Short-Term Effects of Low Intensity Thinning on the Fine Root Dynamics of Pinus massoniana Plantations in the Three Gorges Reservoir Area, China
Abstract
:1. Introduction
2. Materials and Methods
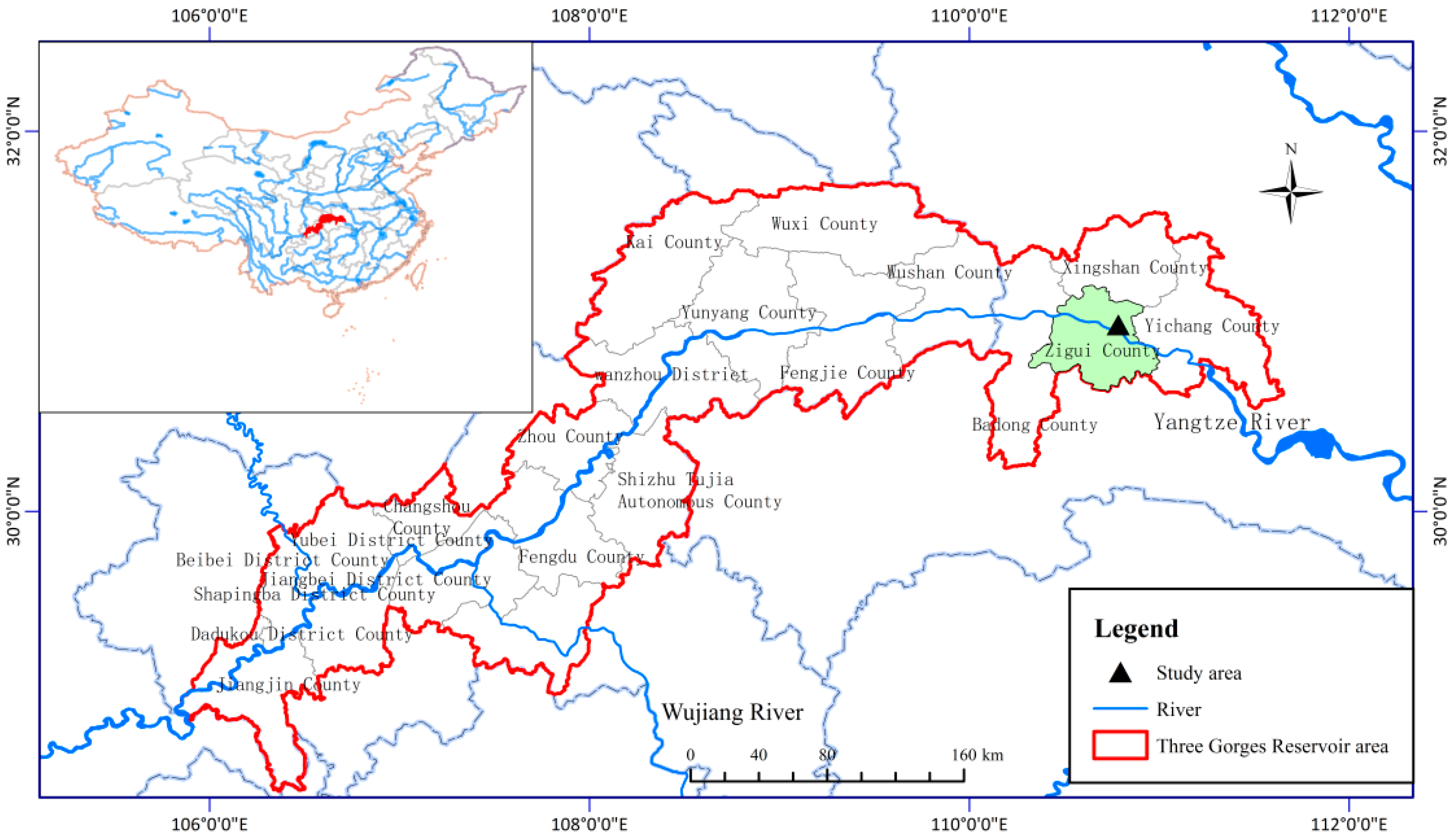
2.1. Study Site
2.2. Fine Root Biomass
2.3. Environmental Factors
2.3.1. Soil Temperature and Moisture
2.3.2. Soil Samples and Chemical Analysis
2.4. Statistical Analysis
3. Results
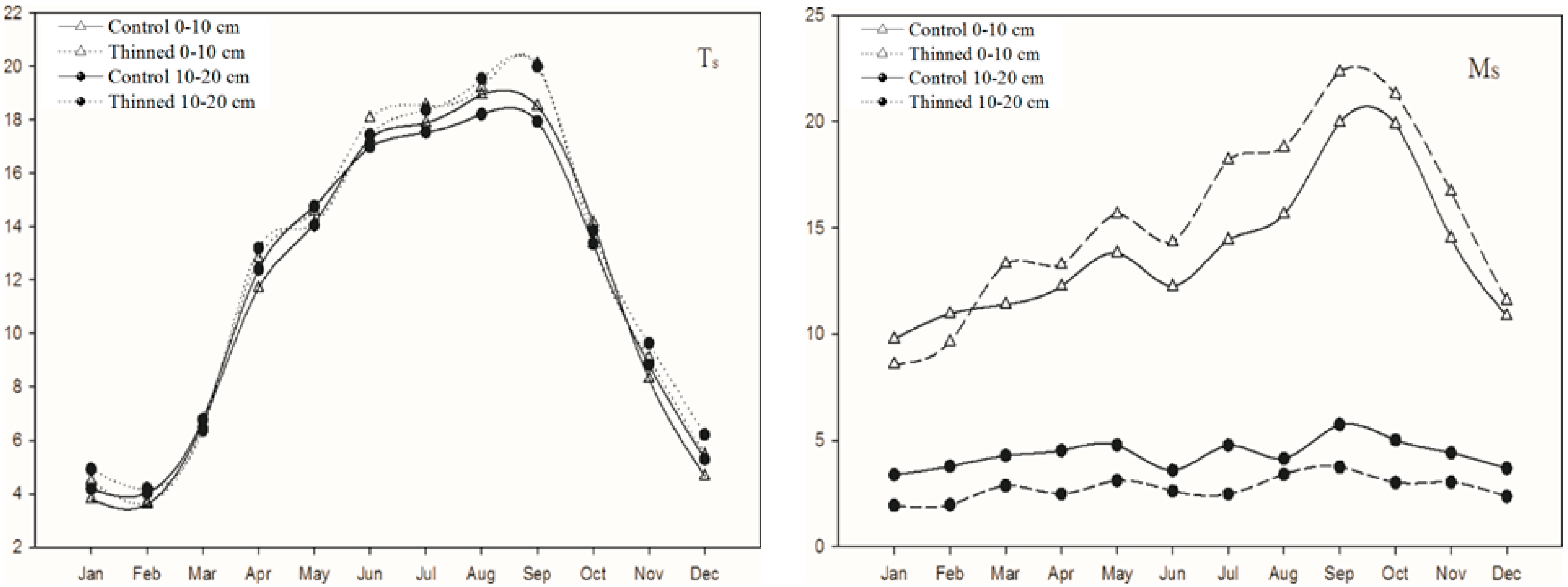
3.1. Soil Temperature and Moisture
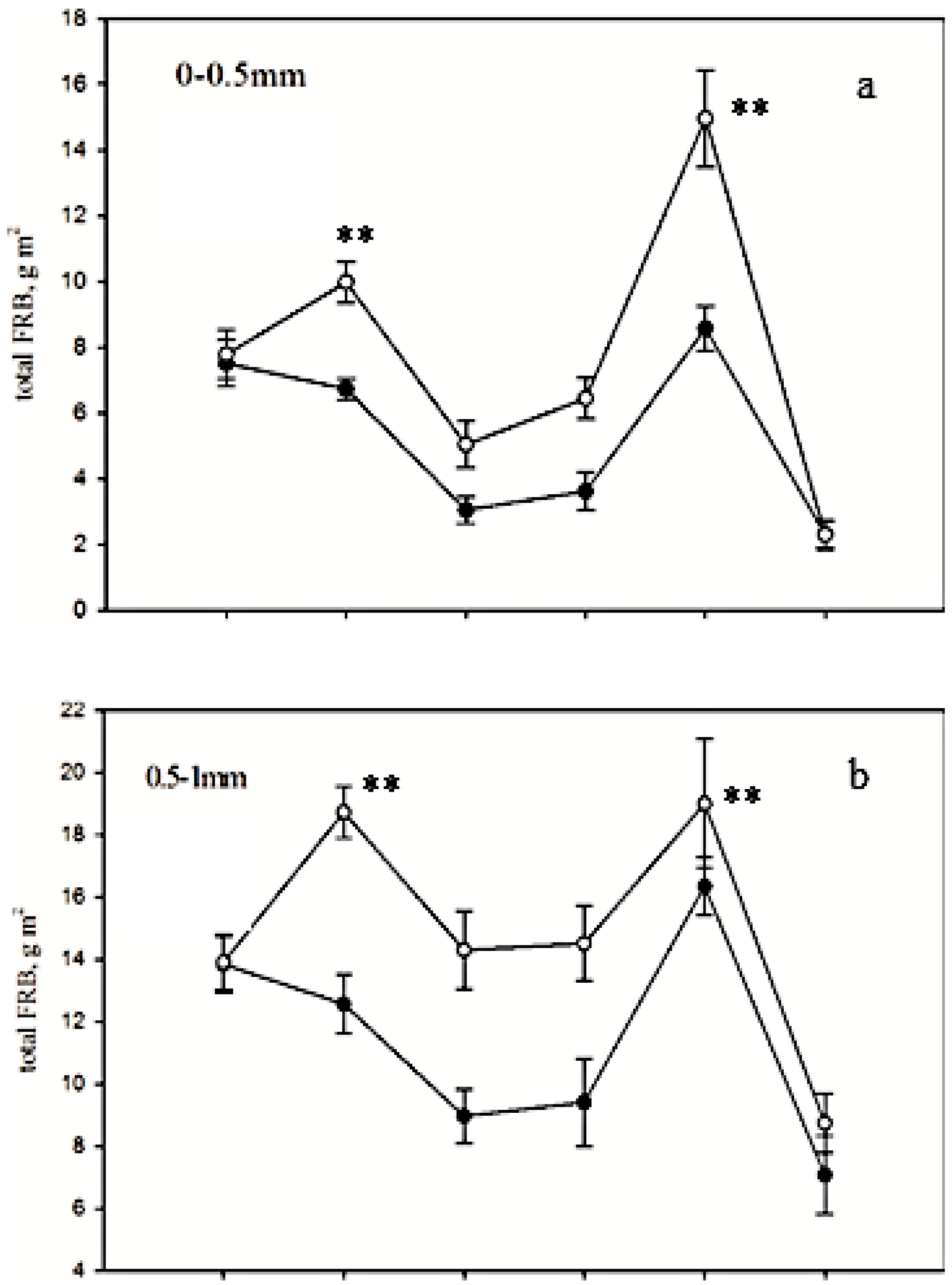
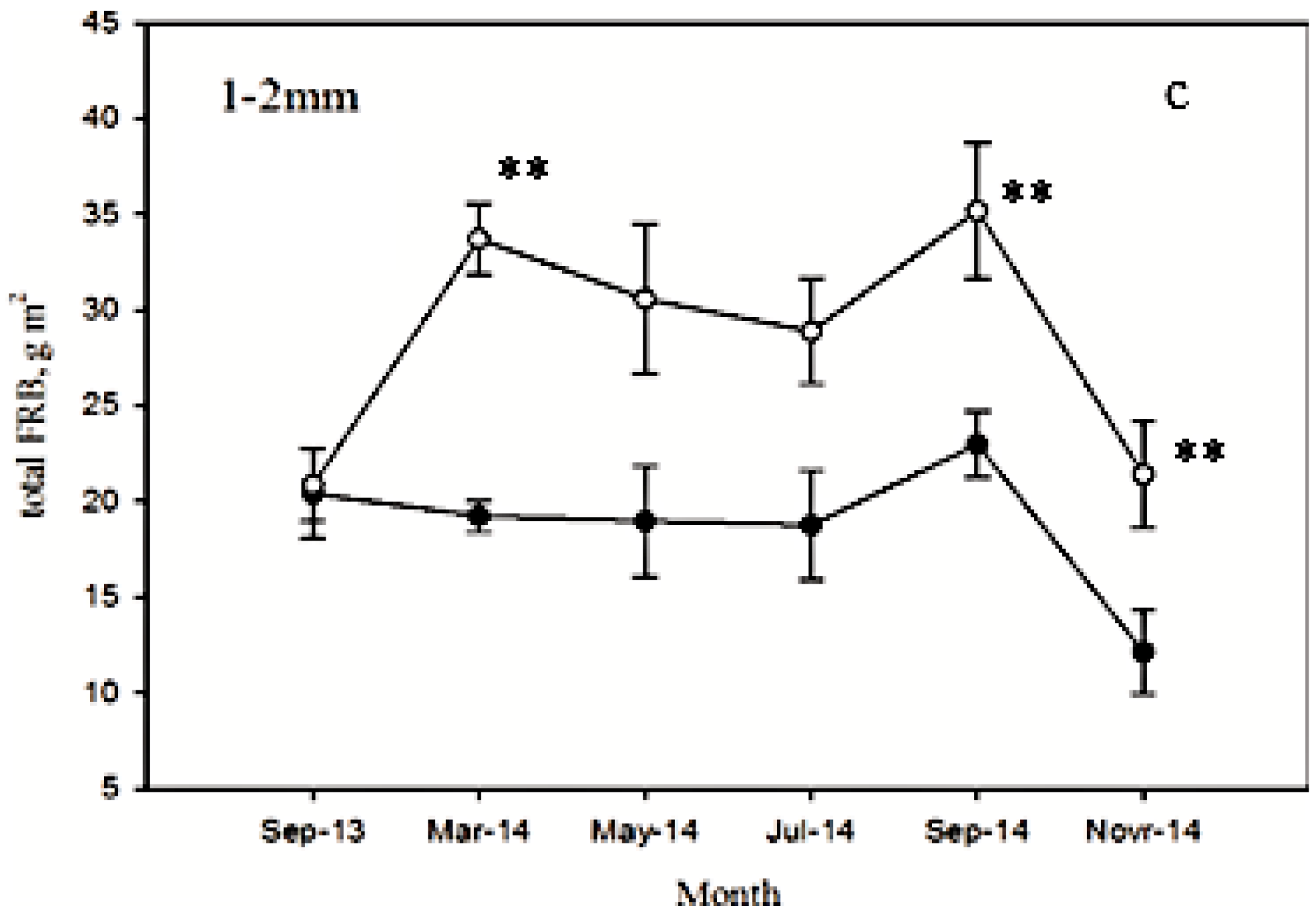
3.2. Seasonal Dynamics of FRB after Low Intensity Thinning
3.3. Seasonal Dynamics of FRB at Different Depths after Low Intensity Thinning
3.4. Relationships between Soil Environmental Factors and FRB
4. Discussion
4.1. Changes in FRB Dynamics in Response to Thinning
4.2. Changes in FRB Dynamics at Different Soil Depths in Response to Thinning
4.3. Correlations of FRB and Soil Factors in Response to Thinning
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. Alarge and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Wan, S.; Wang, J.; Wang, H.; Liu, K. Effects of experimental throughfall reduction and soil warming on fine root biomass and its decomposition in a warm temperate oak forest. Sci. Total Environ. 2016, 574, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Dong, L.; Mao, Z.; Li, Y. Fine root dynamics of trees and understorey vegetation in chronosequence of Betula platyphylla, stands. For. Ecol. Manag. 2015, 346, 1–9. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Feinstein, L.M.; Kershner, M.W.; Blackwood, C.B. Aggregated and complementary: Symmetric proliferation, overyielding, and mass effects explain fine-root biomass in soil patches in a diverse temperate deciduous forest landscape. New Phytol. 2015, 205, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Joslin, J.D.; Gaudinski, J.B.; Torn, M.S.; Tiley, W.J.; Hanson, P.J. Fine-root turnover patterns and their relationship to root diameter and soil depth in a 14C-labeled hardwood forest. New Phytol. 