Dynamics of Understory Shrub Biomass in Six Young Plantations of Southern Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Methods
2.3. Statistics Analysis
3. Results
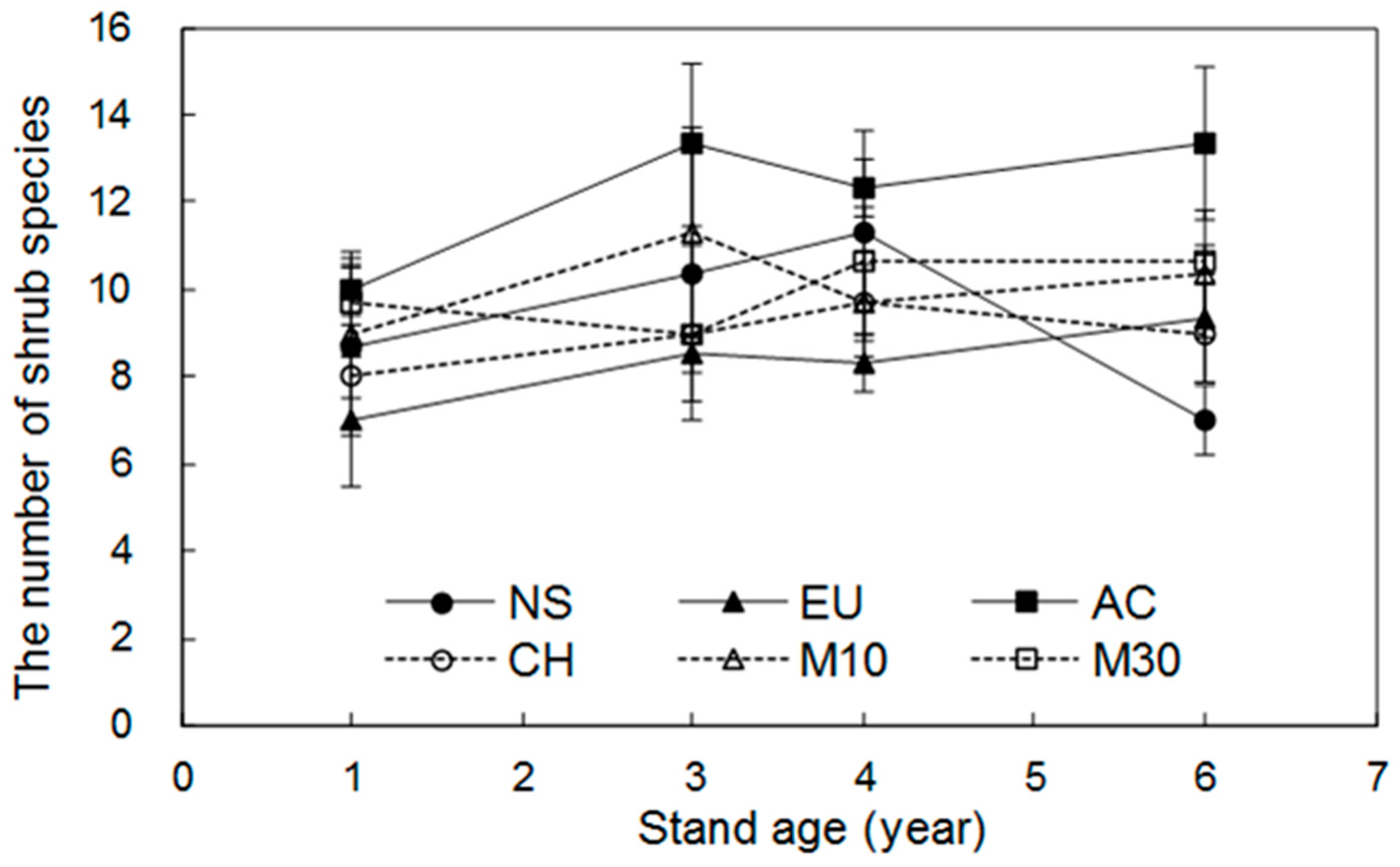
3.1. Dynamics of Shrub Species
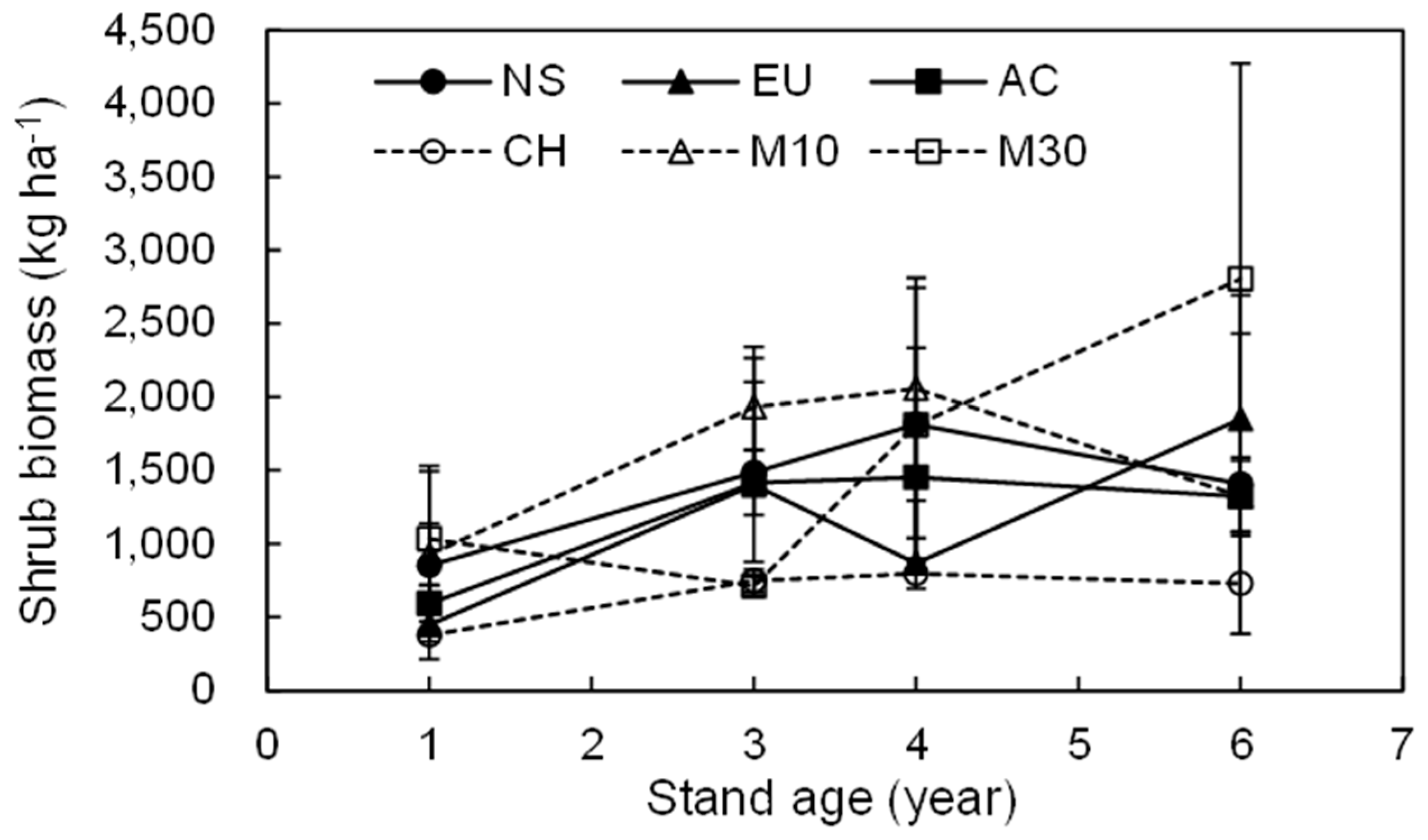
3.2. Dynamics of the Total Shrub Biomass
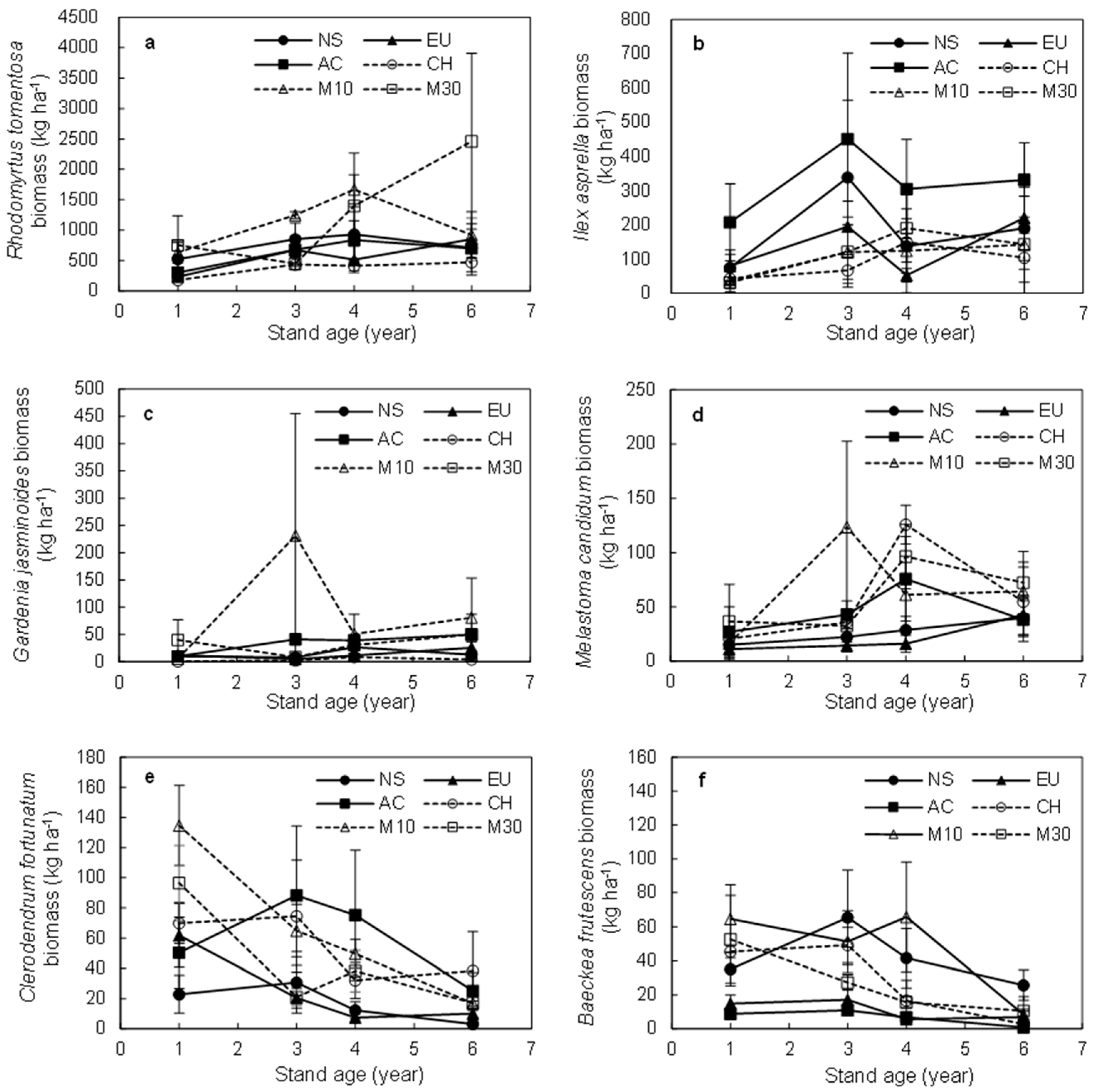
3.3. Biomass of the Dominant Shrub Species
4. Discussion
4.1. Effects of Plantation Type
4.2. Effects of Stand Age
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mandal, G.; Joshi, S.P. Estimation of above-ground biomass and carbon stock of an invasive woody shrub in the subtropical deciduous forests of Doon Valley, western Himalaya, India. J. For. Res. 2015, 26, 291–305. [Google Scholar] [CrossRef]
- Di Castri, F.; Goodall, D.W.; Specht, R.L. Mediterranean Type Shrubland; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1981; p. 56. [Google Scholar]
- Yang, B.; Li, Y.; Ding, B.; Both, S.; Erfmeier, A.; Härdtle, W.; Ma, K.; Schmid, B.; Scholten, T.; Seidler, G.; et al. Impact of tree diversity and environmental conditions on the survival of shrub species in a forest biodiversity experiment in subtropical China. J. Plant Ecol. 2017, 10, 179–189. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Xie, Z. Shrub effects on herbaceous vegetation vary with growth stages and herb relative location. Pol. J. Ecol. 2014, 62, 421–429. [Google Scholar] [CrossRef]
