Effects of Linear Disturbances and Fire Severity on Velvet Leaf Blueberry Abundance, Vigor, and Berry Production in Recently Burned Jack Pine Forests
Abstract
:1. Introduction
2. Materials and Methods
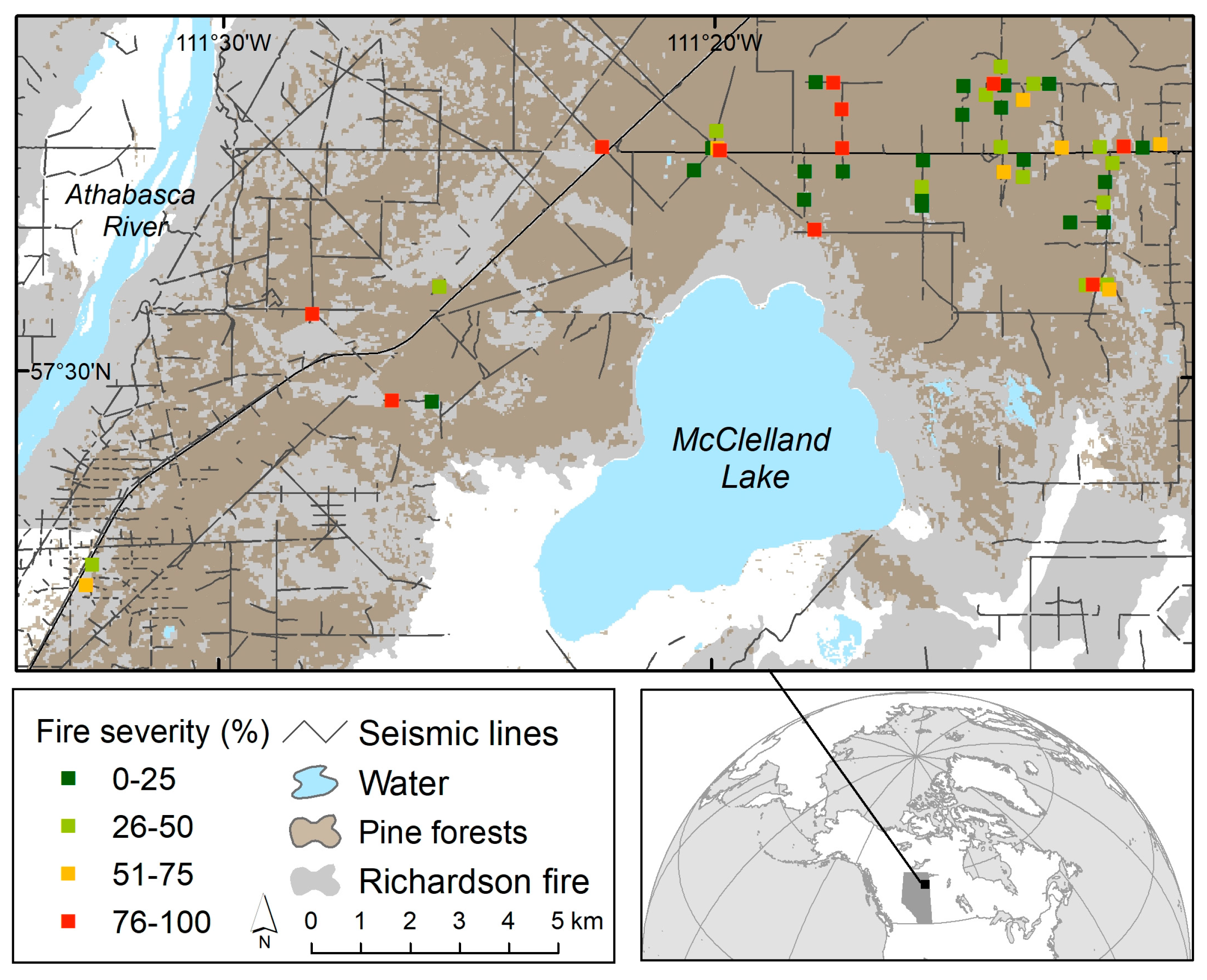
2.1. Study Area
2.2. Experimental Design and Field Measures
2.2.1. Site Selection
2.2.2. Measured Responses in Vaccinium myrtilloides and Treatment Variables
2.3. Data Analysis
2.3.1. Responses in Vaccinium myrtilloides on Seismic Lines and Adjacent Forests
2.3.2. Effects of Seismic Line Width (Forest Gap Size) on Vaccinium myrtilloides
3. Results
3.1. Presence
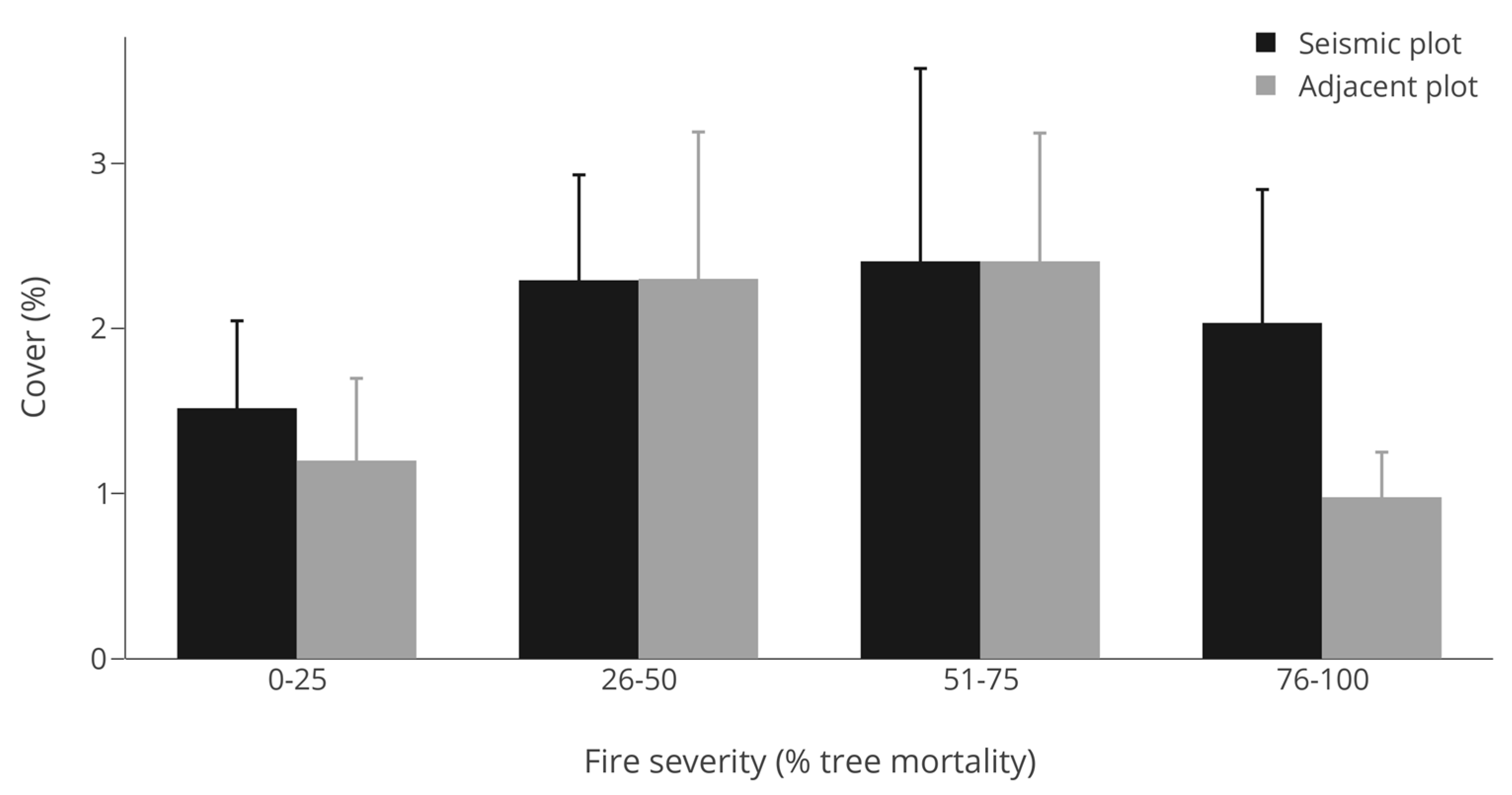
3.2. Abundance (Cover)
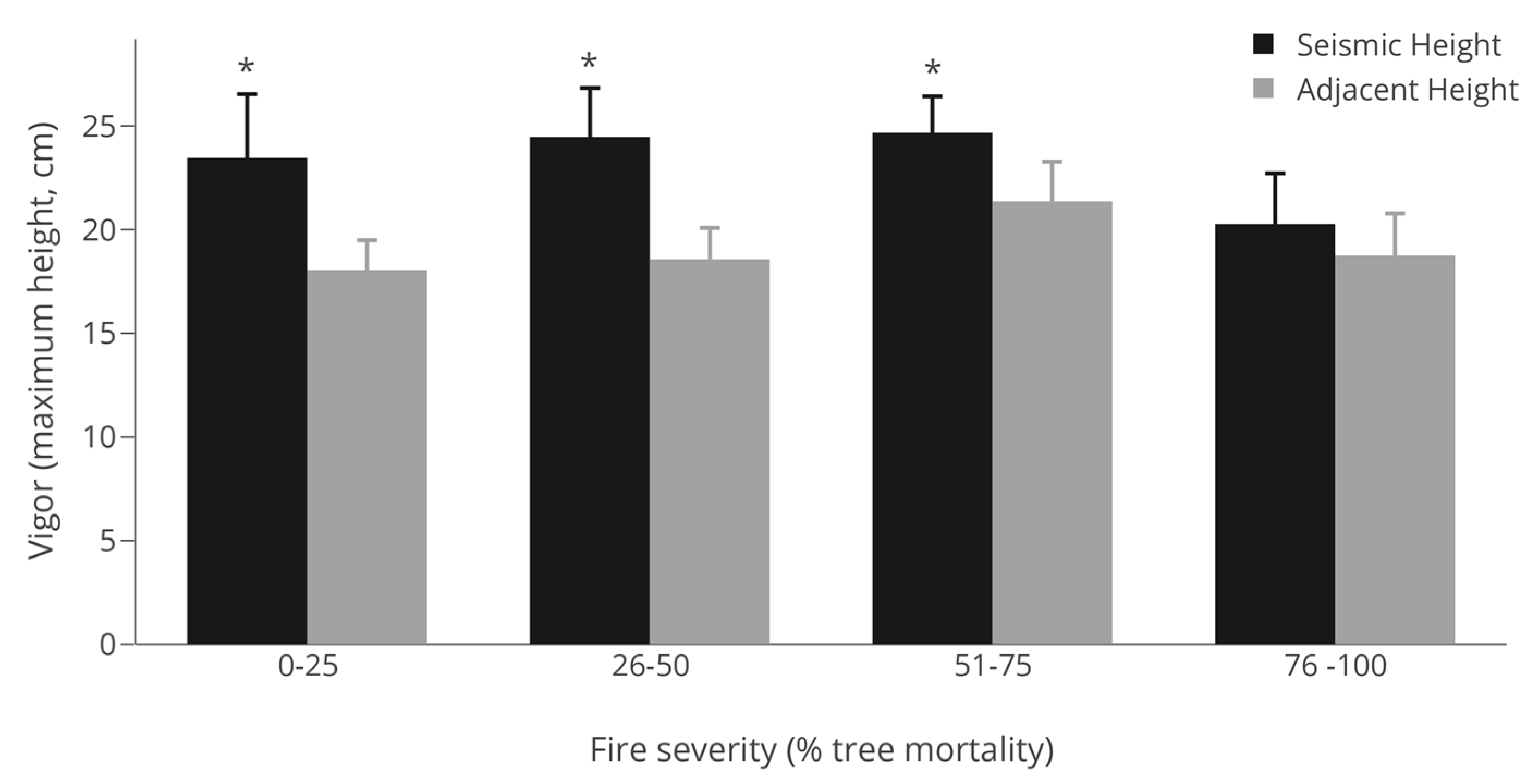
3.3. Vigor (Maximum Height)
3.4. Berry Production Across All Sites
3.5. Responses in Berry Production When Plants Were Present
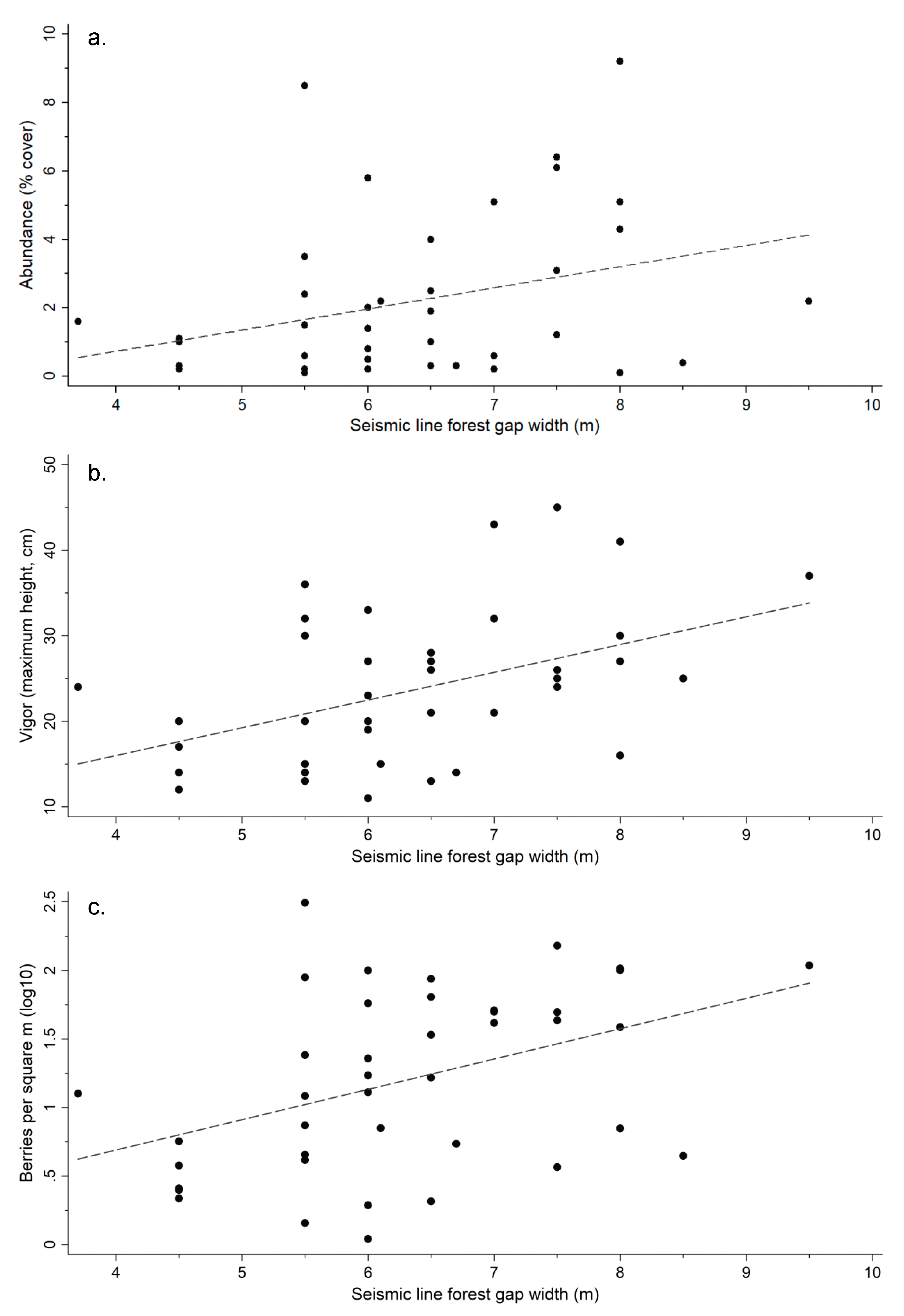
3.6. Effect of Forest Gap Width from Seismic Lines
4. Discussion
4.1. Responses to Fire
4.2. Response to Seismic Lines
4.3. Management Implications: Fire and Seismic Lines
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bradbury, S.M. Response of the Post-Fire Bryophyte Community to Salvage Logging in Boreal Mixedwood Forests of Northeastern Alberta, Canada. For. Ecol. Manag. 2006, 234, 313–322. [Google Scholar] [CrossRef]