2006, 172, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Hirano, Y.; Mizoguchi, T.; Kominami, Y.; Dannoura, M.; Ishii, H. Very fine roots respond to soil depth: Biomass allocation, morphology, and physiology in a broad-leaved temperate forest. Ecol. Res. 2011, 26, 95–104. [Google Scholar] [CrossRef]
- Violita, V.; Triadiati, T.; Anas, I.; Miftahudin, M. Fine root production and decomposition in lowland rainforest and oil palm plantations in Sumatra, Indonesia. Hayati J. Biosci. 2016, 23, 7–12. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.E. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Factors causing variation in fine root biomass in forest ecosystems. For. Ecol. Manag. 2011, 261, 265–277. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Liu, C.; Xiang, W.H.; Lei, P.F.; Deng, X.W.; Tian, D.L.; Fang, X.; Peng, C.H. Standing fine root mass and production in four Chinese subtropical forests along a succession and species diversity gradient. Plant Soil 2014, 376, 445–459. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Iorio, A.D.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey-oak stand in relation to seasonal changes in soil moisture in the southern Apennines, Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Montagnoli, A.; Terzaghi, M.; di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root seasonal pattern, production and turnover rate of European beech (Fagus sylvatica L.) stands in Italy prealps: Possible implications of coppice conversion to high forest. Plant Biosyst. 2012, 146, 1012–1022. [Google Scholar] [CrossRef] [Green Version]
- Montagnoli, A.; Terzaghi, M.; Scippa, G.S.; Chiatante, D. Heterorhizy can lead to underestimation of fine-root production when using mesh-based techniques. Acta Oecol. 2014, 59, 84–90. [Google Scholar] [CrossRef]