- Yang, L.; Ren, H.; Liu, N.; Wang, J. The shrub Rhodomyrtus tomentosa acts as a nurse plant for seedlings differing in shade tolerance in degraded land of South China. J. Veg. Sci. 2010, 21, 262–272. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, W.; Sun, Z.; Yang, L.; Yuan, S.; Ren, H. Canopy size dependent facilitations from the native shrub Rhodomyrtus tomentosa to the early establishment of native trees Castanopsis fissa and Syzygium hancei in tropical China. Restor. Ecol. 2014, 22, 509–516. [Google Scholar] [CrossRef]
- Alday, J.G.; Santana, V.M.; Marrs, R.H.; Martínez-Ruiz, C. Shrub-induced understory vegetation changes in reclaimed mine sites. Ecol. Eng. 2014, 73, 691–698. [Google Scholar] [CrossRef]
- Zhao, J.; Wan, S.; Zhang, C.; Liu, Z.; Zhou, L.; Fu, S. Contributions of understory and/or overstory vegetations to soil microbial PLFA and nematode diversities in eucalyptus monocultures. PLoS ONE 2014, 9, e85513. [Google Scholar] [CrossRef] [PubMed]
- Hortal, S.; Bastida, F.; Moreno, J.L.; Armas, C.; García, C.; Pugnaire, F.I. Benefactor and allelopathic shrub species have different effects on the soil microbial community along an environmental severity gradient. Soil Biol. Biochem. 2015, 88, 48–57. [Google Scholar] [CrossRef]
- Qiao, Y.; Miao, S.; Silva, L.C.; Horwath, W.R. Understory species regulate litter decomposition and accumulation of C and N in forest soils: A long-term dual-isotope experiment. For. Ecol. Manag. 2014, 329, 318–327. [Google Scholar] [CrossRef]