- Kalamees, R.; Püssa, K.; Tamm, S.; Zobel, K. Adaptation to Boreal Forest Wildfire in Herbs: Responses to Post-Fire Environmental Cues in Two Pulsatilla Species. Acta Oecol. 2012, 38, 1–7. [Google Scholar] [CrossRef]
- Wein, R.W.; MacLean, D.A. The Role of Fire in Northern Circumpolar Ecosystems; Published on behalf of the Scientific Committee on Problems of the Environment of the International Council of Scientific Unions; Wiley: Chichester, UK, 1983. [Google Scholar]
- Mallik, A.U. Conifer Regeneration Problems in Boreal and Temperate Forests with Ericaceous Understory: Role of Disturbance, Seedbed Limitation, and Keytsone Species Change. Crit. Rev. Plant Sci. 2003, 22, 341–366. [Google Scholar] [CrossRef]
- Beaufait, W. Some Effects of High Temperatures on the Cones and Seeds of Jack Pine. For. Sci. 1960, 6, 194–198. [Google Scholar]
- Radeloff, V.C.; Mladenoff, D.J.; Guries, R.P.; Boyce, M.S. Spatial Patterns of Cone Serotiny in Pinus Banksiana in Relation to Fire Disturbance. For. Ecol. Manag. 2004, 189, 133–141. [Google Scholar] [CrossRef]
- Mallik, A.U. Ecology of a Forest Weed of Newfoundland: Vegetative Regeneration Strategy of Kalmia angustifolia. Can. J. Bot. 1993, 71, 161–166. [Google Scholar] [CrossRef]
- Moola, F.M.; Vasseur, L. The Importance of Clonal Growth to the Recovery of Gaultheria procumbens L. (Ericaceae) after Forest Disturbance. For. Ecol. Recent Adv. Plant Ecol. 2009, 319–337. [Google Scholar] [CrossRef]
