Dispersal Patterns of Pine Wilt Disease in the Early Stage of Its Invasion in South Korea
Abstract
:1. Introduction
2. Materials and Methods
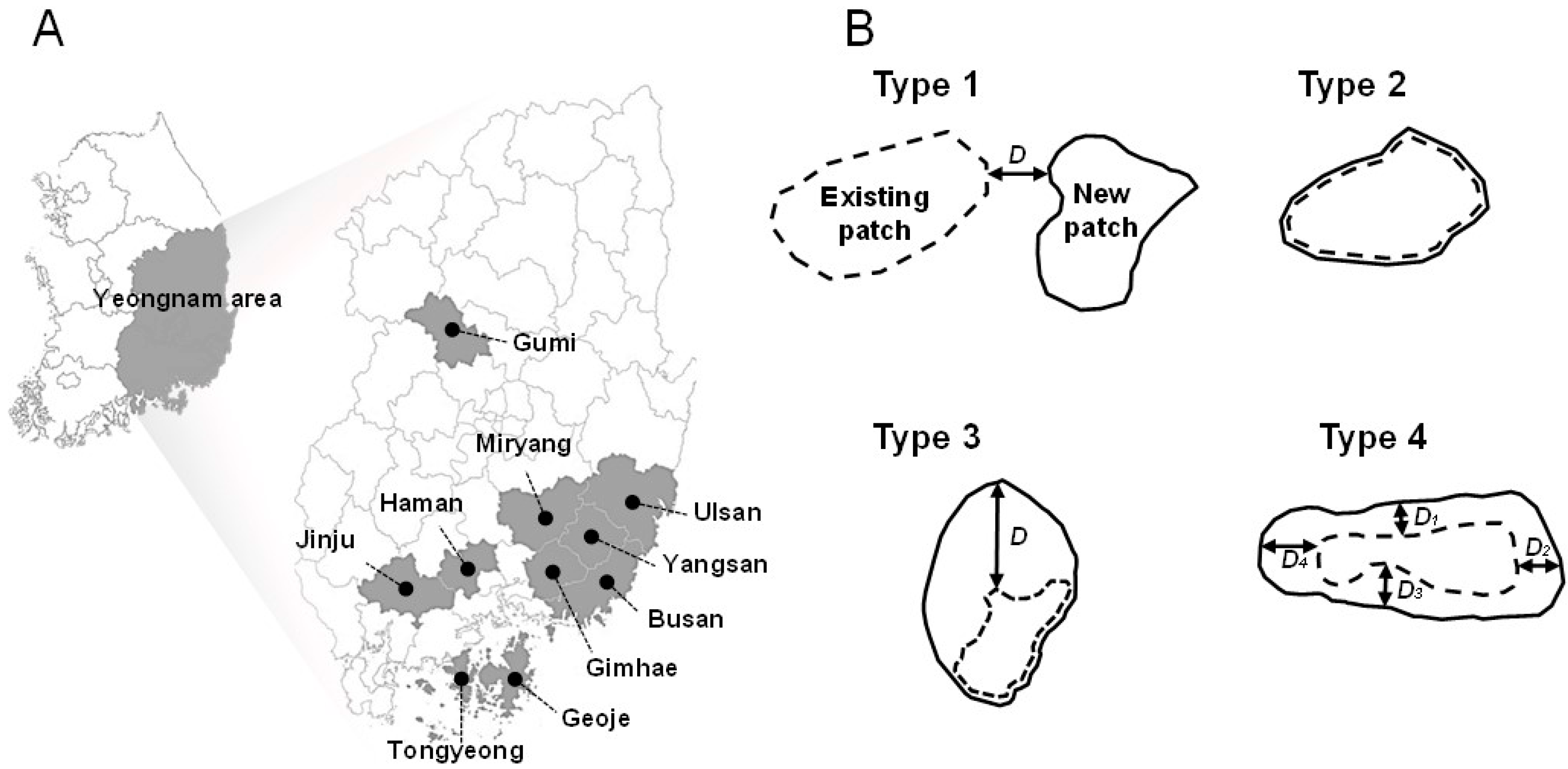
2.1. PWD Occurrence Data
2.2. Dispersal Pattern of Patches
2.3. Statistical Analysis
3. Results
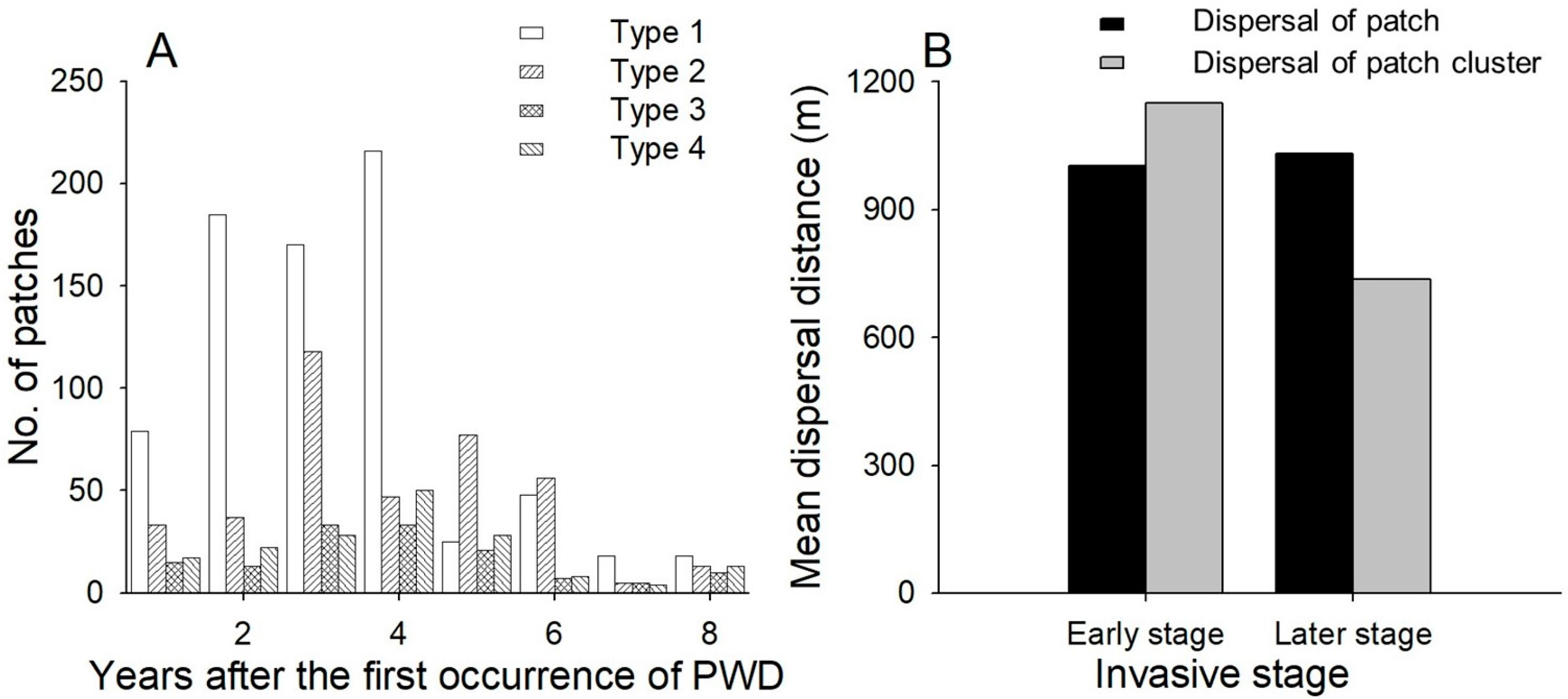
3.1. Characteristics of Dispersal Patterns
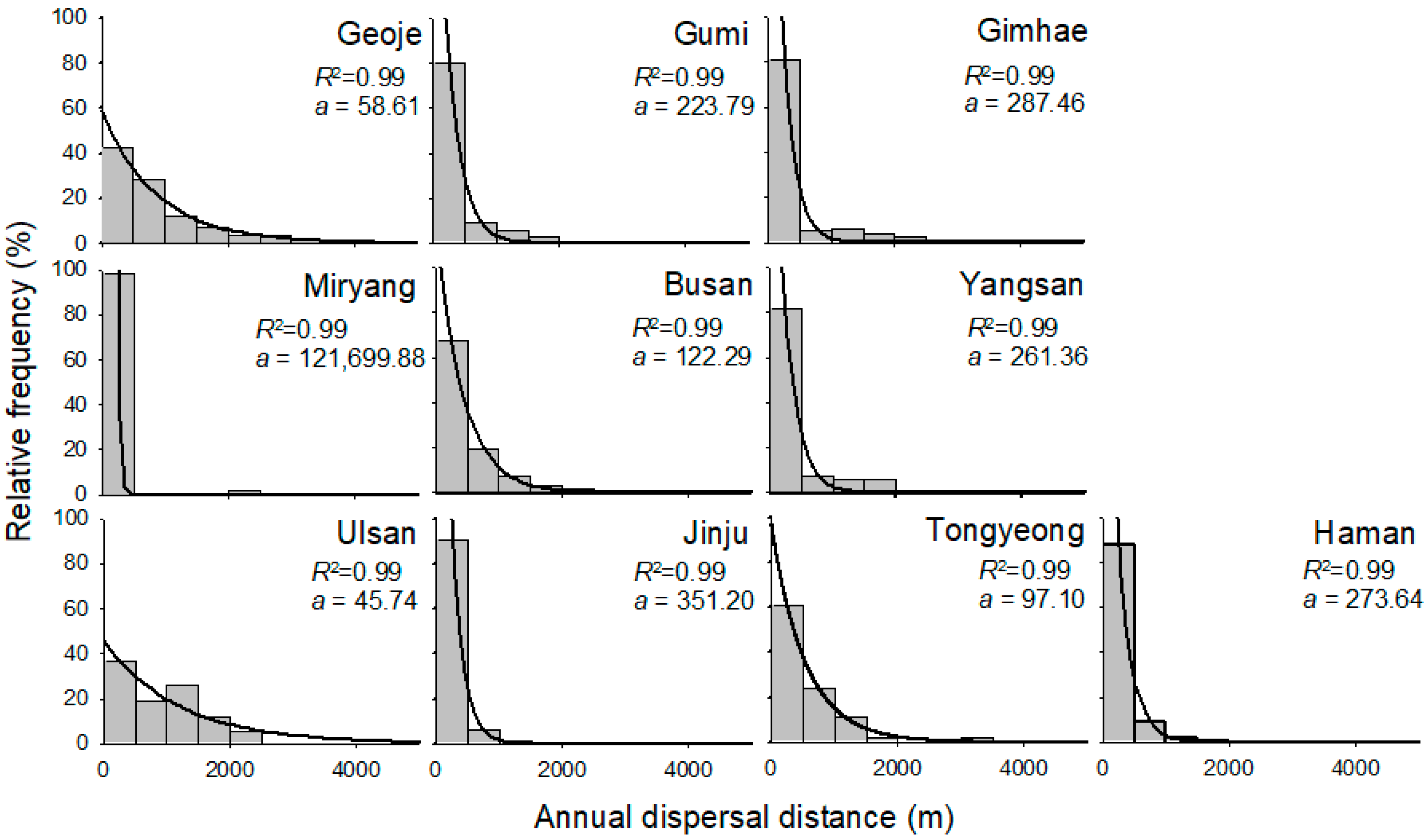
3.2. Annual Dispersal Distance at Patch and Regional Levels
3.3. Influence of Socio-Environmental Factors
4. Discussion
4.1. Characteristics of Dispersal Patterns
4.2. Annual Dispersal Distance at Patch and Regional Levels
4.3. Influence of Socio-Environmental Factors on Dispersal
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic Invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Choi, W.I.; Park, Y.-S. Dispersal patterns of exotic forest pests in South Korea. Insect Sci. 2012, 19, 535–548. [Google Scholar] [CrossRef]
