Growth and Physiology of Senegalia senegal (L.) Britton Seedlings as Influenced by Seed Origin and Salinity and Fertility Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growth Conditions, Treatment, and Experimental Design
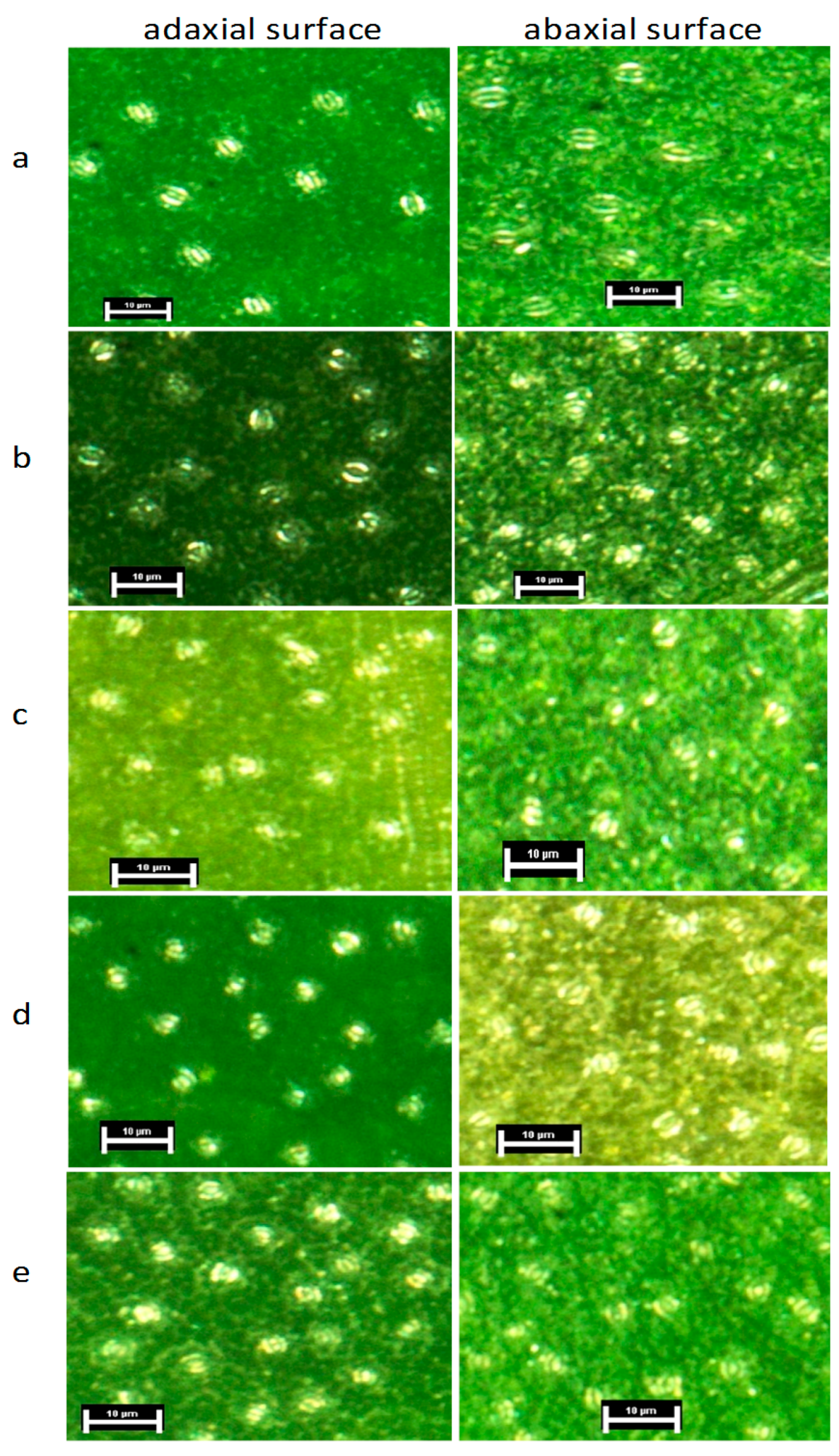
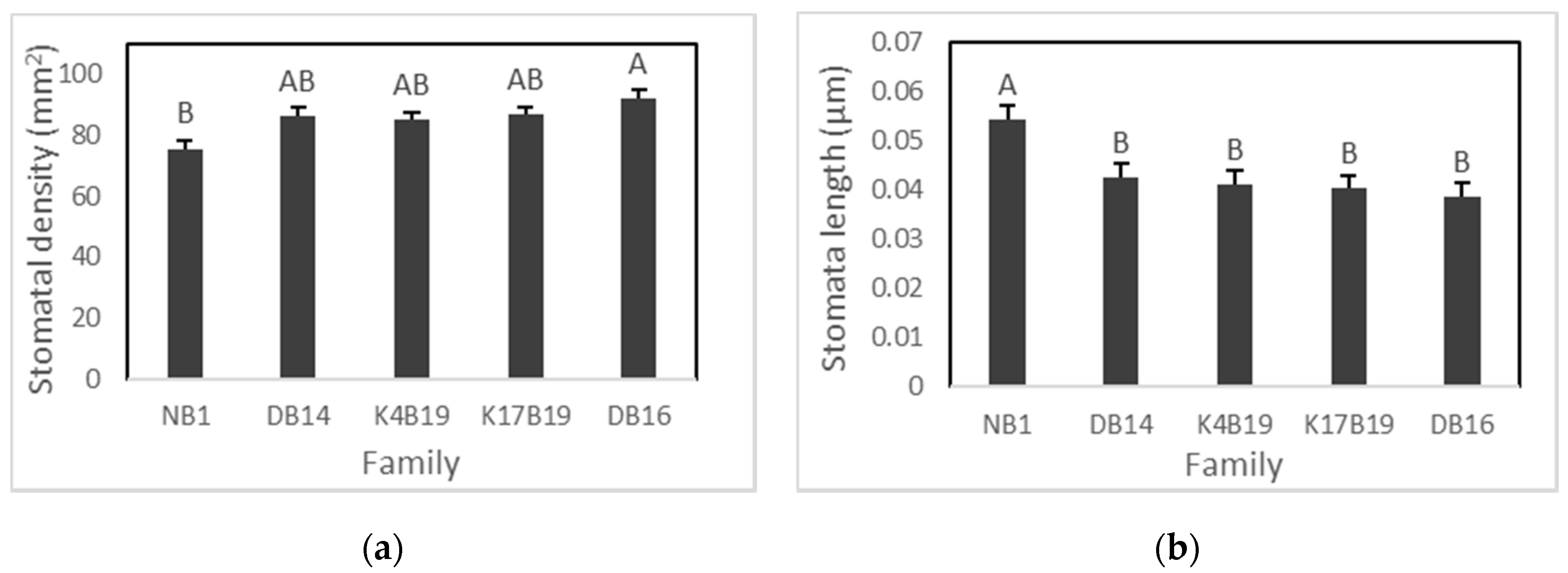
2.3. Stomatal Density and Stomatal Size Measurements
2.4. Leaf Gas Exchanges and Chlorophyll Content Measurements
2.5. Relative Growth Rate and Biomass
2.6. Statistical Analysis
3. Results
3.1. Prior Drought Conditions
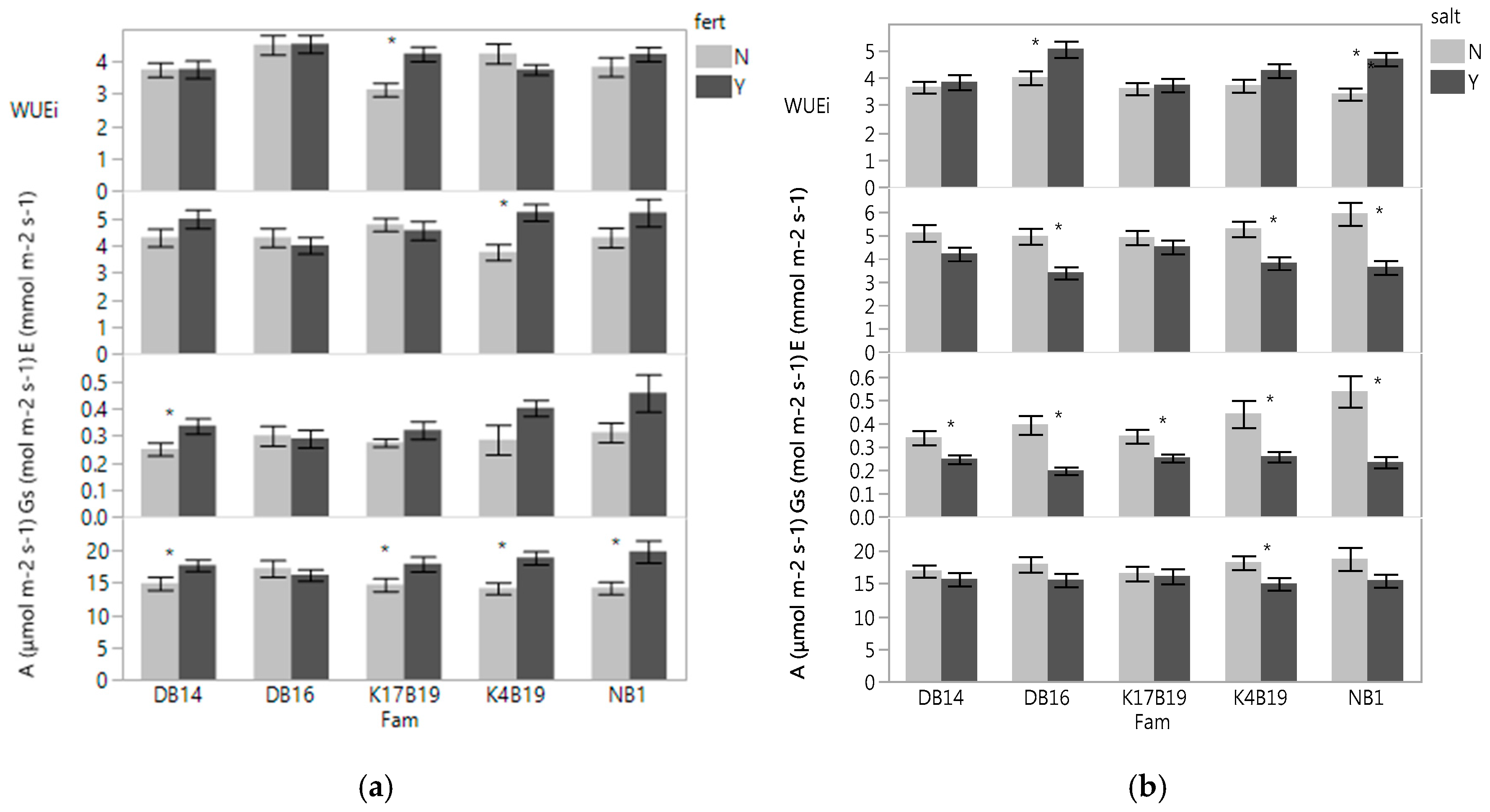
3.1.1. Leaf Gas Exchanges and Chlorophyll Content
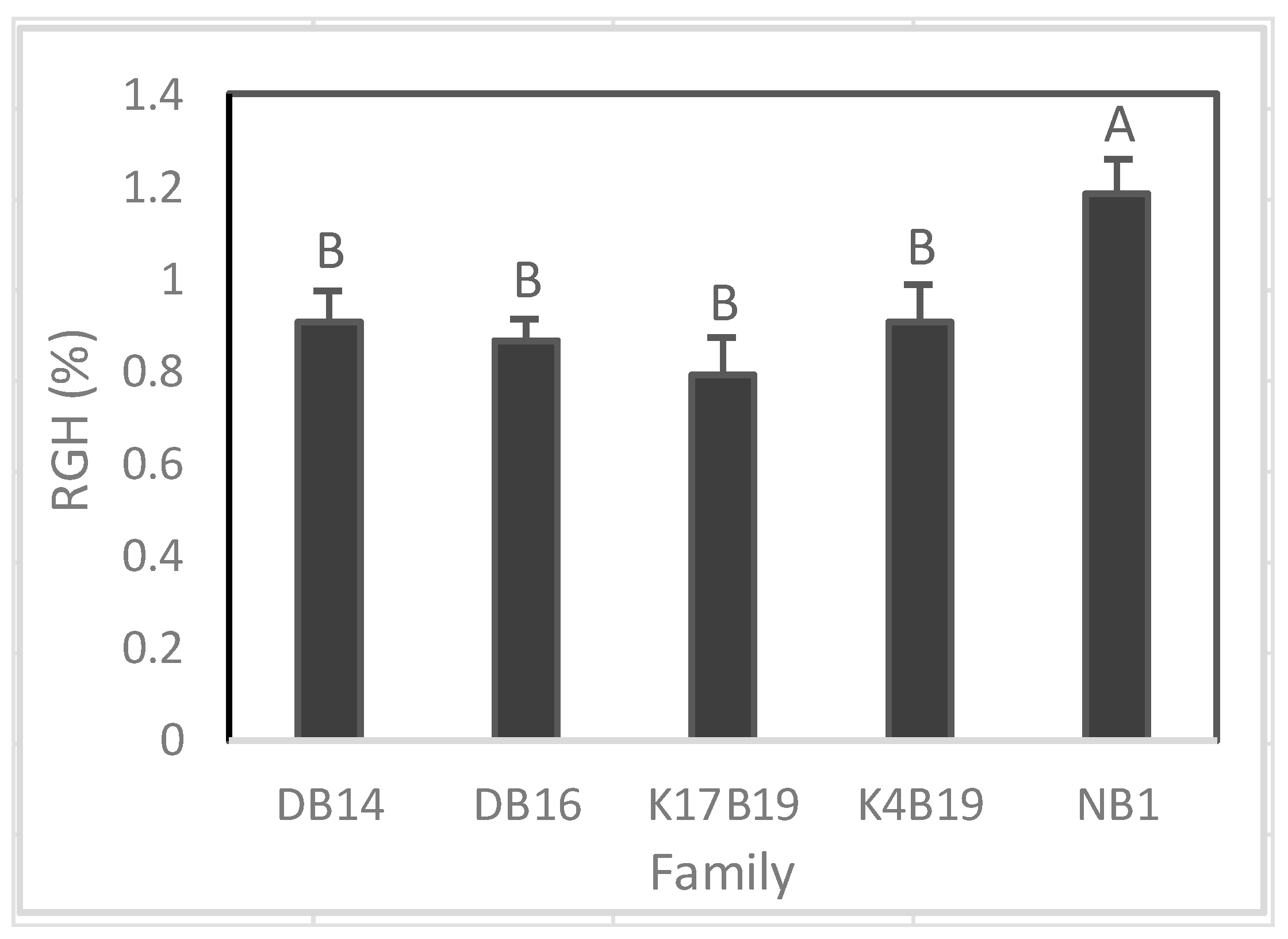
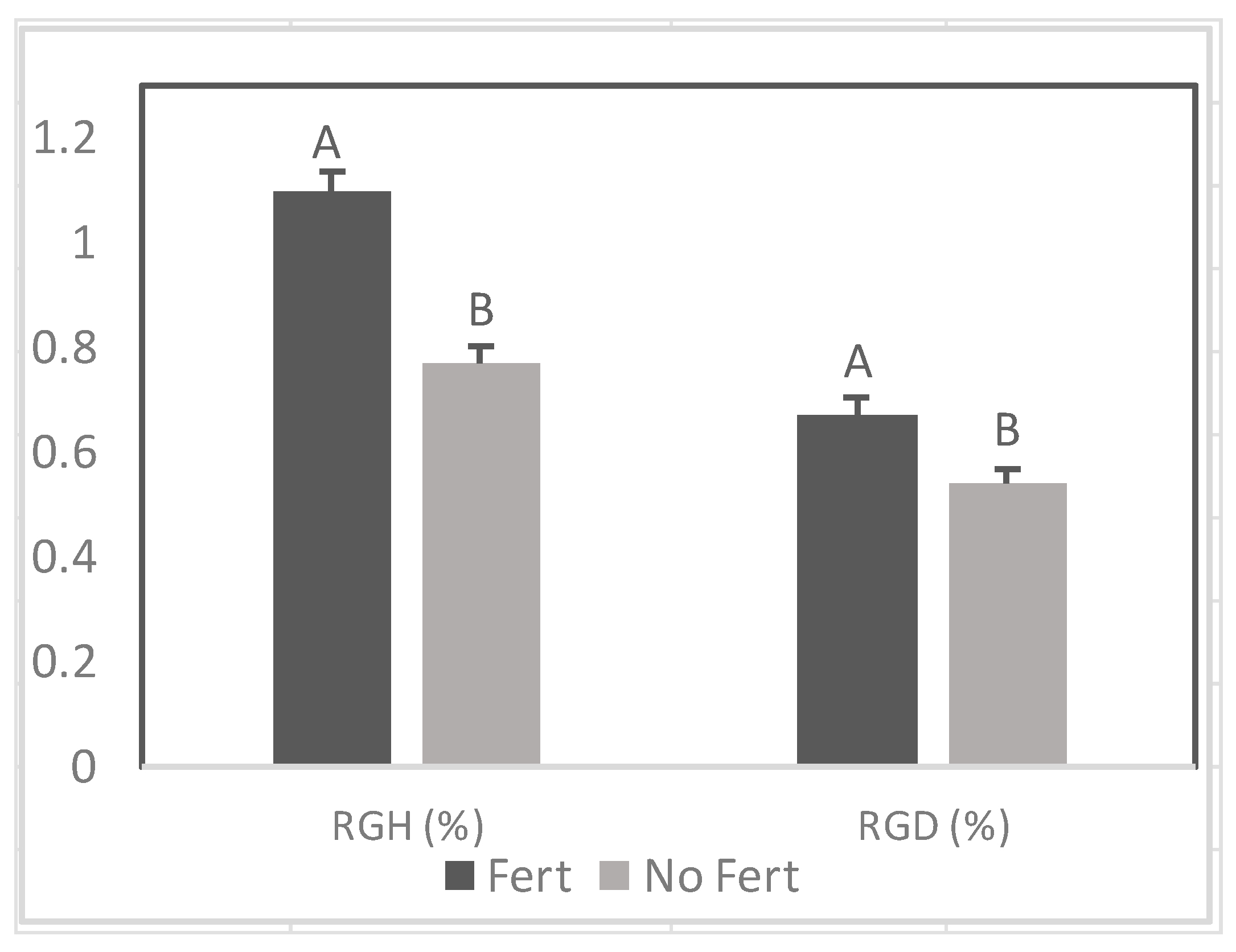
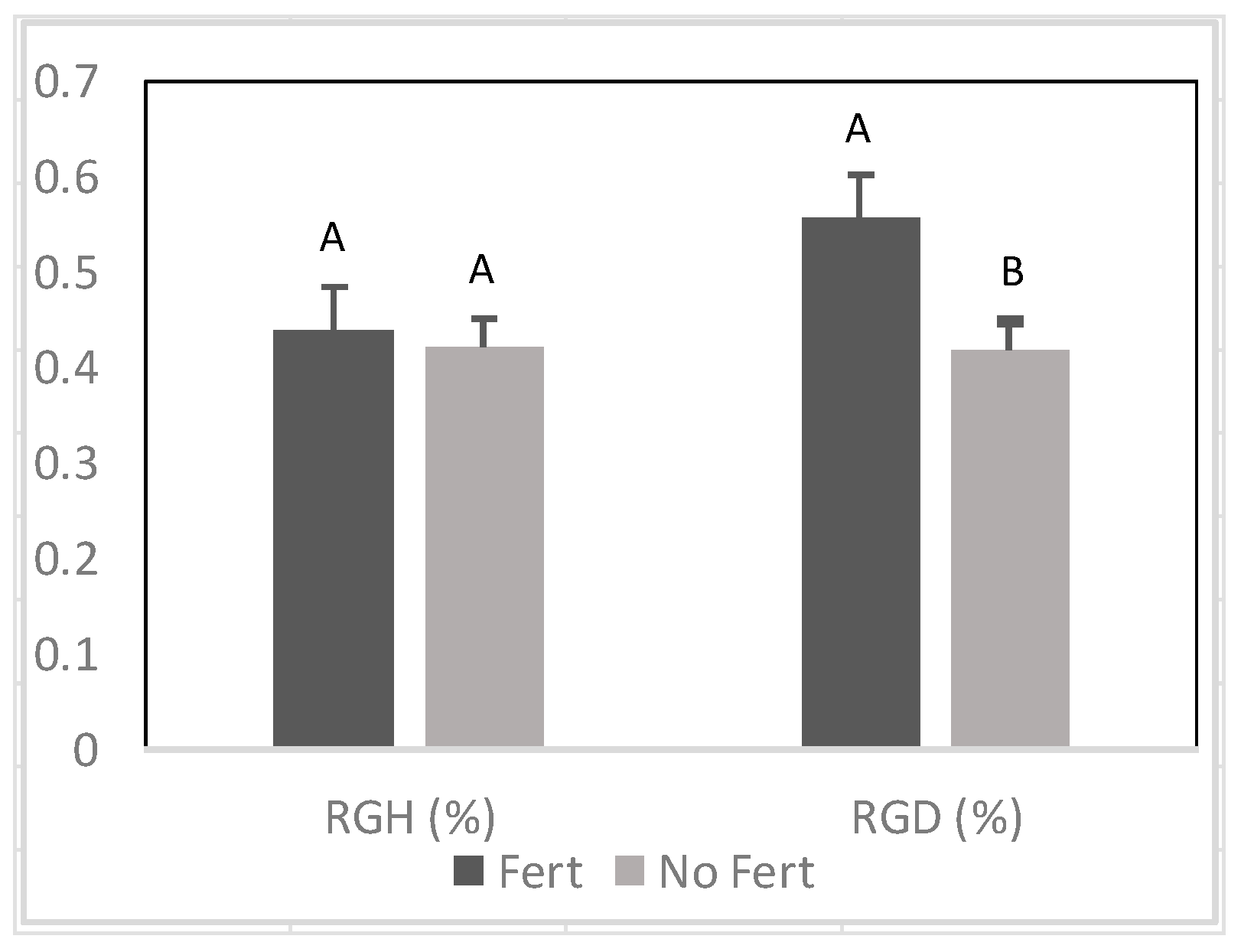
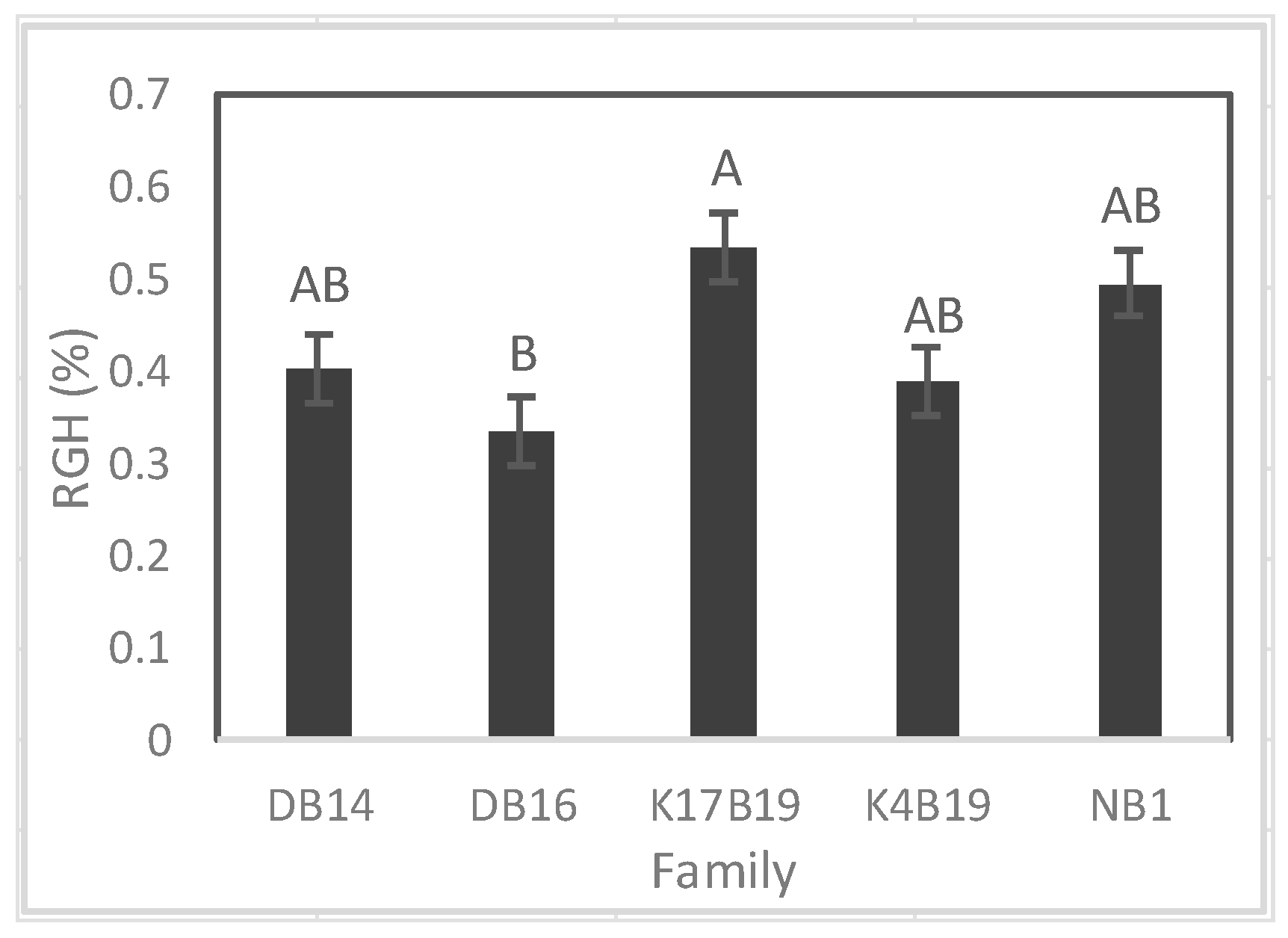
3.1.2. Relative Growth Rate
3.2. Under Drought Conditions
3.2.1. Stomatal Density and Size
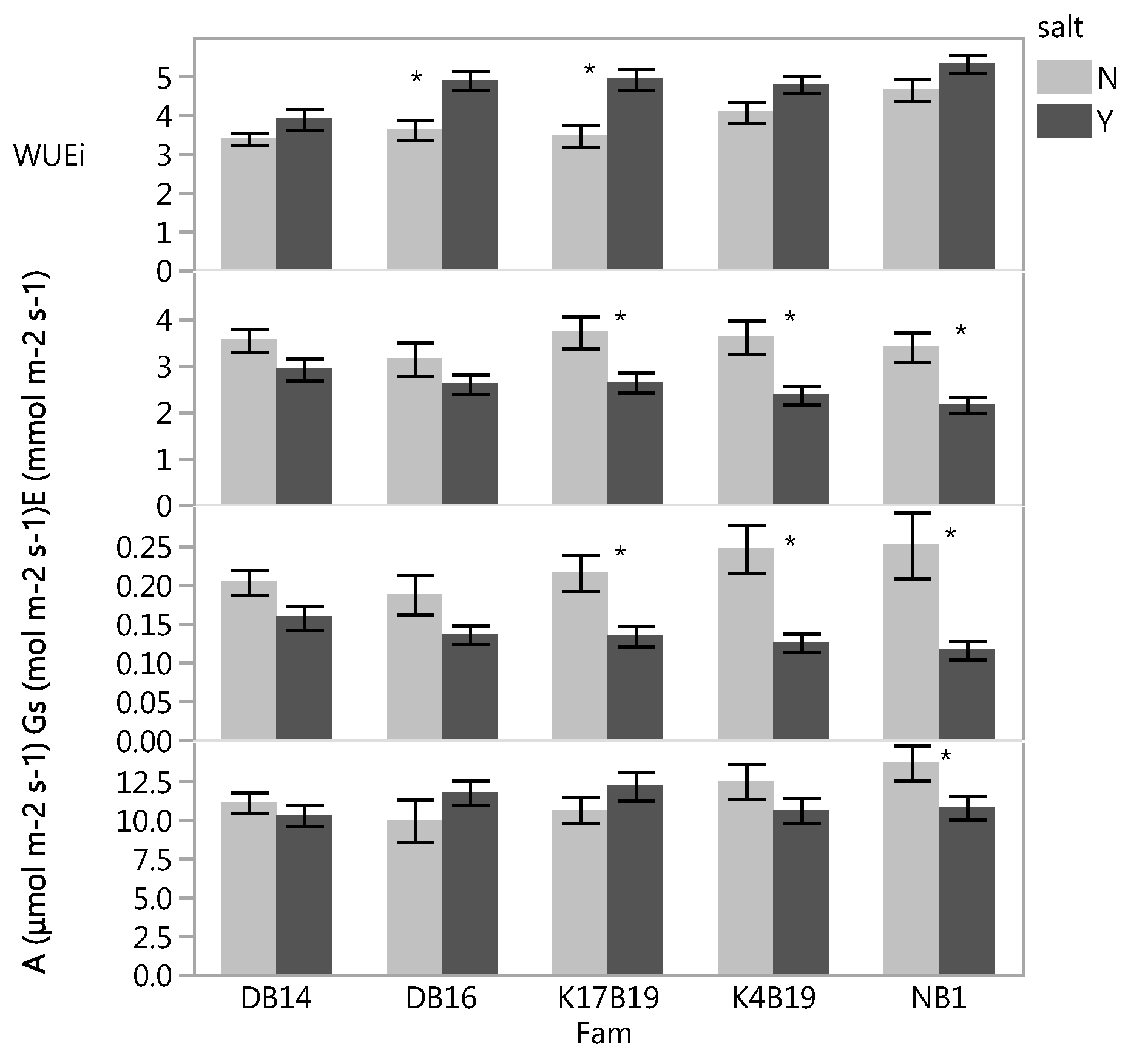
3.2.2. Leaf Gas Exchanges
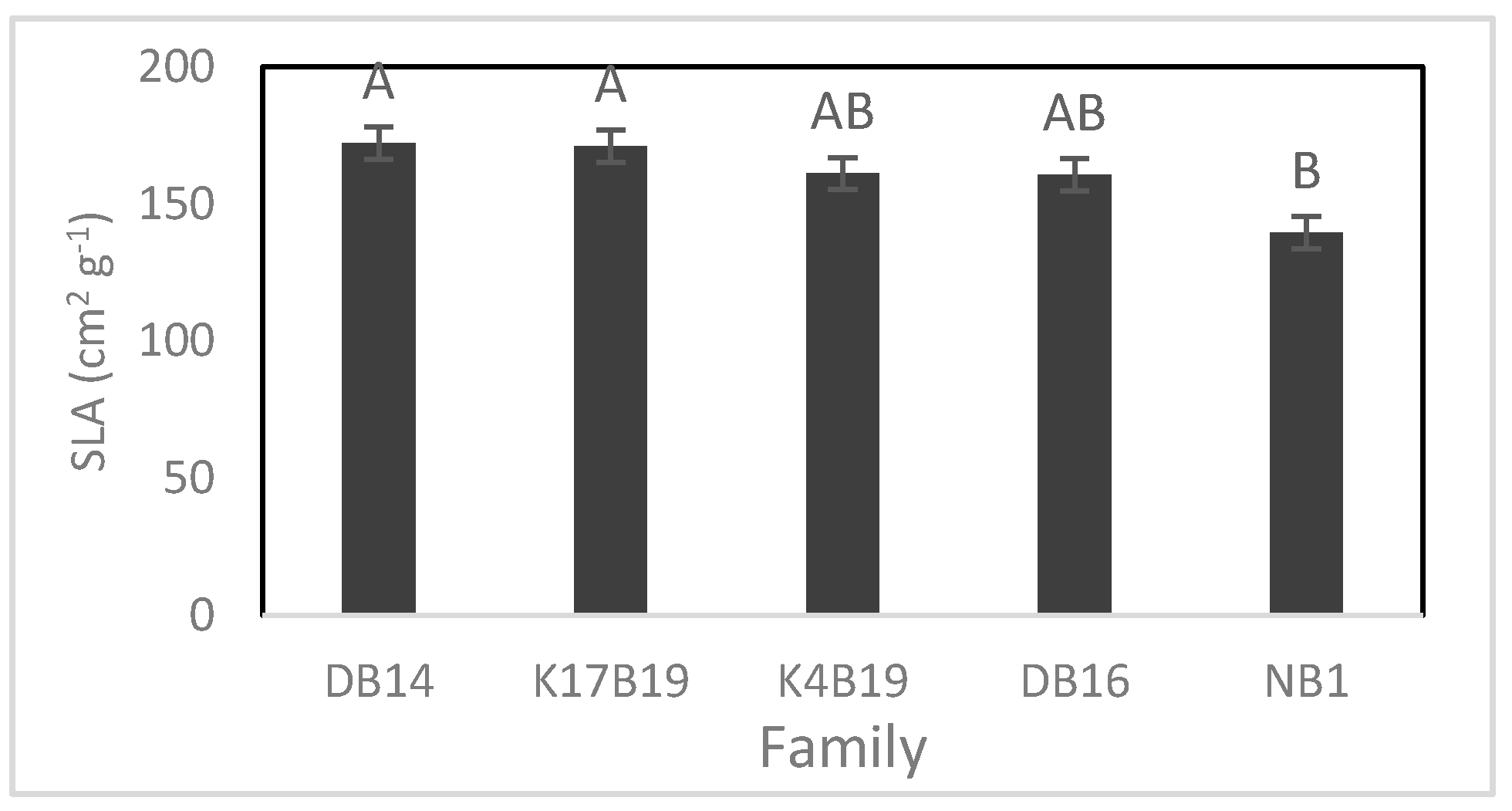
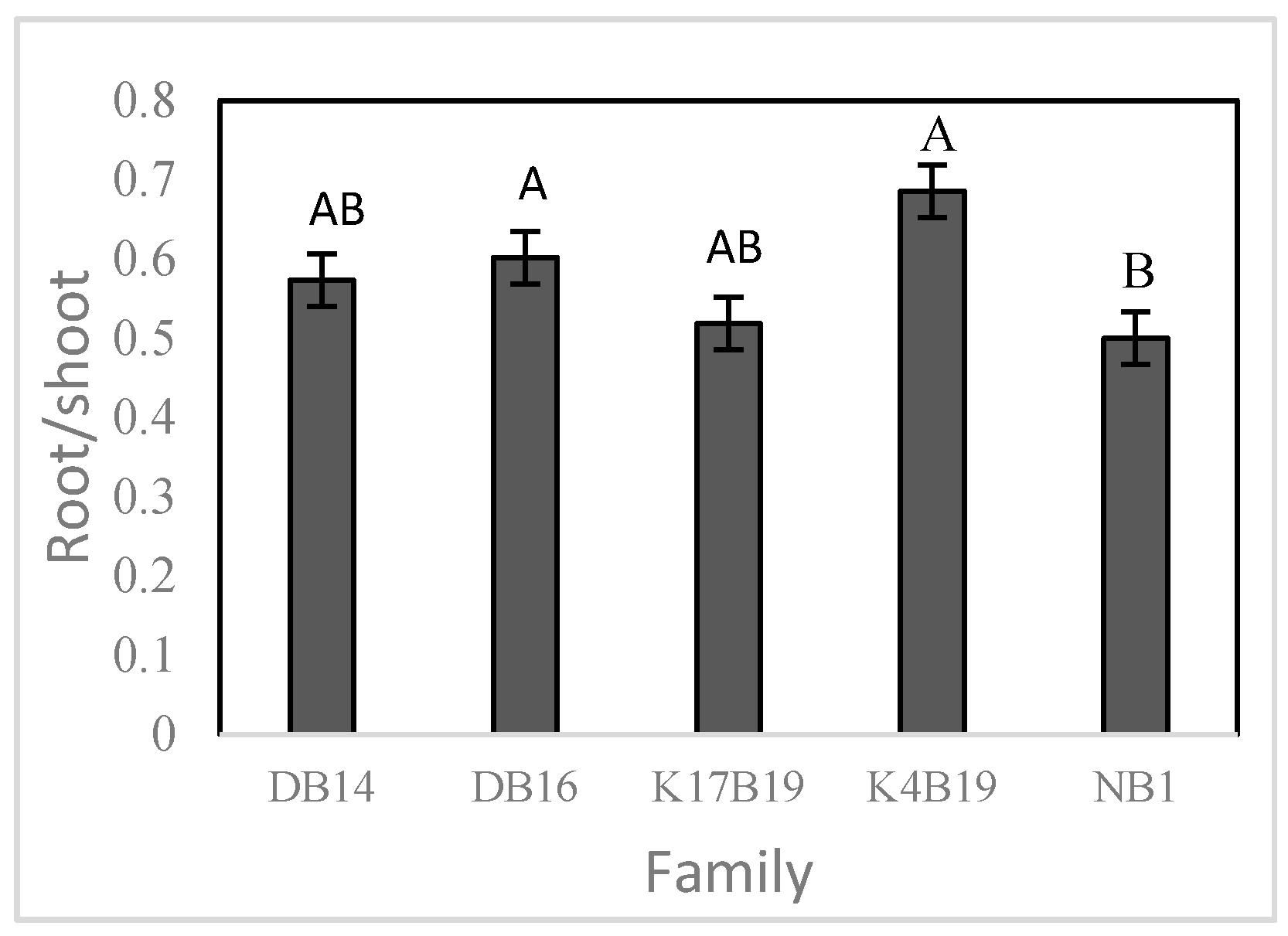
3.2.3. Biomass and Relative Growth Rates
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gonzalez, P. Desertification and a Shift of Forest Species in the West African Sahel. Clim. Res. 2001, 17, 217–228. [Google Scholar] [CrossRef]
- Green Economy Sector Study on Water Resources in Senegal. Available online: https://www.unep.org/greeneconomy/sites/unep.org.greeneconomy/files/publications/ge_water_senegal_final.pdf (accessed on 10 October 2017).
- Bradley, D.; Grainger, A. Social resilience as a controlling influence on desertification in Senegal. Land Degrad. Dev. 2004, 15, 451–470. [Google Scholar] [CrossRef]
- Rapport Exploratoire sur L’Économie Verte au Sénégal. Available online: http://www.cepod.gouv.sn/sites/default/files/L'%C3%A9conomie%20verte%20%20rapport%20provisoire.pdf (accessed on 10 October 2017).
