1. Introduction
Lucidophyllous (evergreen broad-leaved) forests are distributed widely in the subtropical and warm-temperate regions of East Asia [
1]. Lucidophyllous forests are mainly dominated by evergreen species of Fagaceae, Lauraceae, Theaceae, Magnoliaceae, and Hamamelidaceae; beyond that,
Castanopsis cuspidata is one of the typical dominant species from the coastal area of central Japan to southwestern Japan [
2]. Ohsawa [
3] suggested that the tropical lower montane forests that are mainly dominated by evergreen Fagaceae (especially
Castanopsis) can be correlated to the horizontal subtropical/warm-temperate zone of East Asia as lucidophyllous forests, and the latitudinal northern limit reaches sea level at 35° N of central Japan. There have been several long-term studies that investigated the structure and the dynamics of the unique forests using large permanent quadrats in southwestern Japan; e.g., Aya Research Site from 1989 [
4,
5,
6] or Tatera Forest reserve from 1990 [
7,
8,
9]. These studies have revealed that diverse gap-forming processes created environmental heterogeneity in the forest floor and contributed to the maintenance of the species-rich evergreen broad-leaved forests.
However, almost all of these unique natural lucidophyllous forests distributed in human populated areas have been logged or greatly changed by human activities due to the demands for products [
10]. Only remnants of evergreen forests can be found in sanctuaries around temples and shrines or lie on steep slopes which are not easy to access, and secondary forests of coppice woods or pioneer pine forests are dominant in southern and central Japan. Biomass increment in forests through secondary succession is one of the most important components of carbon sequestration of the terrestrial ecosystem [
11]. Particularly, forests in the middle and high latitudes of the northern hemisphere, which are the human-dominated areas, had high potential of carbon sequestration during the end of the 20th century due to enhancement of plantations or forest recovery during secondary succession [
12,
13]. Moreover, it is well known that wood production declines along with ecosystem development [
14]. For example, Ohtsuka et al. [
11,
15] studied net primary production (NPP) in a temperate deciduous coppice forest of 18 years after clear-cutting, and stand increment of wood parts reached 4.8 ton ha
−1 year
−1, which was more than two times higher than that of mature forest. Forest age-sequence studies revealed that NPP reached a peak during early stand development and then gradually declined by as much as 76% [
16]. Magnani et al. [
17] also reported that the age effects accounted for 92% of the total variability in net ecosystem production from boreal coniferous to temperate broadleaf forests. Therefore, the age-related changes of wood production are crucial for global carbon cycling in the near future.
The development process of forests is often based on the chronosequence of various stand ages, and, thus, many studies conducted on the comparisons of secondary and old-growth lucidophyllous forests [
10,
18,
19,
20]. For example, Kubota [
19] investigated the structural changes and growth dynamics in lucidophyllous forests of four stands of different ages in southwestern Japan and revealed that wood production decreased with age from 7.95 (15 years old) to 4.61 (over 100 years old) ton ha
−1 year
−1. Moreover, self-thinning was an important factor in the development of young lucidophyllous forests, and the monopolization of
Castanopsis sieboldii through secondary succession had a negative influence on species diversity [
20]. However, in contrast to these chronosequence studies, there was a rare investigation directly monitoring stand development over several decades, particularly for secondary lucidophyllous forests. Therefore, the long-term investigation of changes in forest structure and the development process of secondary lucidophyllous forests were needed.