- Sundarapandina, S.M.; Swamy, P.S. Fine root biomass disrtibution and Productivity patterns under open and cloesd canopies of tropical forest ecosystems at Kodayar in Western Ghats, South India. For. Ecol. Manag. 1996, 86, 181–192. [Google Scholar] [CrossRef]
- Quan, X.K.; Wang, C.K.; Zhang, Q.Z.; Wang, X.C.; Luo, Y.Q.; Lamberty, B.B. Dynamics of fine roots in five Chinese temperate forests. J. Plant Res. 2010, 123, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.M.; Wang, R.L.; Xiao, W.F.; Feng, X.H.; Liu, Z.B.; Ge, X.G.; Wang, X.R.; Zhang, W.Y. Fine root production and turnover in Pinus massoniana plantation in Three Gorges Reservoir Area of China. Chin. J. Appl. Ecol. 2012, 23, 2346–2352. [Google Scholar]
- Mei, L.; Han, Y.Z.; Yu, Y.Q.; Shi, J.W.; Wang, Z.Q. Impact factors on fine roots seasonal dynamics in Fraxinus mandshurica Plantation. Sci. Silv. Sin. 2006, 42, 7–12. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Derome, J.; Nöjd, P.; Kukkola, M. Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol. 2007, 27, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Han, Y.Z.; Zhang, Y.X.; Wu, X.G. Effects of cutting disturbance on spatial heterogeneity of fine root biomass of Larix principis-rupprechtii. Acta Ecol. Sin. 2012, 32, 64–73. [Google Scholar] [CrossRef]
- John, E.M.; Kurt, H.J.; Debby, C.B.; Moira, C. Fine and coarse root parameters from mature black spruce displaying geneticsoil moisture interaction in growth. Can. J. For. Res. 2012, 42, 1926–1938. [Google Scholar]
- Mattson, K.G.; Smith, H.C. Detritical organic matter and soil CO2 efflux in forests regenerating from cutting in western Virginia. Soil Biol. Biochem. 1993, 25, 1241–1248. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Turril1, N.L.; Adams, M.B. Herbaceous layer and over story species in clearcut and mature central Appalachian hardwood forest. Ecol. Appl. 1995, 5, 947–955. [Google Scholar] [CrossRef]
- Bréda, N.; Granier, A.; Barataud, F.; Moyne, C. Soil water dynamics in an oak stand. I. Soil water content, water potentials and water uptake by roots. Plant Soil 1995, 172, 7–27. [Google Scholar]
