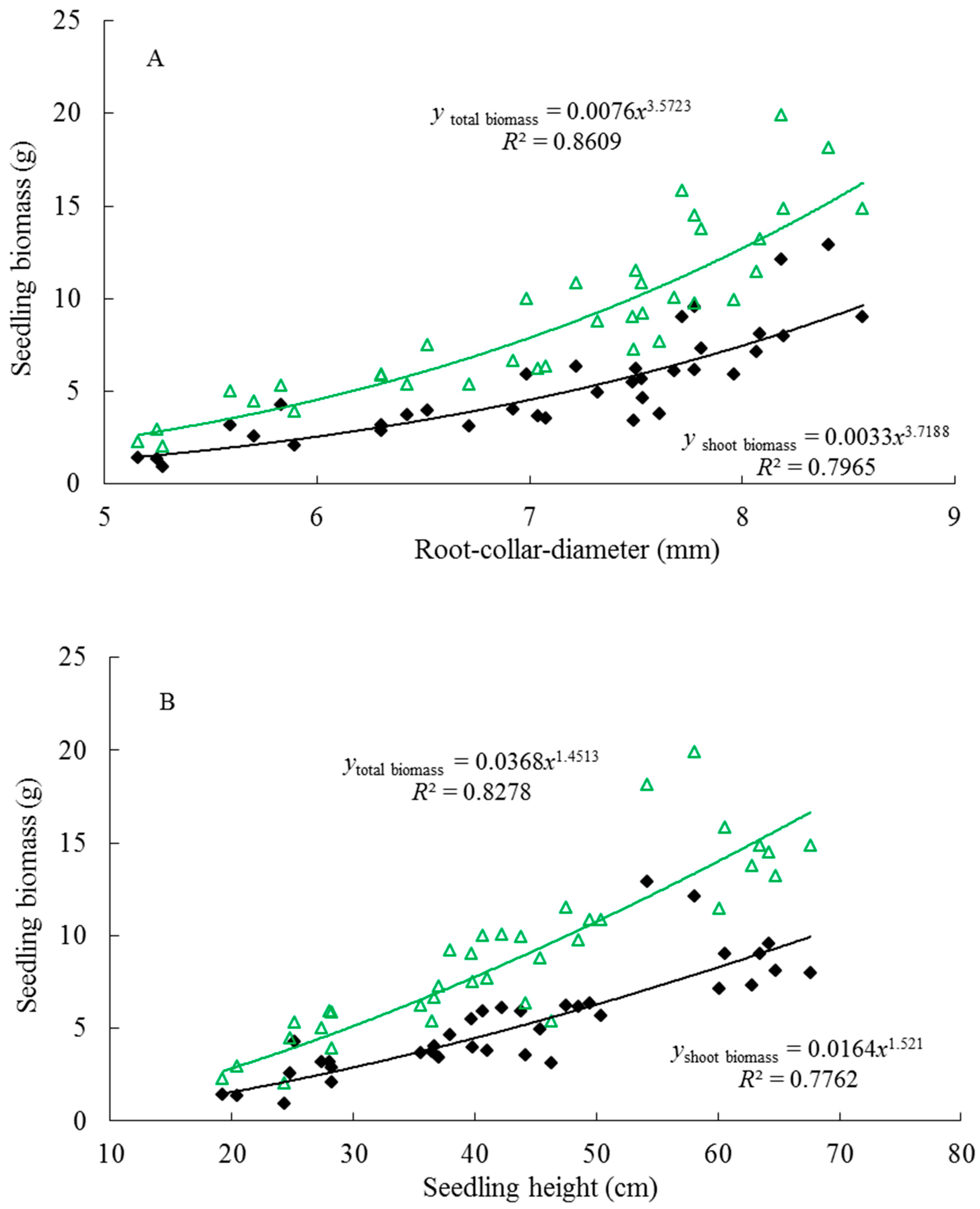
The morphological characteristics of seedling height and root collar diameter were measured on every seedling grown for the experimental phase of this study at the interval of 30 days (n = 1080). Additionally, based on the mean values of seedling height and root-collar diameter that were measured in each treatment, root dry mass, shoot dry mass, and root morphological indexes were measured on a subset of seedlings (n = 36) on 16 October 2015 using destructive sampling techniques. After carefully washing root systems free of all media, biomass was measured after the separated roots and shoots were dried at 60 °C for 72 h. All of the root morphological indexes (total root length, root surface area, root volume, and mean root diameter) were determined and analyzed by WinRHIZO.PRO systems (Version 2007, Regent Instruments Inc., Quebec, QC, Canada). Using the values measured, the ratios of root-to-shoot biomass and height-to-diameter were calculated for all destructively sampled seedlings.
2.4. Statistical Analysis
All of the statistics were performed using the SPSS statistical software package version 15 (SPSS Inc., 2005, Chicago, IL, USA), and the results are reported as mean ± standard deviation. Distribution was tested for normality by Kolmogorov–Smirnov criterion, and the homogeneity of variances was tested by Levene’s test. In order to determine the main effect, as well as identify whether there were significant interactions between container type and growth medium for the measured indexes, a two-way analysis of variance (ANOVA) was adopted. When the interaction was not significant, the one-way ANOVA was conducted for each treatment, and followed by least significant difference tests (LSD) at p < 0.05. However, if the interaction was significant, the one-way ANOVA was only conducted to determine whether the integrated effect of container types and growing media significantly affected seedling growth and root morphology at p < 0.05. Associations between seedling morphological variables were determined by using Pearson’s correlation analysis, while a regression analysis was conducted between seedling growth and biomass.