- Restrepo, C.; Vargas, A. Seeds and seedlings of two neotropical montane understory shrubs respond differently to anthropogenic edges and treefall gaps. Oecologia 1999, 119, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Katahata, S.I.; Naramoto, M.; Kakubari, Y.; Mukai, Y. Seasonal changes in photosynthesis and nitrogen allocation in leaves of different ages in evergreen understory shrub Daphniphyllum humile. Trees 2007, 21, 619–629. [Google Scholar] [CrossRef]
- Knapp, E.E.; Weatherspoon, C.P.; Skinner, C.N. Shrub seed banks in mixed conifer forests of northern California and the role of fire in regulating abundance. Fire Ecol. 2012, 8, 32–48. [Google Scholar] [CrossRef]
- Palmroth, S.; Bach, L.H.; Nordin, A.; Palmqvist, K. Nitrogen-addition effects on leaf traits and photosynthetic carbon gain of boreal forest understory shrubs. Oecologia 2014, 175, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hanley, T.A. Potential management of young-growth stands for understory vegetation and wildlife habitat in southeastern Alaska. Landsc. Urban Plan. 2005, 72, 95–112. [Google Scholar] [CrossRef]
- Hou, L.; Lei, R. Carbon dioxide sequestration of main shrub species in a natural secondary Pinus Tabulaeformis forest at the Huoditang forest zone in the Qinling Moutain. Acta Ecol. Sin. 2009, 29, 6077–6084, (In Chinese with an English Abstract). [Google Scholar]
- Pasalodos-Tato, M.; Ruiz-Peinado, R.; Del Río, M.; Montero, G. Shrub biomass accumulation and growth rate models to quantify carbon stocks and fluxes for the Mediterranean region. Eur. J. For. Res. 2015, 134, 537–553. [Google Scholar] [CrossRef]