- Fall, D.; Bakhoum, N.; Fall, F.; Diouf, F.; Ly, M.O.; Diouf, M.; Gully, D.; Hocher, V.; Diouf, D. Germination, Growth and Physiological Responses of Senegalia senegal (L.) Britton, Vachellia seyal (Delile) P. Hurter and Prosopis Juliflora (Swartz) DC to Salinity Stress in Greenhouse Conditions. Afr. J. Biotechnol. 2016, 15, 2017–2027. [Google Scholar]
- Pandey, S.; Zhang, W.; Assmann, S.M. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007, 581, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Sambou, A.; Ndour, B.; Cheng, S.; Senghor, E. Ligneous species tolerance in acid sulphated and saline soils of sine saloum: Case of rural community of Djilass and Loul Secene. J. Sustain. Dev. 2010, 3, 174. [Google Scholar] [CrossRef]
- Sonneveld, B.G.J.S.; Keyzer, M.A.; Zikhali, P.; Merbis, M. National Land Degradation Assessment Senegal and Review of Global Socio-Economic Parameters in the LADA Data Base–Land Degradation Assessment (LADA) Project Paper; Centre for World Food Studies: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Aroca, R. Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Masle, J.; Gilmore, A.; Farquhar, G. The ERECTA Gene Regulates Plant Transpiration Efficiency in Arabidopsis. Nature 2005, 436, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.Q.; Liu, Y.F.; Liu, P.; Lei, G.; He, S.; Ma, B.; Zhang, W.; Zhang, J.; Chen, S. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J. 2010, 62, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Reginato, M.A.; Reinoso, H.; Llanes, A.S.; Luna, M.V. Stomatal abundance and distribution in Prosopis strombulifera plants growing under different iso-osmotic salt treatments. Am. J. Plant Sci. 2013, 4, 41195. [Google Scholar] [CrossRef]