- Ois Hébert, F.; Thiffault, N.; Ruel, J.-C.; Munson, A.D. Ericaceous Shrubs Affect Black Spruce Physiology Independently from Inherent Site Fertility. For. Ecol. Manag. 2010, 260, 219–228. [Google Scholar] [CrossRef]
- Vila, M.; Terradas, J. Effects of Competition and Disturbance on the Resprouting Performance of the Mediterranean Shrub Erica Multiflora L. (Ericaceae). Am. J. Bot. 1995, 82, 1241. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire Effects on Belowground Sustainability: A Review and Synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Pinno, B.D.; Errington, R.C. Burn Severity Dominates Understory Plant Community Response to Fire in Xeric Jack Pine Forests. Forests 2016, 7. [Google Scholar] [CrossRef]
- Alexander, M.E.; Cruz, M.G. Modelling the Effects of Surface and Crown Fire Behaviour on Serotinous Cone Opening in Jack Pine and Lodgepole Pine Forests. Int. J. Wildland Fire 2012, 21, 709–721. [Google Scholar] [CrossRef]
- Ilisson, T.; Chen, H.Y.H. The Direct Regeneration Hypothesis in Northern Forests. J. Veg. Sci. 2009, 20, 735–744. [Google Scholar] [CrossRef]
- Chiang, J.-M.; McEwan, R.W.; Yaussy, D.A.; Brown, K.J. The Effects of Prescribed Fire and Silvicultural Thinning on the Aboveground Carbon Stocks and Net Primary Production of Overstory Trees in an Oak-Hickory Ecosystem in Southern Ohio. For. Ecol. Manag. 