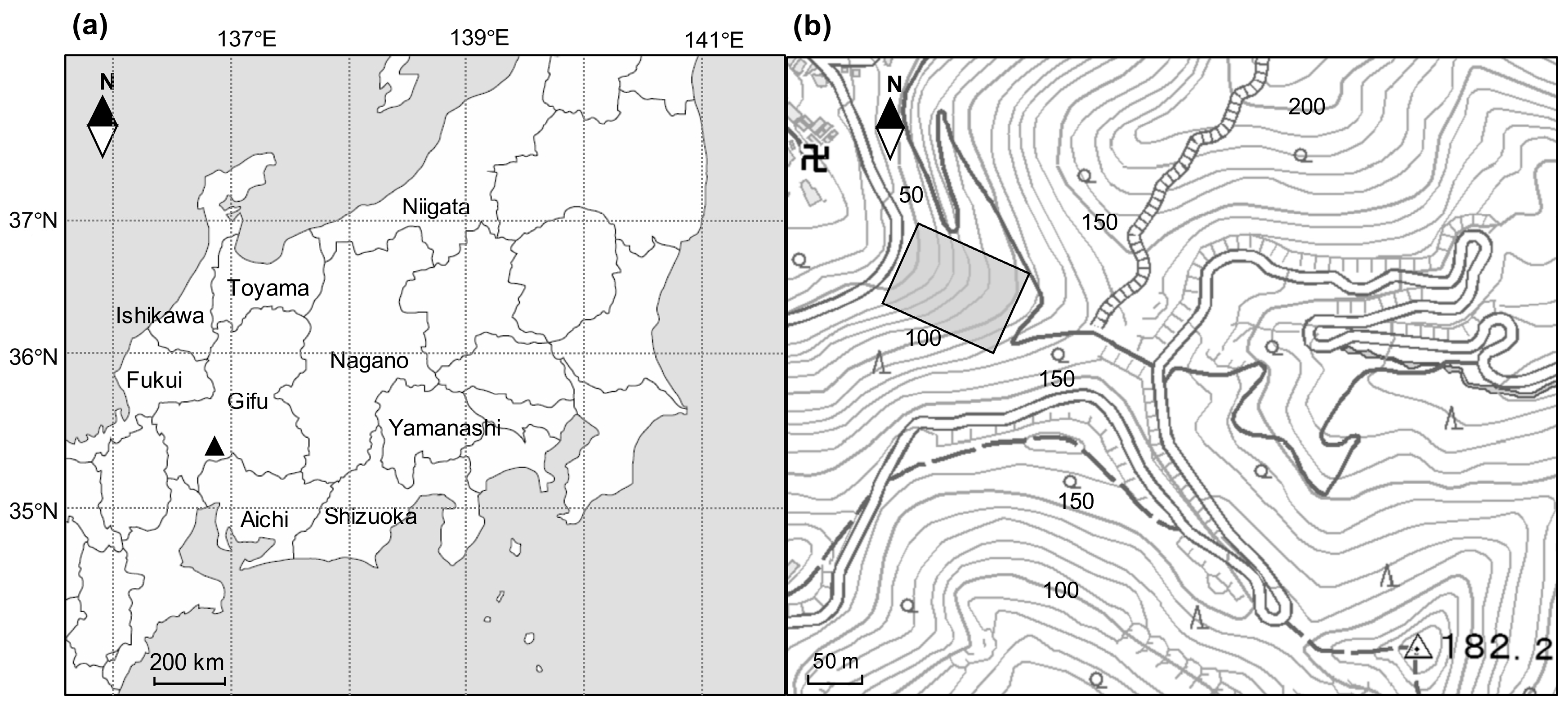
Mt. Kinka (35°26′ N, 136°47′ E, the peak is 329 m) is located in Gifu Prefecture, central Japan. Almost all areas (597 ha) of Mt. Kinka consist of secondary natural forests (93%) and artificial coniferous forests (2%). In particular, the lower slopes of Mt. Kinka are covered by secondary evergreen broad-leaved forests that predominated by
Castanopsis cuspidata, which were mainly recovered after the World War II. Previous studies have showed that the canopy trees of
Castanopsis in the secondary forests grew faster than other species and underwent intraspecific competition (self-thinning) due to the high sprouting capacity [
19]. In addition, the changes in forest structure always accompany production decline [
16]. Therefore, it can be hypothesized that self-thinning may govern the stand development process of this secondary
Castanopsis forest and that age-related wood production decline can be observed due to neighborhood competitions during secondary succession. To test the hypothesis, we demarcated a permanent plot in 1989 and have studied forest dynamics since then on the lower slope of Mt. Kinka. The objectives of this paper were as follows: (1) to assess the community structural change of secondary stands spanning 28 years (1989–2017), and (2) to document temporal trends in biomass accumulation and aboveground woody NPP with special reference to stand development.