- Chaves, M.M.; Costa, J.M.; Saibo, N.J.M. Recent Advances in Photosynthesis under Drought and Salinity. Elsevier Sci. Technol. 2011, 57, 49–104. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, S.A.; Pandey, A.N. Growth, Water Status and Nutrient Accumulation of Seedlings of Acacia senegal (L.) Willd. in Response to Soil Salinity. An. Biol. 2008, 30, 17–28. [Google Scholar]
- Blackman, V.H. The compound interest law and plant growth. Ann. Bot. 1919, 33, 353–360. [Google Scholar] [CrossRef]
- Sarr, M.S. Morphological and Physiological Responses of Senegalia senegal (L.) Britton Provenances to Drought, Salinity, and Fertility. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2017; p. 121. [Google Scholar]
- Flowers, T.J.; Yeo, A.R. Ion Relations of Plants Under Drought and Salinity. Aust. J. Plant Physiol. 1986, 13. [Google Scholar] [CrossRef]
- Briggs, D. Genecological Studies of Salt Tolerance in Groundsel (Senecio vulgaris L.) with Particular Reference to Roadside Habitats. New Phytol. 1978, 81, 381–389. [Google Scholar] [CrossRef]
- Muturi, G.M. Provenance Variation of Salt Tolerance and Seedling Nutrition in Acacia tortilis (Forsk.) Hayne. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 1993. [Google Scholar]
- Cha-um, S.; Somsueb, S.; Samphumphuang, T.; Kirdmanee, C. Salt tolerant screening in eucalypt genotypes (Eucalyptus spp.) using photosynthetic abilities, proline accumulation, and growth characteristics as effective indices. In Vitro Cell. Dev. Biol. Plant 2013, 49, 611–619. [Google Scholar] [CrossRef]
- Fung, L.E.; Wang, S.S.; Altman, A.; Hutterman, A. Effect of NaCl on Growth, Photosynthesis, Ion and Water Relations of Four Poplar Genotypes. For. Ecol. Manag. 1998, 107, 135–146. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of Salt Tolerance in Nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Munns, R. Genes and Salt Tolerance: Bringing them Together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Ghoulam, C.A.F.; Fares, K. Effects of Salt Stress on Growth, Inorganic Ions and Proline Accumulation in Relation to Osmotic Adjustment in Five Sugar Beet Cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Yadav, R.S.; Sehgal, D.; Vadez, V. Using genetic mapping and genomics approaches in understanding and improving drought tolerance in pearl millet. J. Exp. Bot. 2011, 62, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates, Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Barbieri, G.; Vallone, S.; Orsini, F.; Paradiso, R.; De Pascale, S.; Negre-Zakharov, F.; Maggio, A. Stomatal Density and Metabolic Determinants Mediate Salt Stress Adaptation and Water Use Efficiency in Basil (Ocimum basilicum L.). J. Plant Physiol. 2012, 169, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1995. [Google Scholar]
- Yang, H.; Wang, G. Leaf stomatal densities and distribution in Triticum aestivum under drought and CO2 enrichment. Acta Ecol. Sinica 2000, 25, 312–316. [Google Scholar]
- Zhang, H.; Li, J.; Yoo, J.H.; Yoo, C.C.; Cho, S.H.; Koh, H.J.; Seo, H.S.; Paek, N.C. Rice Chlorina-1 and Chlorina-9 Encode ChlD and ChlI Subunits of mg Chelatase, a Key Enzyme for Chlorophyll Synthesis and Chloroplast Development. Plant Mol. Biol. 2006, 62, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.N.; Udaykumar, M.; Farquhar, G.D.; Talwar, H.S.; Prasad, T.G. Variation in carbon isotope discrimination and its relationship to specific leaf area and ribulose-1, 5-bisphosphate carboxylase content in groundnut genotypes. Funct. Plant Biol. 1995, 22, 545–551. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Hall, A.E.; Farquhar, G.D. Stable Isotopes and Plant Carbon/Water Relations; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Li, C. Carbon isotope composition, water-use efficiency and biomass productivity of Eucalyptus microtheca populations under different water supplies. Plant Soil 1999, 214, 165–171. [Google Scholar] [CrossRef]