2008, 255, 1584–1594. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Chapin, F.S., III; Foote, J.; Kemmett, S.; Price, K.; Viereck, L. Decadal Observations of Tree Regeneration Following Fire in Boreal Forests. Can. J. For. Res. 2004, 34, 267–273. [Google Scholar] [CrossRef]
- Mallik, A.U. Conversion of Temperate Forests into Heaths: Role of Ecosystem Disturbance and Ericaceous Plants. Environ. Manag. 1995, 19, 675–684. [Google Scholar] [CrossRef]
- Feurdean, A.; Florescu, G.; Vannière, B.; Tanţău, I.; O‘Hara, R.B.; Pfeiffer, M.; Hutchinson, S.M.; Gałka, M.; Moskal-del Hoyo, M.; Hickler, T. Fire Has Been an Important Driver of Forest Dynamics in the Carpathian Mountains during the Holocene. For. Ecol. Manag. 2017, 389, 15–26. [Google Scholar] [CrossRef]
- Vizcaíno, E.A.D.; Rodríguez, A.I.; Fernández, M. Interannual Variability in Fire-Induced Germination Responses of the Characteristic Ericaceae of the NW Iberian Peninsula. For. Ecol. Manag. 2006, 234, S179. [Google Scholar] [CrossRef]
- Bunnell, F.L. Reproduction of Salal (Gaultheria Shallon) under Forest Canopy. Can. J. For. Res. 1990, 20, 91–100. [Google Scholar] [CrossRef]
- Schimmel, J.; Granström, A. Fire Severity and Vegetation Response in the Boreal Swedish Forest. Ecology 1996, 77, 1436–1450. [Google Scholar] [CrossRef]