- Wen, Y.G.; Yuan, C.A.; Li, X.X.; He, T.P.; Lai, J.Y.; Huang, M. Development of species diversity in vegetation restoration proeess in midmoutain region of Damingshan, Guang XI. Acta Phyoecol. Sin. 1998, 22, 33–40. [Google Scholar]
- Baldwin, V.C.; Peterson, K.D. The effect of spacing and thinning on stand and tree characteristic of 38 year old loblolly Pine. For. Ecol. Manag. 2000, 137, 91–102. [Google Scholar] [CrossRef]
- Zhang, D.H.; Ye, Z.F.; Fan, B.Y. Wei Tinglin. Influence of thining on soil fertility in artificial forests. Chin. J. Appl. Ecol. 2001, 12, 672–676. [Google Scholar]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 15991. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Oviedo, A.; Ruiz-Peinado, R.; Modrego, P.; Alonso, R.; Montero, G. Forest thinning impact on carbon stock and soil condition in Southern European populations of P. sylvestris L. For. Ecol. Manag. 2015, 357, 259–267. [Google Scholar] [CrossRef]
- Zhang, J.; Webster, J.; Young, D.H.; Fiddler, G.O. Effect of thinning and soil treatments on Pinus ponderosa plantations: 15-year results. For. Ecol. Manag. 2016, 368, 123–132. [Google Scholar] [CrossRef]
- López, B.; Sabaé, S.; Gracia, C.A. Thinning effects on carbon allocation to fine roots in a Quercus ilex forest. Tree Physiol. 2003, 23, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, K.; Shibata, H.; Takagi, K.; Nomura, M.; Kurima, N.; Fukazawa, T.; Satoh, F.; Sasa, K. Effects of clear-cutting on nitrogen leaching and fine root dynamics in a cool-temperate forested watershed in northern Japan. For. Ecol. Manag. 2006, 225, 257–261. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, R.M.; Xiao, W.F.; Liu, Z.B.; Zhang, W.Y. A review of Fine-root of Pinus massoniana. World For. Res. 2014, 27, 25–29. [Google Scholar]
- Cheng, R.M.; Wang, R.L.; Xiao, W.F.; Feng, X.H.; Liu, Z.B.; Ge, X.G.; Wang, X.R.; Zhang, W.Y. Spatial distribution of root biomass of Pinus massoniana plantation in Three Gorges Reservoir Area, China. Acta Ecol. Sin. 2012, 32, 823–832. [Google Scholar] [CrossRef]