- Flexas, J.; Niinemets, U.; Galle, A.; Barbour, M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmes, J.; Ribas-Carbo, M.; Rodrigues, P.L.; et al. Diffusional Conductances to CO2 as a Target for Increasing Photosynthesis and Photosynthetic Water Use Efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Pallardy, S.G. Physiology of Woody Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Flexas, J.; Diaz-Espejo, A.; Conesa, M.A.; Coopman, R.E.; Douthe, C.; Gago, J.; Galle, A.; Galmes, J.; Medrano, H.; Ribas-Carbo, M.; et al. Mesophyll Conductance to CO2 and Rubisco as Targets for Improving Intrinsic Water Use Efficiency in C3 Plants. Plant Cell Environ. 2016, 39, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Ferrio, J.P.; Pou, A.; Florez-Sarasa, I.; Gessler, A.; Kodama, N.; Flexas, J.; Ribas-Carbo, M. The Péclet Effect on Leaf Water Enrichment Correlates with Leaf Hydraulic Conductance and Mesophyll Conductance for CO2. Plant Cell Environ. 2012, 35, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.T.; Orcutt, D.M.; Hale, M.G. The Physiology of Plants under Stress; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Daldoum, D.M.A.; Hammad, G.H. Performance of Acacia senegal (L.) Wild Seedlings Growth under some Tree Manures and NPK Fertilizers in Nursery Site. J. Environ. Sci. 2015, 31, 303–311. [Google Scholar] [CrossRef]
- Fernández, M.; Novillo, C.; Pardos, J.A. Effects of Water and Nutrient Availability in Pinus pinaster Ait. Open Pollinated Families at an Early Age: Growth, Gas Exchange and Water Relations. New For. 2006, 31, 321–342. [Google Scholar] [CrossRef]