- Hall, I.V. Floristic Changes Following the Cutting and Burning of a Woodlot for Blueberry Production. Can. J. Agric. Sci. 1955, 35, 143–152. [Google Scholar]
- Moola, F.M.; Mallik, A.U. Morphological Plasticity and Regeneration Strategies of Velvet Leaf Blueberry (Vaccinium myrtiltoides Michx.) Following Canopy Disturbance in Boreal Mixedwood Forests. For. Ecol. Manag. 1998, 111, 35–50. [Google Scholar] [CrossRef]
- Stocks, B.J.; Mason, J.A.; Todd, J.B.; Bosch, E.M.; Wotton, B.M.; Amiro, B.D.; Flannigan, M.D.; Hirsch, K.G.; Logan, K.A.; Martell, D.L.; et al. Large Forest Fires in Canada, 1959–1997. J. Geophys. Res. 2002, 108, 8149. [Google Scholar] [CrossRef]
- Bayne, E.M.; Habib, L.; Boutin, S. Impacts of Chronic Anthropogenic Noise from Energy-Sector Activity on Abundance of Songbirds in the Boreal Forest. Conserv. Biol. 2008, 22, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, R.E.; Geldart, L.P. Background Mathematics. In Exploration Seismology; Cambridge University Press: Cambridge, UK, 1995; pp. 517–568. [Google Scholar]
- Canham, C.D.; Denslow, J.S.; Platt, W.J.; Runkle, J.; Spies, T.A.; White, P.S. Light Regimes beneath Closed Canopies and Tree-Fall Gaps in Temperate and Tropical Forests. Can. J. For. Res. 1990, 20, 620. [Google Scholar] [CrossRef]