4. Discussion
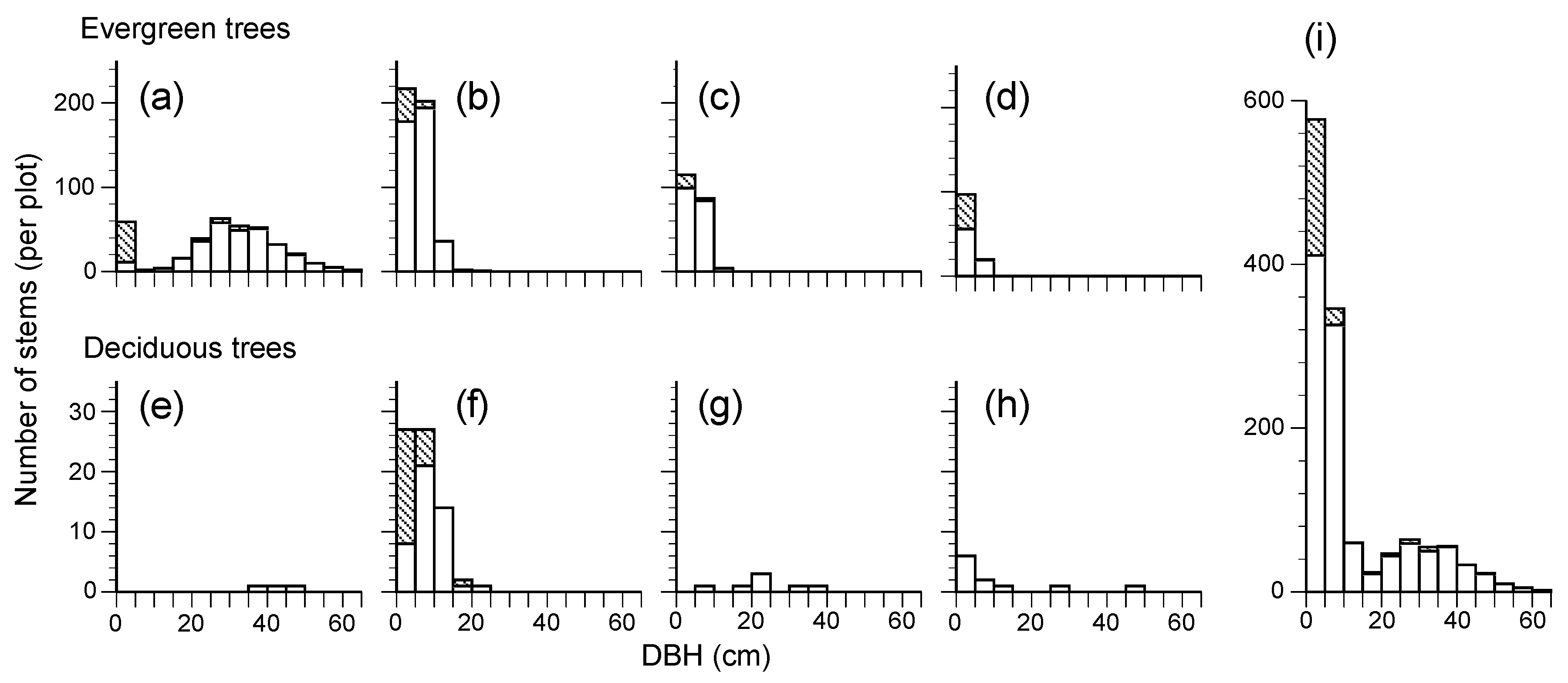
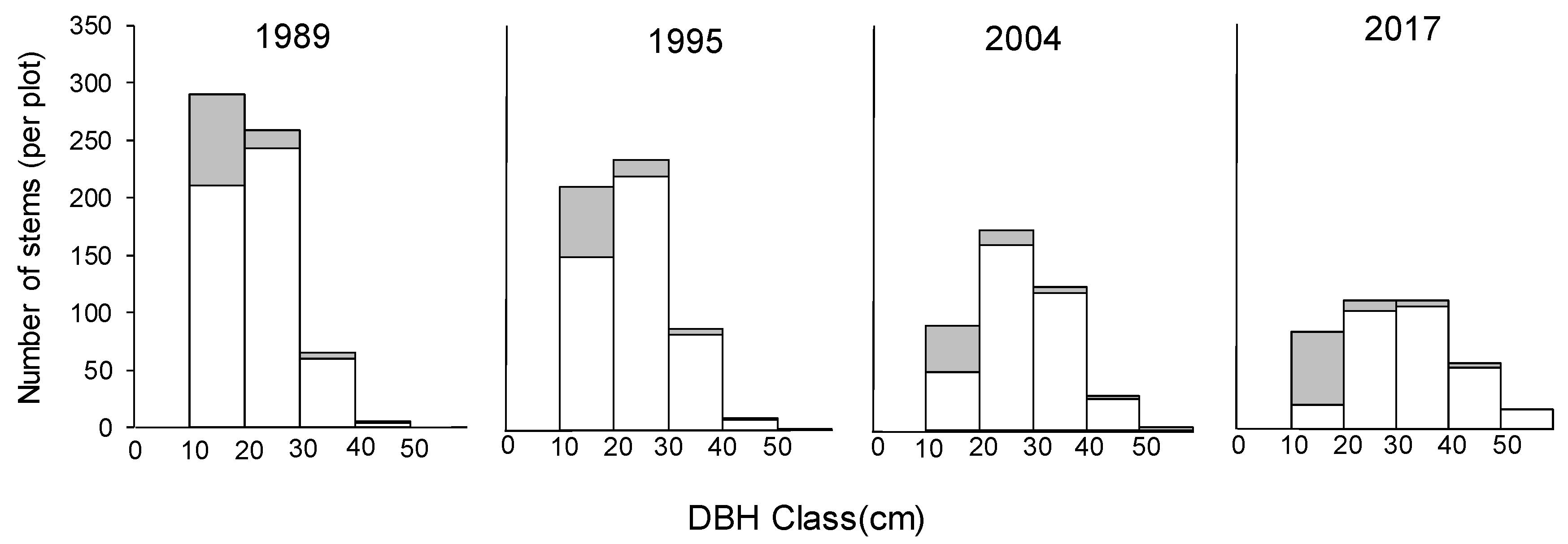
This 28-year study showed us the developmental process of a secondary lucidophyllous forest. In 2017, there were few canopy trees other than
Castanopsis cuspidata, with some pioneer deciduous species (
Ilex micrococca,
Magnolia obovata and
Eleutherococcus sciadophylloides) and only one stem of an evergreen species,
Quercus glauca (
Figure 2d). The long-term direct monitoring illustrated that there was a simplification of species composition, with the number of species over 10 cm DBH decreasing from 17 to 11 due to the exclusion of deciduous species (
Table 3). A predominance of the tree
C. cuspidata also accompanied a decrease in tree density (
Figure 3) during the 28-year study, which was consistent with our hypothesis. These results further support the previous studies, which suggested that the simplification of the species composition in the
Castanopsis zone was caused by the lack of lucidophyllous elements in the northern limit of their distribution and strong sprouting capacity [
10,
18]. In addition, Kubota et al. [
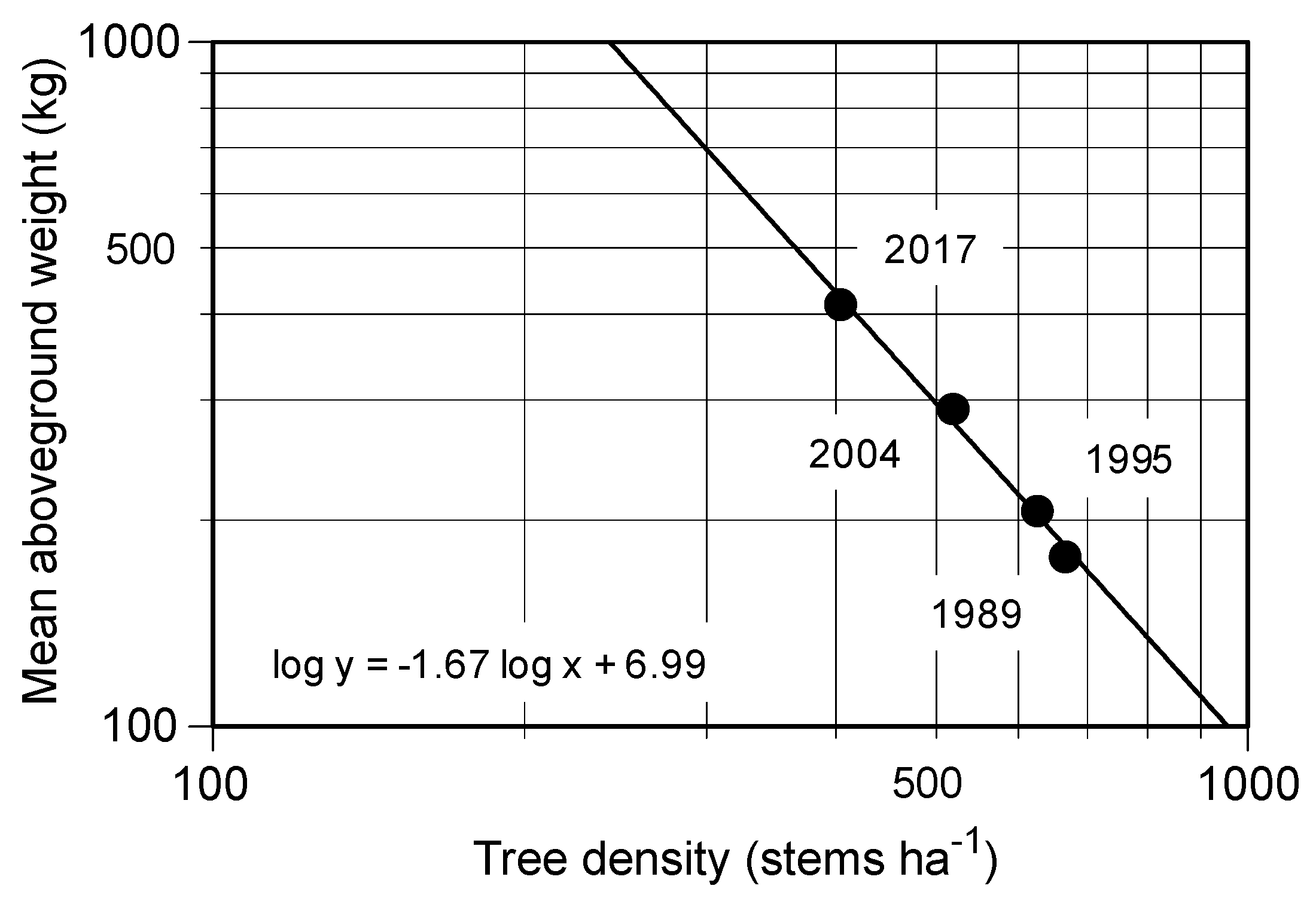