- Stovall, J.P.; Fox, T.R.; Seiler, J.R. Short Term Changes in Biomass Partitioning of Two Full Sib Clones of Pinus taeda L. Under Differing Fertilizer Regimes over 4 months. Trees 2012, 26, 951–961. [Google Scholar] [CrossRef]
- Dvorak, W.S.; Uruena, H.; Moreno, L.A.; Goforth, J. Provenance and family variation in Sterculia apetala in Colombia. For. Ecol. Manag. 1998, 111, 127–135. [Google Scholar] [CrossRef]
| Provenance | Families | Tree Label | 2013 Gum Yield | 2014 Gum Yield | 2015 Gum Yield | 2016 Gum Yield |
|---|---|---|---|---|---|---|
| Diamenar | Diamenar27B16 | DB16 | 29.3 | 9.7 | 0 | - |
| Diamenar | Diamenar27B14 | DB14 | 899.5 | 791.7 | 386.1 | 758.3 |
| Kidira | Kidira4B19 | K4B19 | 555.3 | 551.8 | 583.3 | 990.3 |
| Kidira | Kidira17B19 | K17B19 | 131.3 | 62.1 | 22.2 | 51.7 |
| Ngane | Ngane21B1 | NB1 | 522.2 | 476.4 | 945.2 | 1103.5 |
| Sources of Variation | df | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| RGH | RGD | A | Gs | E | WUEi | Chlorophyll Content | ||
| Salinity | 1 | 0.5807 | 0.2407 | 0.0025 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Fertility | 1 | <0.0001 | 0.0023 | <0.0001 | 0.0008 | 0.0133 | 0.1899 | <0.0001 |
| Family | 4 | 0.0004 | 0.3738 | 0.9632 | 0.0375 | 0.3877 | 0.0054 | 0.0922 |
| Family × salinity | 4 | 0.6415 | 0.7013 | 0.622 | 0.0188 | 0.0494 | 0.079 | 0.3469 |
| Family × fertility | 4 | 0.7818 | 0.1669 | 0.0302 | 0.1636 | 0.0265 | 0.0246 | 0.4621 |
| Family × salinity × fertility | 4 | 0.1288 | 0.6976 | 0.8522 | 0.5166 | 0.9227 | 0.8606 | 0.5800 |
| Sources of Variation | df | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stomatal Density | Stomata Size | Total Biomass | SLA | Root/Shoot | RGH | RGD | A | Gs | E | WUEi | Chlorophyll Content | ||