- Montané, F.; Guixé, D.; Camprodon, J. Canopy Cover and Understory Composition Determine Abundance of Vaccinium myrtillus L., a Key Plant for Capercaillie (Tetrao urogallus), in Subalpine Forests in the Pyrenees. Plant Ecol. Divers. 2016, 9, 187–198. [Google Scholar] [CrossRef]
- Arienti, M.C.; Cumming, S.G.; Krawchuk, M.A.; Boutin, S. Road Network Density Correlated with Increased Lightning Fire Incidence in the Canadian Western Boreal Forest. Int. J. Wildland Fire 2009, 18, 970–982. [Google Scholar] [CrossRef]
- Lee, P.; Boutin, S. Persistence and Developmental Transition of Wide Seismic Lines in the Western Boreal Plains of Canada. J. Environ. Manag. 2006, 78, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Van Rensen, C.K.; Nielsen, S.E.; White, B.; Vinge, T.; Lieffers, V.J. Natural Regeneration of Forest Vegetation on Legacy Seismic Lines in Boreal Habitats in Alberta’S Oil Sands Region. Biol. Conserv. 2015, 184, 127–135. [Google Scholar] [CrossRef]
- Dabros, A.; Hammond, H.E.J.; Pinzon, J.; Pinno, B.; Langor, D. Edge Influence of Low-Impact Seismic Lines for Oil Exploration on Upland Forest Vegetation in Northern Alberta (Canada). For. Ecol. Manag. 2017, 400, 278–288. [Google Scholar] [CrossRef]
- Historical Wildfire Database 1999–2014. AAF—Agriculture and Forestry. Available online: http://wildfire.alberta.ca/resources/historical-data/historical-wildfire-database.aspx (accessed on 14 July 2017).