- Gong, Z.T. Chinese Soil Taxonomy; China Science Press: Beijing, China, 2003. [Google Scholar]
- Li, Z.Y.; Wang, Y.H.; Yu, P.T. Soil chemical properties and growth characteristics of mixed plantation of Pinus massoniana and Cinnamomum camphora in the acid rain region of Chongqing, China. Chin. J. Plant Ecol. 2010, 34, 387–395. [Google Scholar]
- Nelson, D.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; ASA-SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Zhu, T.B.; Zhang, J.B.; Meng, T.Z.; Zhang, Y.C.; Yang, J.J.; Müller, C.; Cai, Z.C. Tea plantation destroys soil retention of NO3—And increases N2O emissions in subtropical China. Soil Biol. Biochem. 2014, 73, 106–114. [Google Scholar] [CrossRef]
- Zhang, W.R.; Yang, G.C.; Tu, X.N. Adiministration Forestry Standard of People’s Republic of China—Method of Forest Soil Analysis; Chinese Standard Press: Beijing, China, 1999. [Google Scholar]
- López, B.; Sabaé, S.; Gracia, C.A. Annual and seasonal changes in fine root biomass of Quercus ilex L. Plant Soil 2001, 230, 125–134. [Google Scholar] [CrossRef]
- Noguchi, K.; Han, Q.M.; Araki, M.G.; Kawasaki, T.; Kaneko, S.; Takahashi, M.; Chiba, Y. Fine-root dynamics in a young Hinoki cypress (Chamaecyparis obtusa) stand for 3 years following thinning. J. For. Res. 2011, 16, 284–291. [Google Scholar] [CrossRef]
- Liu, Y.K.; Fan, C.; Li, X.W.; Ling, Y.H.; Zhou, Y.G.; Feng, M.S.; Huang, C.D. Effects of thinning on fine root biomass and carbon storage of subalpine Picea asperataplantation in Western Sichuan Province, China. Chin. J. Plant Ecol. 2012, 36, 645–654. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y. Influence of environmental variability on root dynamics in northern forests. Crit. Rev. Plant Sci. 2009, 28, 179–197. [Google Scholar] [CrossRef]
- Lv, S.X.; Yu, X.B. The relationship of planting density of Fir and roots growth. J. Nanjing For. Univ. 2010, 18, 1–3. [Google Scholar]