- Bai, X.; Brenes-Arguedas, T.; Ye, J.; Wang, X.; Lin, F.; Yuan, Z.; Shi, S.; Xing, D.; Hao, Z. Dynamics of two multi-stemmed understory shrubs in two temperate forests. PLoS ONE 2014, 9, e98200. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, Q.; Feng, Z.; Ma, Z. Biomass equations for four shrub species in subtropical China. J. For. Res. 2010, 15, 83–90. [Google Scholar] [CrossRef]
- Pang, H.; Wang, X.; Zhang, J.; Zheng, L.; Cui, H. Characteristics of shrub layer biomass and carbon density in different forest types and different regions in Hubei province. J. Northwest For. Univ. 2014, 29, 46–51, (In Chinese with an English Abstract). [Google Scholar]
- Zandler, H.; Brenning, A.; Samimi, C. Quantifying dwarf shrub biomass in an arid environment: Comparing empirical methods in a high dimensional setting. Remote Sens. Environ. 2015, 158, 140–155. [Google Scholar] [CrossRef]
- Li, A.; Glenn, N.F.; Olsoy, P.J.; Mitchell, J.J.; Shrestha, R. Aboveground biomass estimates of sagebrush using terrestrial and airborne LiDAR data in a dryland ecosystem. Agric. For. Meteorol. 2015, 213, 138–147. [Google Scholar] [CrossRef]
- Greaves, H.E.; Vierling, L.A.; Eitel, J.U.; Boelman, N.T.; Magney, T.S.; Prager, C.M.; Griffin, K.L. High-resolution mapping of aboveground shrub biomass in Arctic tundra using airborne lidar and imagery. Remote Sens. Environ. 2016, 184, 361–373. [Google Scholar] [CrossRef]
- State Forestry Administration. The Main Results of the 8th National Forest Resources Inventory (from 2009 to 2013). 2014. Available online: http://www.forestry.gov.cn/main/65/content-659670.html (accessed on 1 August 2017).