20] studied the biomass accumulation in proportion to decreasing tree density in young secondary lucidophyllous forests. They concluded that trees died because of self-thinning, which implied intraspecific competition following the –3/2 power low [
25]. The slope of the relationship between the stem density and dry weight of
C. cuspidata during the study period was nearly –3/2 (
Figure 3), possibly suggesting that self-thinning governs the process of this stand’s development.
Previous studies of AGB and NPP in lucidophyllous forests are summarized in
Table 6. Woody NPP means the increments of new organic matter are retained by the biomass of stems and branches [
24], and is also one of the most important components of carbon sequestration in forest ecosystems [
26]. Biomass and NPP of old-growth lucidophyllous forests have been well documented, especially in Japan (
Table 6), and their production ecology has flourished since the 1960s within the IBP (International Biological Program), e.g., Minamata Forest [
27] and Aya Research site [
5]. These old-growth forests have a fairly high AGB of more than 300 ton ha
−1, and have a woody NPP ranging from 3.4 to 8.8 ton ha
−1 year
−1 (
Table 6). In contrast, AGB of young secondary lucidophyllous forests, including this study site, were less than 150 ton ha
−1 under developmental stages in human populated areas, and woody NPP tended to be higher than old-growth forests, which ranged from 7.5 to 14.1 ton ha
−1 year
−1 (
Table 6).
Age-related decline of woody NPP seems more important because only a few old-growth lucidophyllous forests occur in human populated areas. For example, Tadaki [
28,
29] revealed that the density depended on mortality and a change of production structure in a very young stand of
C. cuspidata, which had regenerated naturally by coppice-shoots and seeds. An 11-year stand of 42,000 stems ha
−1 decreased to 24,667 stems in a 14-year stand in proportion to biomass increments of 56.4 to 80.5 ton ha
−1 year
−1 over three years, and the woody NPP was much higher (14.1 ton ha
−1 year
−1) than it was in the mature lucidophyllous forests. Moreover, the woody NPP reached up to 11.8 ton ha
−1 year
−1 in a 12-year young stand (67.1 ton ha
−1 year
−1 of AGB) of
C. echidnocarpa in subtropical China [
30].