- Peet, R.K.; Wentworth, T.R.; White, P.S. A Flexible, Multipurpose Method for Recording Vegetation Composition and Structure. Castanea 1998, 63, 262–274. [Google Scholar]
- Pitelka, L.F.; Stanton, D.S.; Peckenham, M.O. Effects of Light and Density on Resource Allocation in a Forest Herb, Aster Acuminatus (Compositae). Am. J. Bot. 1980, 67, 942. [Google Scholar] [CrossRef]
- Christensen, N.L. Fire and Soil-Plant Nutrient Relations in a Pine-Wiregrass Savanna on the Coastal Plain of North Carolina. Oecologia 1977, 31, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Simms, E.L. The Effect of Nitrogen and Phosphorus Addition on the Growth, Reproduction, and Nutrient Dynamics of Two Ericaceous Shrubs. Oecologia 1987, 71, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Faison, E.K.; Del Tredici, P.; Foster, D.R. To Sprout or Not to Sprout: Multiple Factors Determine the Vigor of Kalmia Latifolia (Ericaceae) in Southwestern Connecticut. Rhodora 2014, 116, 148–162. [Google Scholar] [CrossRef]
- Ballester, A.; Vieitez, A.M.; Vieitez, E. Allelopathic Potential ofErica Vagans, Calluna Vulgaris, and Daboecia Cantabrica. J. Chem. Ecol. 1982, 8, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.A.; Casper, B.B. Morphogenetic Constraints on Patterns of Carbon Distribution in Plants. Annu. Rev. Ecol. Syst. 1984, 15, 233–258. [Google Scholar] [CrossRef]
- Reader, R.J. Bog Ericad Flowers: Self-Compatibility and Relative Attractiveness to Bees. Can. J. Bot. 1977, 55, 2279–2287. [Google Scholar] [CrossRef]
- Usui, M.; Kevan, P.G.; Obbard, M. Pollination and Breeding System of Lowbush Blueberries, Vaccinium angustifolium Ait. and V. myrtilloides Michx. (Ericacaeae), in the Boreal Forest. Can. Field Nat. 2005, 119, 48. [Google Scholar] [CrossRef]
- Brodeur, V.; Ouellet, J.-P.; Courtois, R.; Fortin, D. Habitat Selection by Black Bears in an Intensively Logged Boreal Forest. Can. J. Zool. 2008, 86, 1307–1316. [Google Scholar] [CrossRef]
- Tigner, J.; Bayne, E.M.; Boutin, S. Black Bear Use of Seismic Lines in Northern Canada. J. Wildl. Manag. 2014, 78, 282–292. [Google Scholar] [CrossRef]
- Turner, N.J.; Cocksedge, W. Aboriginal Use of Non-Timber Forest Products in Northw Estern North America. J. Sustain. For. 2001, 13, 31–58. [Google Scholar] [CrossRef]
- Gottesfeld, L.M.J. Aboriginal Burning for Vegetation Management in Northwest British Columbia. Hum. Ecol. 1994, 22, 171–188. [Google Scholar] [CrossRef]
- Environment Canada. Recovery Strategy for the Woodland Caribou (Rangifer tarandus caribou), Boreal Population, in Canada; Species at Risk Act Recovery Strategy Series; Environment Canada: Ottawa, ON, Canada, 2012. [Google Scholar]
- Hebblewhite, M. Billion Dollar Boreal Woodland Caribou and the Biodiversity Impacts of the Global Oil and Gas Industry. Biol. Conserv. 2016, 206, 102–111. [Google Scholar] [CrossRef]
- James, A.R.C.; Stuart-Smith, A.K. Distribution of Caribou and Wolves in Relation to Linear Corridors. J. Wildl. Manag. 2000, 64, 154. [Google Scholar] [CrossRef]
- Edmonds, E.J. Population Status, Distribution, and Movements of Woodland Caribou in West Central Alberta. Can. J. Zool. 1988, 66, 817–826. [Google Scholar] [CrossRef]