- Wang, H.; Liu, S.R.; Mo, J.M.; Wang, J.X.; Makeschin, F.; Wolff, M. Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China. Ecol. Res. 2010, 25, 1071–1079. [Google Scholar] [CrossRef]
- Nilsson, M.; Wardle, D. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Zhao, J.; Wan, S.; Li, Z.; Shao, Y.; Xu, G.; Liu, Z.; Zhou, L.; Fu, S. Dicranopteris-dominated understory as major driver of intensive forest ecosystem in humid subtropical and tropical region. Soil Biol. Biochem. 2012, 49, 78–87. [Google Scholar] [CrossRef]
- Chastain, R.A., Jr.; Currie, W.S.; Townsend, P.A. Carbon sequestration and nutrient cycling implications of the evergreen understory layer in Appalachian forests. For. Ecol. Manag. 2006, 231, 63–77. [Google Scholar] [CrossRef]
- Zhou, C.; Wei, X.; Zhou, G.; Yan, J.; Wang, X.; Wang, C.; Liu, H.; Tang, X.; Zhang, Q. Impacts of a large-scale reforestation program on carbon storage dynamics in Guangdong, China. For. Ecol. Manag. 2008, 255, 847–854. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Wu, J.; Chen, H.; Lin, Y.; Zhou, L.; Fu, S. Impacts of understory species removal and/or addition on soil respiration in a mixed forest plantation with southern China. For. Ecol. Manag. 2011, 261, 1053–1060. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Chen, D.; Huang, G.; Zhou, L.; Fu, S. Understory plants can make substantial contributions to soil respiration: Evidence from two subtropical plantations. Soil Biol. Biochem. 2011, 43, 2355–2357. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Rao, X.; Wang, X.; Liang, C.; Lin, Y.; Zhou, L.; Cai, X.; Fu, S. Carbon storage and allocation pattern in plant biomass among different forest plantation stands in Guangdong, China. Forests 2015, 6, 794–808. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Peng, S.; Zobel, K. Climate warming may facilitate invasion of the exotic shrub Lantana camara. PLoS ONE 2014, 9, e105500. [Google Scholar] [CrossRef] [PubMed]
- Michalet, R.; Brooker, R.W.; Lortie, C.J.; Maalouf, J.P.; Pugnaire, F.I. Disentangling direct and indirect effects of a legume shrub on its understorey community. Oikos 2015, 124, 1251–1262. [Google Scholar] [CrossRef]
- Fernández, M.E.; Gyenge, J.E.; Salda, G.D.; Schlichter, T.M. Silvopastoral systems in northwestern Patagonia I: Growth and photosynthesis of Stipa speciosa under different levels of Pinus ponderosa cover. Agrofor. Syst. 2002, 55, 27–35. [Google Scholar] [CrossRef]
- Dyer, L.A.; Letourneau, D.K.; Chavarria, G.V.; Amoretti, D.S. Herbivores on a dominant understory shrub increase local plant diversity in rain forest communities. Ecology 2010, 91, 3707–3718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, F.; Zhou, J.; Kuang, X.; Niu, D.; Ye, C.; Guo, X. A Study on Species Diversity of Shrubs in Eucalyptus spp. Plantation under Different Management Practices. Acta Agric. Univ. Jiangxiensis 2012, 34, 59–65, (In Chinese with an English Abstract). [Google Scholar]