- Sharov, A.A.; Leonard, D.; Liebhold, A.M.; Roberts, E.A.; Dickerson, W. “SLOW THE SPREAD” a national program to contain the gypsy moth. J. For. 2002, 100, 30–35. [Google Scholar]
- Sharov, A.A.; Liebhold, A.M. Model of slowing the spread of gypsy moth (Lepidoptera: Lymantriidae) with a barrier zone. Ecol. Appl. 1998, 8, 1170–1179. [Google Scholar] [CrossRef]
- Hengeveld, B. Dynamics of Biological Invasions; Chapman and Hall: London, UK, 1989; 160p. [Google Scholar]
- Tobin, P.C.; Blackburn, L.M. Long-distance dispersal of the gypsy moth (Lepidoptera: Lymantriidae) facilitated its initial invasion of Wisconsin. Environ. Entomol. 2008, 37, 87–93. [Google Scholar] [CrossRef]
- Suarez, A.V.; Holway, D.A.; Case, T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc. Natl. Acad. Sci. USA 2001, 98, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Walton, A. Mountain pine beetle dispersal: Spatiotemporal patterns and role in the spread and expansion of the present outbreak. Ecosphere 2011, 2, 1–17. [Google Scholar] [CrossRef]
- Jung, C.S.; Koh, S.-H.; Nam, Y.; Ahn, J.J.; Choi, W.I. A forecasting model for predicting the spring emergence of Monochamus saltuarius (Coleoptera: Cerambycidae) on Korean white pine, Pinus koraiensis. J. Econ. Entomol. 2015, 108, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Shi, J.; Luo, Y.-Q.; Ren, L.-L.; Shi, Y.-M. Effect of pine wood nematode invasion on pine community functions in the Pinghu region, Zhejiang Province, Eastern China. For. Sci. Pract. 2013, 15, 302–309. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2011, 42, 89–99. [Google Scholar] [CrossRef]
- David, G.; Giffard, B.; Piou, D.; Jactel, H. Dispersal capacity of Monochamus galloprovincialis, the European vector of the pine wood nematode, on flight mills. J. Appl. Entomol. 2014, 138, 566–576. [Google Scholar] [CrossRef]
- Park, Y.-S.; Chung, Y.-J.; Moon, Y.-S. Hazard ratings of pine forests to a pine wilt disease at two spatial scales (individual trees and stands) using self-organizing map and random forest. Ecol. Inform. 2013, 13, 40–46. [Google Scholar] [CrossRef]
- Enda, N. The status of pine wilting disease caused by Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its control in Korea. J. Korean For. Soc. 1989, 78, 248–253. [Google Scholar]
- Environmental Systems Research Institute (ESRI). ArcGIS 9.1, ESRI: Redlands, CA, USA, 2005.
- Kim, D.Y. Development of Pine Wilt Disease Spatial Information Management System Using Web-GIS. Master’s Thesis, The Graduate School, Kyungil University, Gyeongsan, Korea, 2008. (In Korean). [Google Scholar]
- SAS Institute. SAS/STATVR 9.2 User’s Guide; SAS Institute: Cary, NC, USA, 2010. [Google Scholar]
- Hyames, D. CurveExpert Version 1.4, A Comprehensive Curve Fitting System for Windows; Daniel G. Hyams: Hixson, TN, USA, 2009.