| Salinity | 1 | 0.0158 | 0.2604 | <0.0001 | 0.0059 | <0.0001 | 0.0435 | 0.6073 | 0.452 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Fertility | 1 | 0.6565 | 0.91 | <0.0001 | 0.6169 | <0.0001 | 0.623 | 0.0012 | 0.2827 | 0.0142 | 0.0057 | <0.0001 | <0.0001 |
| Family | 4 | 0.0091 | <0.0001 | <0.0001 | 0.0159 | 0.023 | 0.0421 | 0.0019 | 0.4944 | 0.7874 | 0.3642 | <0.0001 | 0.2614 |
| Family × Salinity | 4 | 0.2459 | 0.5518 | 0.0634 | 0.9215 | 0.2997 | 0.2108 | 0.4326 | 0.0438 | 0.1426 | 0.5021 | 0.233 | 0.6956 |
| Family × fertility | 4 | 0.7811 | 0.0733 | 0.6723 | 0.2896 | 0.1454 | 0.1914 | 0.5758 | 0.9961 | 0.4089 | 0.514 | 0.2803 | 0.5889 |
| Family × Salinity × fertility | 4 | 0.5933 | 0.6437 | 0.0002 | 0.2343 | 0.7375 | 0.0925 | 0.1375 | 0.4873 | 0.1361 | 0.3306 | 0.0410 | 0.7770 |
| Variable | Treatment | Families | ||||
|---|---|---|---|---|---|---|
| DB14 | DB16 | K4B19 | K17B19 | NB1 | ||
| Leaf (g) | Control | 1.4 ± 0.47Aa | 1.7 ± 0.28Aab | 2.3 ± 0.61ABab | 1.5 ± 0.17Aa | 3.7 ± 0.40Bb |
| Salt | 1.4 ± 0.24Aa | 1.6 ± 0.12Aa | 1.7 ± 0.18Aa | 2.1 ± 0.16Aab | 1.6 ± 0.17Aa | |
| Fert | 4.2 ± 0.11Ab | 4.9 ± 0.45Ac | 4.2 ± 0.33Abc | 4.8 ± 0.41Ac | 5.3 ± 0.30Ab | |
| Salt × fert | 2.9 ± 0.09BCab | 2.3 ± 0.21Aab | 3.7 ± 0.36ABbc | 3.3 ± 0.30ABCbc | 4.2 ± 0.32Cb | |
| Stem (g) | Control | 1.3 ± 0.40Aa | 1.9 ± 0.34Aa | 1.7 ± 0.47Aa | 1.2 ± 0.16Aa | 2.7 ± 0.23Aab |
| Salt | 1.3 ± 0.23Aa | 2.0 ± 0.11Ba | 1.8 ± 0.14ABa | 2.1 ± 0.15Bab | 1.5 ± 0.17ABa | |
| Fert | 3.9 ± 0.24Ab | 5.3 ± 0.65Ac | 4.8 ± 0.62Ab | 5.0 ± 0.52Ac | 5.2 ± 0.25Ac | |
| Salt × fert | 3.0 ± 0.17ABab | 2.5 ± 0.21Aab | 4.0 ± 0.35Bb | 3.3 ± 0.31ABbc | 4.2 ± 0.35Bbc | |
| Root (g) | Control | 2.0 ± 0.35ABab | 2.7 ± 0.17ABab | 2.9 ± 0.45ABa | 1.7 ± 0.25Aa | 3.3 ± 0.29Bab |
| Salt | 1.5 ± 0.30Aa | 2.4 ± 0.22ABab | 2.6 ± 0.38Ba | 2.5 ± 0.25ABab | 1.7 ± 0.07ABa | |