- Carneiro, M.; Fabião, A.; Martins, M.C.; Cerveira, C.; Santos, C.; Nogueira, C.; Lousã, M.; Hilário, L.; Fabião, A.; Abrantes, M.; et al. Species richness and biomass of understory vegetation in a Eucalyptus globulus Labill. coppice as affected by slash management. Eur. J. For. Res. 2007, 126, 475–480. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, F.; Xue, C.; Liu, P. Species diversity of shrub layer in forest communities, Guangdong Province in South China. Guangdong For. Technol. 2014, 30, 8–14, (In Chinese with an English Abstract). [Google Scholar]
- Becknell, J.M.; Powers, J.S. Stand age and soils as drivers of plant functional traits and aboveground biomass in secondary tropical dry forest. Can. J. For. Res. 2014, 44, 604–613. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, S.; Liu, S.; Wang, X.; Zhang, Y.; Liu, T.; Zhou, L.; Zhang, W.; Fu, S. Reforestation makes a minor contribution to soil carbon accumulation in the short term: Evidence from four subtropical plantations. For. Ecol. Manag. 2017, 384, 400–405. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, W.; Xia, H.; Fu, S.; Li, Z. Nitrogen mineralization and leaching in the early stages of a subtropical reforestation in Southern China. Restor. Ecol. 2010, 18, 313–322. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Y.; Zhao, J.; Fu, S.; Li, Z.; Xia, H.; Zhou, L. Temperature sensitivity of total soil respiration and its heterotrophic and autotrophic components in six vegetation types of subtropical China. Sci. Total Environ. 2017, 607, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S. Understory development in young Cordia alliodora plantations. New For. 2000, 19, 159–170. [Google Scholar] [CrossRef]
- Suchar, V.A.; Crookston, N.L. Understory cover and biomass indices predictions for forest ecosystems of the Northwestern United States. Ecol. Indic. 2010, 10, 602–609. [Google Scholar] [CrossRef]
- Liu, Z.W.; Chen, R.S.; Song, Y.X.; Han, C.T. Distribution and estimation of aboveground biomass of alpine shrubs along an altitudinal gradient in a small watershed of the Qilian Mountains, China. J. Mt. Sci. 2015, 12, 961–971. [Google Scholar] [CrossRef]
- Halpern, C.B.; Lutz, J.A. Canopy closure exerts weak controls on understory dynamics: A 30-year study of overstory-understory interactions. Ecol. Monogr. 2013, 83, 221–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, F.; Han, H.; Cheng, X.; Zhou, B.; Li, Y.; Liu, K.; Yin, X. Analysis on influence factors of undergrowth vegetation biomass in Pinus tabulaeformis plantation in Mount Taiyue. J. Central South Univ. For. Technol. 2015, 35, 104–108, 125, (In Chinese with an English Abstract). [Google Scholar]
- Cheng, X.; Yu, M.; Wang, G.G.; Wu, T.; Zhang, C. Growth, morphology and biomass allocation in response to light gradient in five subtropical evergreen broadleaved tree seedlings. J. Trop. For. Sci. 2013, 25, 537–546. [Google Scholar]
- Hamelin, C.; Gagnon, D.; Truax, B. Aboveground biomass of Glossy Buckthorn is similar in open and understory environments but architectural strategy differs. Forests 2015, 6, 1083–1093. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; Gong, G.; Chen, J.; Tang, B.; Zhu, Z.; Wu, X.; Mu, C. Biomass and its allocation of undergrowth Vitex negundo L. in different age classes of mixed cypress forest. Acta Ecol. Sin. 2010, 30, 2809–2818, (In Chinese with an English Abstract). [Google Scholar]