- Shibata, E. Spatial distribution pattern of the Japanese pine sawyer, Monochamus alternatus Hope (Coleoptera: Cearambycidae), on dead pine trees. Appl. Entomol. Zool. 1984, 19, 361–366. [Google Scholar] [CrossRef]
- Shibata, E. Dispersal movement of the adult Japanese pine sawyer, Monochamus alternates Hope (Coleoptera: Cerambycidae) in a young pine forest. Appl. Entomol. Zool. 1986, 21, 184–186. [Google Scholar] [CrossRef]
- Togashi, K. A field experiment on dispersal of newly emerged adults of Monochamus alternatus (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1990, 32, 184–186. [Google Scholar]
- Etxebeste, I.; Sanchez-Husillos, E.; Álvarez, G.; Mas i Gisbert, H.; Pajares, J. Dispersal of Monochamus galloprovincialis (Col.: Cerambycidae) as recorded by mark–release–recapture using pheromone traps. J. Appl. Entomol. 2016, 140, 485–499. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Zugasti, C.; De-Juan, J.M.; Oliva, M.J.; Montero, C.; Mendiola, F.J.; Conejo, Y.; Sánchez, Á.; Fernández, F.; Ponce, F.; et al. Mark-recapture of Monochamus galloprovincialis with semio chemical baited traps: Population density, attraction distance, flight behavior and mass trapping efficiency. Forestry 2015, 88, 224–236. [Google Scholar] [CrossRef]
- Humphry, S.J.; Linit, M.J. Effect of pinewood nematode density on tethered flight of Monochamus carolinensis (Coleoptera: Cerambycidae). Environ. Entomol. 1989, 18, 670–673. [Google Scholar] [CrossRef]
- Kwon, T.S.; Shin, J.H.; Lim, J.H.; Kim, Y.K.; Lee, E.J. Management of pine wilt disease in Korea through preventative silvicultural control. For. Ecol. Manag. 2011, 261, 562–569. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef] [PubMed]
- Robinet, C.; Van Opstal, N.; Baker, R.; Roques, A. Applying a spread model to identify the entry points from which the pinewood nematode, the vector of pine wilt disease, would spread most rapidly across Europe. Biol. Invasions 2011, 13, 2981–2995. [Google Scholar] [CrossRef]
- Prasad, A.M.; Iverson, L.R.; Peters, M.P.; Bossenbroek, J.M.; Matthews, S.N.; Sydnor, T.D.; Schwartz, M.W. Modeling the invasive emerald ash borer risk of spread using a spatially explicit cellular model. Landsc. Ecol. 2010, 25, 353–369. [Google Scholar] [CrossRef]
- Robinet, C.; Suppo, C.; Darrouzet, E. Rapid spread of the invasive yellow-legged hornet in France: The role of human-mediated dispersal and the effects of control measures. J. Appl. Ecol. 2017, 54, 205–215. [Google Scholar] [CrossRef]
- Horvitz, N.; Wang, R.; Wan, F.-H.; Nathan, R. Pervasive human-mediated large-scale invasion: Analysis of spread patterns and their underlying mechanisms in 17 of China’s worst invasive plants. J. Ecol. 2017, 105, 85–94. [Google Scholar] [CrossRef]
- Korea Forest Service (KFS). Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Korea, 2014; 473p, (In Korean with English summary).
- Verheggen, F.; Verhaeghe, A.; Glordanengo, P.; Tassus, X.; Escobar-Gutiérrez, A. Walnut husk fly, Rhagoletis complete (Diptera: Tephritidae), invades Europe: Invasion potential and control strategies. Appl. Entomol. Zool. 2017, 52, 1–7. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.I.; Song, H.J.; Kim, D.S.; Lee, D.-S.; Lee, C.-Y.; Nam, Y.; Kim, J.-B.; Park, Y.-S. Dispersal Patterns of Pine Wilt Disease in the Early Stage of Its Invasion in South Korea. Forests 2017, 8, 411. https://doi.org/10.3390/f8110411
Choi WI, Song HJ, Kim DS, Lee D-S, Lee C-Y, Nam Y, Kim J-B, Park Y-S. Dispersal Patterns of Pine Wilt Disease in the Early Stage of Its Invasion in South Korea. Forests. 2017; 8(11):411. https://doi.org/10.3390/f8110411
Chicago/Turabian StyleChoi, Won Il, Hye Jung Song, Dong Soo Kim, Dae-Sung Lee, Cha-Young Lee, Youngwoo Nam, Joon-Bum Kim, and Young-Seuk Park. 2017. "Dispersal Patterns of Pine Wilt Disease in the Early Stage of Its Invasion in South Korea" Forests 8, no. 11: 411. https://doi.org/10.3390/f8110411