| Fert | 3.9 ± 0.36Ac | 5.4 ± 0.40Ac | 5.2 ± 0.34Ac | 5.1 ± 0.68Ac | 5.2 ± 0.20Ac | |
| Salt × fert | 1.8 ± 0.19Aab | 1.7 ± 0.15Aa | 3.3 ± 0.14Bab | 2.0 ± 0.22Aab | 3.6 ± 0.41Babc | |
| Leaf area (cm2) | Control | 242.2 ± 111.3Aa | 279.9 ± 57.1Aa | 366.9 ± 106.9Aa | 295.1 ± 52.9Aa | 551.5 ± 61.6Aab |
| Salt | 263.2 ± 58.7Aa | 237.7 ± 22.4Aa | 380.6 ± 90.6Aa | 346.0 ± 72.1Aab | 226.2 ± 50.5Aa | |
| Fert | 891.6 ± 80.6Ac | 909.9 ± 113.8Ac | 686.8 ± 111.3Aab | 818.0 ± 134.8Ac | 756.1 ± 106.1Ab | |
| Salt × fert | 435.1 ± 74.0Aab | 323.9 ± 38.4Aab | 560.8 ± 118.0Aab | 522.5 ± 49.2Aabc | 546.6 ± 92.7Aab | |
| Total dry matter (g) | Control | 4.6 ± 1.16Aa | 6.2 ± 0.75ABa | 6.8 ± 1.41ABab | 4.4 ± 0.49Aa | 9.7 ± 0.76Bb |
| Salt | 4.2 ± 0.71Aa | 6.0 ± 0.22ABa | 6.2 ± 0.63ABa | 6.7 ± 0.53Bab | 4.8 ± 0.38Aa | |
| Fert | 12.0 ± 0.57Ab | 15.6 ± 1.39Ab | 14.1 ± 0.90Acd | 14.9 ± 1.51Ac | 15.7 ± 0.43Ac | |
| Salt × fert | 7.8 ± 0.30ABab | 6.5 ± 0.45Aa | 11.0 ± 0.77BCbc | 8.6 ± 0.80ABb | 12.0 ± 0.99Cbc | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarr, M.S.; Seiler, J.R.; Sullivan, J. Growth and Physiology of Senegalia senegal (L.) Britton Seedlings as Influenced by Seed Origin and Salinity and Fertility Treatments. Forests 2017, 8, 388. https://doi.org/10.3390/f8100388
Sarr MS, Seiler JR, Sullivan J. Growth and Physiology of Senegalia senegal (L.) Britton Seedlings as Influenced by Seed Origin and Salinity and Fertility Treatments. Forests. 2017; 8(10):388. https://doi.org/10.3390/f8100388
Chicago/Turabian StyleSarr, Mame Sokhna, John R. Seiler, and Jay Sullivan. 2017. "Growth and Physiology of Senegalia senegal (L.) Britton Seedlings as Influenced by Seed Origin and Salinity and Fertility Treatments" Forests 8, no. 10: 388. https://doi.org/10.3390/f8100388