- Chen, F.; Luo, Y.; Li, Q. Allometric equations for estimating biomass of dominant shrub species in subtropical forests in eastern Guangdong Province, China. J. Central South Univ. For. Technol. 2013, 33, 5–11, (In Chinese with an English Abstract). [Google Scholar]
- Limsuwan, S.; Trip, E.N.; Kouwen, T.R.; Piersma, S.; Hiranrat, A.; Mahabusarakam, W.; Voravuthikunchai, S.P.; van Dijl, J.M.; Kayser, O. Rhodomyrtone: A new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine 2009, 16, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.P.; Li, G.; Li, X.; Hu, Q.P.; Liu, J.X.; Zhang, F.X.; Su, Z.R.; Lai, X.P. The roots of Ilex asprella extract lessens acute respiratory distress syndrome in mice induced by influenza virus. J. Ethnopharmacol. 2014, 155, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, W.; Fan, J.; Wang, H.; Ma, D.; Li, Q. Comparison on photosynthetic and fluorescence characteristics between one cultivar and one species in Gardenia. China For. Sci. Technol. 2015, 29, 18–23, (Chinese with an English Abstract). [Google Scholar]
- Zhu, X. Ecological Characteristics of Five Shrubs in Eucalyptus Urophylla Forest. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, May 2015; p. 26. [Google Scholar]
- Zhu, X.; Liang, C.; Ca, X.; Fu, S.; Zhou, L. Soil microbial community characteristics of 5 tree species in Eucalyptus urophylla plantation. Ecol. Environ. Sci. 2015, 24, 617–623, (Chinese with an English Abstract). [Google Scholar]
- Liang, H.L.; Liu, H.N.; Yang, Q.H.; Huang, R.Z.; Wei, X.; Ye, W.H.; Luo, W.H.; Xiong, Z.C. Seed germination of Rhodomyrtus tomentosa. Seed Sci. Technol. 2013, 41, 188–198. [Google Scholar] [CrossRef]
- Li, K.X.; Liang, X.J.; Wei, X.J.; Huang, J.; Liang, W.H. Research Progress on Baeckea frutesce. Guangxi For. Sci. 2013, 42, 38–42, (Chinese with an English Abstract). [Google Scholar]
| Plantations | Stand Age (Year) | ||
|---|---|---|---|
| 1 | 4 | 6 | |
| NS | 1.20 ± 0.38 | 0.99 ± 0.12 | 1.05 ± 0.02 |
| EU | 4.76 ± 0.29 | 10.21 ± 1.15 | 11.69 ± 1.03 |
| AC | 2.58 ± 0.2 | 7.41 ± 0.33 | 9.15 ± 0.90 |
| CH | 0.79 ± 0.01 | 3.97 ± 0.22 | 5.61 ± 0.09 |
| M10 | 0.90 ± 0.06 | 2.81 ± 0.19 | 3.63 ± 0.32 |
| M30 | 0.90 ± 0.10 | 2.94 ± 0.03 | 3.98 ± 0.03 |
| Species Name | Plantations | |||||
|---|---|---|---|---|---|---|
| NS | EU | AC | CH | M10 | M30 | |
| Mallotus apelta | √ | √ | √ | √ | √ | √ |
| Ficus variolosa | √ | ○ | √ | ○ | √ | √ |
| Cassia alata | ○ | ○ | ○ | ○ | ○ | √ |
| Rhaphiolepis indica | √ | √ | √ | √ | √ | √ |
| Baeckea frutescens | √ | √ | √ | √ | √ | √ |
| Clerodendrum fortunatum. | √ | √ | √ | √ | √ | √ |
| Breynia fruticosa | √ | ○ | √ | ○ | √ | ○ |
| Symplocos chinensis | ○ | ○ | ○ | ○ | ○ | √ |
| Gardenia jasminoides | √ | √ | √ | √ | √ | √ |
| Psychotria rubra | ○ | ○ | ○ | ○ | ○ | √ |
| Phyllodium pulchellum | ○ | ○ | √ | √ | ○ | ○ |
| Wikstroemia indica | √ | √ | √ | ○ | √ | √ |
| Glochidion eriocarpum | ○ | ○ | √ | ○ | ○ | ○ |
| Ilex asprella | √ | √ | √ | √ | √ | √ |