- Bloomfield, J.K.; Vogt, K.A.; Wargo, P.M. Tree root turnover and Senescence. In Plant Roots: The Hidden Half, 2nd ed.; Waisel, Y., Eshel, A., Kaafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 363–381. [Google Scholar]
- Gower, S.T.; Vogt, K.A.; Grier, C.C. Carbon dynamics of Rocky Mountain Douglas-fir: Influence of water and nutrient availability. Ecol. Monogr. 1992, 62, 43–65. [Google Scholar] [CrossRef]
- Mallonen, K.; Helmisaari, H.S. Seasonal and yearly variations of fine-root biomass and necromass in a Scots pine( Pinus sylvestris L.) stand. For. Ecol. Manag. 1998, 102, 283–290. [Google Scholar]
- Yang, Y.S.; Chen, G.S.; Liu, P.; Huang, R.Z.; Chen, Y.X.; He, Z.M. Fine root distribution, seasonal pattern and production in a native forest and monoculture plantations in subtropical China. Acta Ecol. Sin. 2003, 23, 1719–1730. [Google Scholar]
- Marquard, E.; Weigelt, A.; Temperton, V.M.; Roscher, C.; Schumacher, J.; Buchmann, N.; Fischer, M.; Weisser, W.W.; Schmid, B. Plant species richness and functional composition drive overyielding in a six-year grassland experiment. Ecology 2009, 90, 3290–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iorio, A.D.; Montagnoli, A.; Terzaghi, M.; Scippa, G.S.; Chiatante, D. Effect of tree density on root distribution in fagus sylvatica, stands: A semi-automatic digitising device approach to trench wall method. Trees 2013, 27, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.F.; Wang, N.; Cheng, R.M.; Xiao, W.F.; Yang, S.; Guo, Y.; Lei, L.; Zeng, L.X.; Wang, X.R. Characteristics of Fine Roots of Pinus massoniana in the Three Gorges Reservoir Area, China. Forests 2017, 8, 183. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y. Fine Root Biomass, Production, Turnover Rates, and Nutrient Contents in Boreal Forest Ecosystems in Relation to Species, Climate, Fertility, and Stand Age: Literature Review and Meta-Analyses. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Burton, A.J.; Pregitzer, K.S.; Hendrick, R.L. Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests. Oecologia 2000, 125, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.R.; McCarthy, H.; Hendrey, G.; McNulty, S.G.; et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Dukes, J.S.; Shaw, M.R.; Luo, Y.Q.; Field, C.B. Nitrogen and climate change. Science 2003, 302, 1512–1513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Han, Y.Z.; Wang, Q.C.; Wang, Z.Q. Seasonal dynamics of fine root biomass, root lenght density, specific root lenght and soil resource availablity in a Larix gmelini plantation. Acta Phytoecol. Sin. 2005, 29, 403–410. [Google Scholar]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Borken, W.; Beese, F. Methane and nitrous oxide fluxes of soils in pure and mixed stands of European beech and Norway spruce. Eur. J. Soil Sci. 2006, 57, 617–625. [Google Scholar] [CrossRef]
- Persson, H. Adaptive tactics and characteristics of tree fine roots. In The Supporting Roots of Trees and Woody Plants: Form, Function and Physiology; Stokes, A., Ed.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 337–346. [Google Scholar]
- Yang, X.Y.; Han, Y.Z.; Wu, X.G. Response of fine root biomass tochanges in spatial heterogeneity of soil moisture and nitrogen in Larix principis-rupprechtii forest. Chin. J. Plant Ecol. 2012, 36, 965–972. [Google Scholar] [CrossRef]
- Serrasolses, I. Fertilitat dels Sòls Afectats pel foc. Dinàmica del Nitrogen i del Fósfor. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 1994. [Google Scholar]
- Zhang, X.Q. Fine-root production and turnover for forest ecosystems. Sci. Silv. Sin. 2001, 37, 126–138. [Google Scholar]
- Norby, R.J.; Jackson, R.B. Root dynamics and global change: Seeking an ecosystem perspective. New Phytol. 2000, 147, 3–12. [Google Scholar] [CrossRef]

| Soil Depth (cm) | Control | Thinned | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤0.5 mm | 0.5–1 mm | 1–2 mm | Sum of the Size Sub-Classes | ≤0.5 mm | 0.5–1 mm | 1–2 mm | Sum of the Size Sub-Classes | |