- Pinard, V.; Dussault, C.; Ouellet, J.-P.; Fortin, D.; Courtois, R. Calving Rate, Calf Survival Rate, and Habitat Selection of Forest-Dwelling Caribou in a Highly Managed Landscape. J. Wildl. Manag. 2012, 76, 189–199. [Google Scholar] [CrossRef]
- Machtans, C.S. Songbird Response to Seismic Lines in the Western Boreal Forest: A Manipulative Experiment. Can. J. Zool. 2006, 84, 1421–1430. [Google Scholar] [CrossRef]


| Variable | Presence | Abundance (Cover) | Vigor (Max. Height) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef. | S.E. | p | Coef. | S.E. | p | Coef. | S.E. | p | |
| Seismic line | 4.167 | 2.88 | 0.148 | 0.323 | 0.428 | 0.451 | 4.516 | 1.357 | 0.001 |
| Fire severity | 1.010 | 0.175 | <0.001 | 0.004 | 0.008 | 0.642 | −0.006 | 0.028 | 0.832 |
| Fire severity2 | −0.007 | 0.001 | <0.001 | ||||||
| Seismic × Fire | −0.105 | 0.043 | 0.014 | ||||||
| Constant | −9.446 | 3.935 | 0.016 | 1.509 | 0.524 | 0.004 | 19.255 | 1.754 | <0.001 |
| Model fit, R2 | 0.41 | 0.01 | 0.09 | ||||||
| Variable | Number of Berries (Plot) | ||
|---|---|---|---|
| Coef. | S.E. | p | |
| Seismic line (binary) | 0.753 | 0.213 | <0.001 |
| Fire severity (tree mortality) | 0.007 | 0.003 | 0.009 |
| Seismic × Fire severity | −0.008 | 0.004 | 0.032 |
| Constant (intercept) | 0.391 | 0.163 | 0.016 |
| Model fit, R2 | 0.15 | ||
| Variable | Number of Berries (Where Present) | ||
|---|---|---|---|
| Coef. | S.E. | p | |
| Seismic line (binary) | 0.422 | 0.134 | 0.002 |
| Fire severity (tree mortality) | 0.006 | 0.002 | 0.001 |
| Seismic × Fire severity | −0.004 | 0.002 | 0.097 |
| Constant (intercept) | −0.426 | 0.165 | 0.010 |
| Shrub abundance (cover) | 0.105 | 0.024 | <0.001 |
| Shrub vigor (max. height) | 0.037 | 0.008 | <0.001 |
| Model fit, R2 | 0.59 | ||
| Variable | Abundance (Cover) | Vigor (Max. Height) | ||||
|---|---|---|---|---|---|---|
| Coef. | S.E. | p | Coef. | S.E. | p | |
| Seismic line width | 0.676 | 0.219 | 0.003 | 3.244 | 1.019 | 0.003 |
| Constant (intercept) | −2.774 | 1.357 | 0.045 | 3.010 | 6.549 | 0.648 |
| Model fit, R2 (RMSE) | 0.12 (2.02) | 0.19 (8.09) | ||||
| Variable | Number of Berries (All Sites) | Number of Berries (Where Present) | ||||
|---|---|---|---|---|---|---|
| Coef. | S.E. | p | Coef. | S.E. | p | |
| Seismic line width | 0.285 | 0.077 | <0.001 | 0.064 | 0.061 | 0.298 |
| Shrub abundance (cover) | 0.115 | 0.038 | 0.004 | |||
| Shrub vigor (max. height) | 0.027 | 0.011 | 0.018 | |||
| Constant (intercept) | −0.993 | 0.478 | 0.042 | −0.076 | 0.358 | 0.833 |
| Model fit, R2 (RMSE) | 0.16 (0.71) | 0.57 (0.43) | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawe, C.A.; Filicetti, A.T.; Nielsen, S.E. Effects of Linear Disturbances and Fire Severity on Velvet Leaf Blueberry Abundance, Vigor, and Berry Production in Recently Burned Jack Pine Forests. Forests 2017, 8, 398. https://doi.org/10.3390/f8100398
Dawe CA, Filicetti AT, Nielsen SE. Effects of Linear Disturbances and Fire Severity on Velvet Leaf Blueberry Abundance, Vigor, and Berry Production in Recently Burned Jack Pine Forests. Forests. 2017; 8(10):398. https://doi.org/10.3390/f8100398
Chicago/Turabian StyleDawe, Charlotte A., Angelo T. Filicetti, and Scott E. Nielsen. 2017. "Effects of Linear Disturbances and Fire Severity on Velvet Leaf Blueberry Abundance, Vigor, and Berry Production in Recently Burned Jack Pine Forests" Forests 8, no. 10: 398. https://doi.org/10.3390/f8100398