| Eurya chinensis | √ | √ | √ | √ | √ | √ |
| Evodia lepta | √ | √ | √ | √ | √ | ○ |
| Helicteres angustifolia | √ | √ | √ | ○ | √ | √ |
| Garcinia oligantha | ○ | ○ | ○ | √ | ○ | ○ |
| Glochidion puberum | ○ | ○ | √ | ○ | ○ | √ |
| Rhodomyrtus tomentosa | √ | √ | √ | √ | √ | √ |
| Ficus hirta | √ | √ | √ | √ | √ | √ |
| Glochidion zeylanicum | √ | ○ | ○ | ○ | ○ | ○ |
| Melastoma candidum | √ | √ | √ | √ | √ | √ |
| Shrub Species | PT | SA | PT × SA | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Rhodomyrtus tomentosa | 2.682 | 0.032 | 2.754 | 0.053 | 1.162 | 0.332 |
| Ilex asprella | 2.586 | 0.039 | 3.198 | 0.032 | 0.413 | 0.967 |
| Gardenia jasminoides | 1.479 | 0.215 | 0.535 | 0.661 | 0.682 | 0.789 |
| Melastoma candidum | 1.669 | 0.160 | 3.276 | 0.029 | 1.169 | 0.327 |
| Clerodendrum fortunatum | 2.910 | 0.023 | 8.359 | <0.001 | 1.477 | 0.152 |
| Baeckea frutescens | 5.537 | <0.001 | 6.188 | 0.001 | 0.875 | 0.594 |
| Shrub Species | Plantation | Stand Age (Year) | |||
|---|---|---|---|---|---|
| 1 | 3 | 4 | 6 | ||
| Rhodomyrtus tomentosa | NS | 6853 | 4437 | 3789 | 1991 |
| EU | 4652 | 5416 | 0.4193 | 3151 | |
| AC | 9481 | 7756 | 6770 | 4000 | |
| CH | 6104 | 3281 | 2570 | 1526 | |
| M10 | 14148 | 8207 | 7415 | 2370 | |
| M30 | 12207 | 2637 | 4089 | 5015 | |
| Ilex asprella | NS | 189 | 517 | 316 | 317 |
| EU | 430 | 489 | 228 | 489 | |
| AC | 415 | 785 | 533 | 578 | |
| CH | 178 | 156 | 311 | 193 | |
| M10 | 267 | 422 | 230 | 356 | |
| M30 | 133 | 178 | 341 | 193 | |
| Melastoma candidum | NS | 789 | 411 | 415 | 333 |
| EU | 578 | 444 | 656 | 575 | |
| AC | 741 | 511 | 919 | 644 | |
| CH | 1096 | 578 | 756 | 630 | |
| M10 | 385 | 1415 | 896 | 548 | |
| M30 | 1822 | 570 | 1059 | 578 | |
| Gardenia jasminoides | NS | 244 | 98 | 231 | 206 |
| EU | 415 | 185 | 156 | 254 | |
| AC | 519 | 593 | 548 | 526 | |
| CH | 89 | 74 | 96 | 207 | |
| M10 | 222 | 496 | 215 | 296 | |
| M30 | 726 | 267 | 193 | 400 | |
| Clerodendrum fortunatum | NS | 3459 | 3693 | 1849 | 289 |
| EU | 6040 | 2159 | 548 | 800 | |
| AC | 5452 | 3941 | 2807 | 1030 | |
| CH | 12415 | 4793 | 2415 | 2000 | |
| M10 | 10622 | 1785 | 3207 | 711 | |
| M30 | 11615 | 4096 | 2681 | 852 | |
| Baeckea frutescens | NS | 5933 | 5867 | 5485 | 2133 |
| EU | 3072 | 2244 | 1800 | 565 | |
| AC | 1733 | 1593 | 1741 | 156 | |
| CH | 9274 | 4037 | 2644 | 222 | |
| M10 | 13244 | 8356 | 7296 | 748 | |
| M30 | 9244 | 2541 | 1926 | 333 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cai, X.; Zhang, Y.; Rao, X.; Fu, S. Dynamics of Understory Shrub Biomass in Six Young Plantations of Southern Subtropical China. Forests 2017, 8, 419. https://doi.org/10.3390/f8110419
Chen Y, Cai X, Zhang Y, Rao X, Fu S. Dynamics of Understory Shrub Biomass in Six Young Plantations of Southern Subtropical China. Forests. 2017; 8(11):419. https://doi.org/10.3390/f8110419
Chicago/Turabian StyleChen, Yuanqi, Xi’an Cai, Yanju Zhang, Xingquan Rao, and Shenglei Fu. 2017. "Dynamics of Understory Shrub Biomass in Six Young Plantations of Southern Subtropical China" Forests 8, no. 11: 419. https://doi.org/10.3390/f8110419