The AGB of the studied stand (131.7 ton ha
−1 to 180.6 ton ha
−1) was at a medium level compared with other lucidophyllous forests (
Table 6), and it steadily accumulated during the study period (
Table 5). Woody NPP ranged from 2.40 ± 0.13 ton ha
−1 year
−1 (1989 to 1995) to 3.93 ± 0.33 ton ha
−1 year
−1 (1995 to 2004) at the study site. Contrary to expectations, age-related wood production decline was not observed during the 28-year investigation. The woody NPP of
C. cuspidate increased from 2.23 to 3.06 ha
−1 year
−1 during the 28-year period irrespective of the significant decrease in density of the species (666 ha
−1 to 404 ha
−1) due to self-thinning (
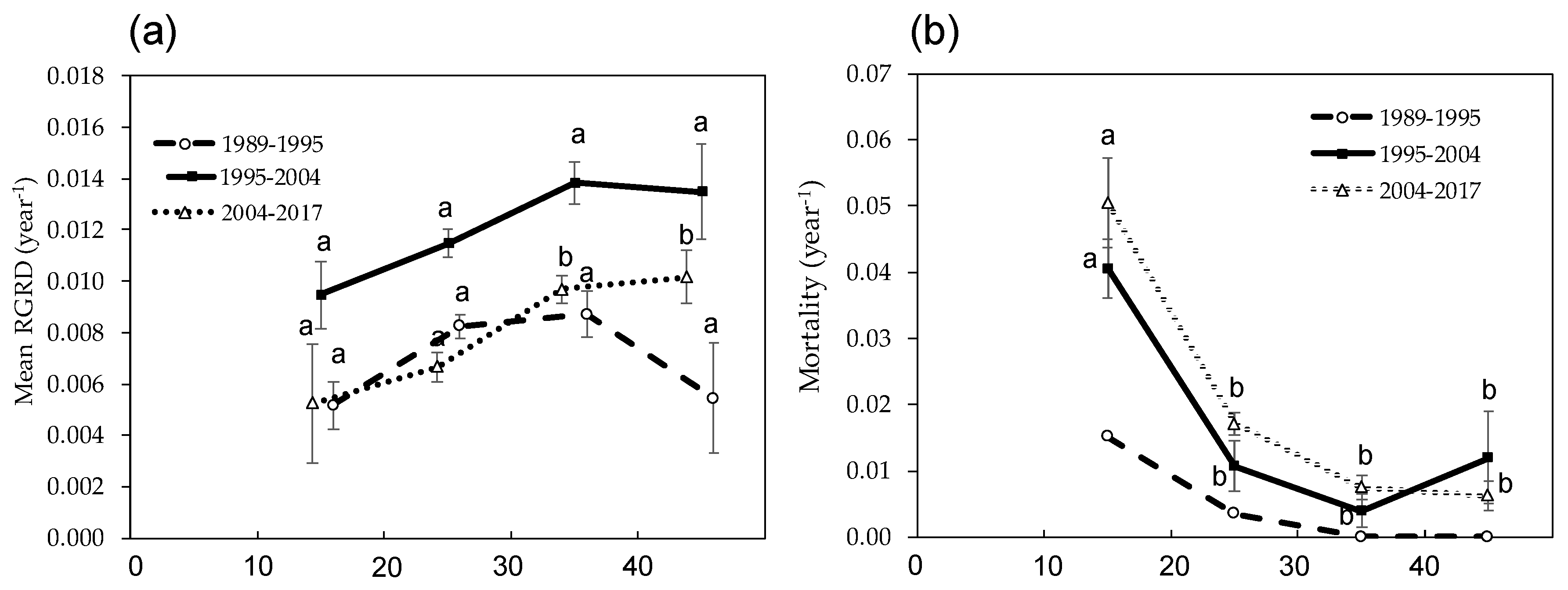
Table 2) in our study site. One reason is that the size of dead trees concentrated on the 10–20 cm class, and the size of all tree variability increased. Therefore, bigger trees (20 cm ≤ DBH ≤ 40 cm) contributed significantly to wood production, and the growth can successfully compensate for the stem wood loss (
Figure 4a,b). Moreover, age-related decline in wood production is related to intrinsic growth limitation of individual trees and/or growth reduction due to local competition among neighboring trees (e.g., self-thinning process) [
31]. Berger et al. [
32] studied the effects of neighborhood competition considering self-thinning and natural recruitment, which has not been considered for age-related production decline. They found that the wood production decline starts after self-thinning; it can thus be suggested that the study site was still during the self-thinning process and the growth of dominant individual tree (
C. cuspidata) still increased. However, the woody NPP at the study site was relatively low while at a developmental stage with medium biomass. This may be due to the low growth of
C. cuspidata because the lucidophyllous forest in this study is located near the northern limit of its distribution. Moreover, Clark et al. [
23] suggested that the long intervals typical of forest production studies might result in the underestimation of woody NPP. This may be because the biomass increment of dead trees during the intervals is possibly ignored, especially with regards to the competitive exclusion of trees with high mortality during the study period in the study area (
Figure 3).