| 0–10 | 0.020 | 0.102 | 0.143 | 0.109 | 0.003 | 0.014 | 0.283 | 0.154 |
| 10–20 | 0.018 | 0.210 | 0.678 | 0.268 | 0.006 | 0.115 | 0.889 | 0.547 |
| 20–30 | 0.461 | 0.605 | 0.990 | 0.621 | 0.291 | 0.555 | 0.429 | 0.401 |
| Parameter | Correlation Coefficient | ||||||||
| rOC | rTN | rTP | rAP | rTK | rAK | rOC+TN+TK+TP+AK+AP | |||
| Fine root ≤0.5 mm biomass/(t·ha−1) | 0.332 * | 0.492 * | 0.289 | 0.619 * | 0.357 | 0.094 | 0.701 ** | ||
| Fine root 0.5-1 mm biomass/(t·ha−1) | 0.583 * | 0.563 * | 0.316 | 0.597 * | 0.085 | 0.063 | 0.797 ** | ||
| Fine root 1-2 mm biomass/(t·ha−1) | 0.124 | 0.165 | 0.248 | 0.276 | 0.072 | 0.183 | 0.299 | ||
| Soil Depth (cm) | rT | rW | rT+W | rT | rW | rT+W | rT | rW | rT+W |
| Fine Root ≤0.5 mm Biomass/(t·ha−1) | Fine Root 0.5–1 mm Biomass/(t·ha−1) | Fine Root 1–2 mm Biomass/(t·ha−1) | |||||||
| 0–10 | 0.701 * | 0.831 ** | 0.749 ** | 0.685 * | 0.814 ** | 0.643 * | 0.501 * | 0.662 ** | 0.497 * |
| 10–20 | 0.521 * | 0.552 * | 0.516 * | 0.503 * | 0.101 | 0.467 * | 0.287 | 0.194 | 0.196 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Wang, N.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y. Short-Term Effects of Low Intensity Thinning on the Fine Root Dynamics of Pinus massoniana Plantations in the Three Gorges Reservoir Area, China. Forests 2017, 8, 428. https://doi.org/10.3390/f8110428
Shen Y, Wang N, Cheng R, Xiao W, Yang S, Guo Y. Short-Term Effects of Low Intensity Thinning on the Fine Root Dynamics of Pinus massoniana Plantations in the Three Gorges Reservoir Area, China. Forests. 2017; 8(11):428. https://doi.org/10.3390/f8110428
Chicago/Turabian StyleShen, Yafei, Na Wang, Ruimei Cheng, Wenfa Xiao, Shao Yang, and Yan Guo. 2017. "Short-Term Effects of Low Intensity Thinning on the Fine Root Dynamics of Pinus massoniana Plantations in the Three Gorges Reservoir Area, China" Forests 8, no. 11: 428. https://doi.org/10.3390/f8110428




