1. Introduction
Fusarium circinatum Nirenberg & O’Donnell [
1] (teleomorph
Gibberella circinata Nirenberg & O’Donnell) is the causal agent of the disease called Pine Pitch Canker (PPC), which affects up to 60 species of pines and Douglas fir (
Pseudotsuga menziesii (Mirb.) Franco) [
2]. In adult trees, the main symptoms of PPC are pitch soaked cankers on the main stem or big lateral branches [
3], which may girdle both stem and branches. Roots, shoots, flowers, cones and seeds may result infected as well. PPC is often responsible for the retarded growth of mature trees and massive mortality of saplings in forest nurseries, causing serious economic losses [
4].
Originating naturally in Central America [
5], nowadays,
F. circinatum has a worldwide distribution. Since its discovery in the USA in 1946 [
6], it has been introduced to Japan [
7], South Africa [
8], South Korea [
9], and Chile [
10]. In 2005, it was reported in Spain [
11], although it was observed in forest nurseries in the Basque country (Spain) in 1997 [
12]. After that,
F. circinatum rapidly appeared in other European countries, including France [
13], Italy [
14] and Portugal [
15], alarming the European forest authorities. Nowadays, the pathogen is mentioned in the EPPO (European and Mediterranean Plant Protection Organization) A2-list as a quarantine organism present in the area of EPPO countries but not yet widely distributed [
16]. Although apparently eradicated in Italy and France, it is still present in Portugal and Spain [
17].
Transport of infected plant material seems to be the most effective way for PPC introduction, especially for long distances [
18]. In forest stands, however, the pathogen can also spread by natural means.
F. circinatum is a seedborne pathogen that can survive both superficially and internally in seeds [
19], favouring the spread of the disease to the following pine generation. Its macro- and microconidia (asexual spores) are also spread by wind, water and insect vectors that infect trees through weather-related injuries and wounds associated with insect feeding and pruning [
20,
21,
22,
23].
Understanding the temporal and spatial spore dispersal is, thus, critical for fine tuning efficient control measures that limit the disease expansion. Such knowledge could be efficiently applied to design silvicultural guidelines aimed at minimizing the risk of infection of trees by the pathogen. As an example, Gonthier et al. [
24] suggested a shift in timing of thinning operation in Norway spruce forests according to the temporal pattern of spore dispersal of
Heterobasidion parviporum Niemelä & Korhonen. Furthermore, environmental factors determining the temporal dispersal of a pathogen play a crucial role in the estimation and modelling of future spread and extent of possible epidemics [
23,
25] and possible development of the disease under global climate changes [
23,
26,
27].
Understanding the temporal and spatial spore dispersal is, thus, critical for fine tuning efficient control measures that limit the disease expansion. Such knowledge could be efficiently applied to design silvicultural guidelines aimed at minimizing the risk of infection of trees by the pathogen. As an example, Gonthier et al. [
24] suggested a shift in timing of thinning operation in Norway spruce forests according to the temporal pattern of spore dispersal of
Heterobasidion parviporum Niemelä & Korhonen. Furthermore, environmental factors determining the temporal dispersal of a pathogen play a crucial role in the estimation and modelling of future spread and extent of possible epidemics [
23,
25] and possible development of the disease under global climate changes [
23,
26,
27].
The seasonal spore dispersal pattern of
F. circinatum has been previously investigated in northern California (USA), where more spores were detected in October than in June and July [
18]. This pattern was confirmed by the whole year sampling carried out by Garbelotto et al. [
28], who detected the highest spore presence in the same area during the cold and wet weather from November to March. In San Francisco, Garbelotto et al. [
28] showed the importance of sea fog, which can alleviate the water deficit during dry periods in summer, and enhance the fungal sporulation. Wingfield et al. [
29] emphasize, however, that the life cycle of the pathogen may largely vary among different geographical areas, host species and particular conditions of forest stands. To the best of our knowledge, studies exploring the temporal dynamics of spore dispersal in Europe are lacking.
Spatial spread of
F. circinatum spores in forest stands has only been partly investigated. Although it is not known how far the airborne spores may be dispersed [
2], it was concluded that the conidia of
F. circinatum have limited flight distance potential [
2,
23,
28]. According to Garbelotto et al. [
28], its dispersal is influenced little by the wind direction and speed. These authors did not find differences in spore occurrence 100, 200 and 300 m from the infested stand. In any case, from an epidemiological point of view, long distance transfer of the spores is probably less important due the possibly low viability of the thin walled and hyaline spores [
2].
The main aim of the present study was to investigate the seasonal spore dispersal pattern of F. circinatum, which may help to develop effective control measures of PPC in European pine forests. Particularly, the objectives of this study were (i) to describe the seasonality of the occurrence of F. circinatum spores during a one-year sampling in an infested locality in Galicia (north western Spain), (ii) to investigate the correlation between air inoculum quantity and meteorological variables and (iii) to investigate the spatial patterns of spore dispersal and the influence of the wind in the spread of the inoculum. To this end, we used an active air-sampling trapping system especially designed for fungal spore assessments, but never used before with F. circinatum. Quantification of the spores collected in these traps was done by quantitative PCR (qPCR) techniques. Two surveys were conducted: one designed for covering the within-annual variation in spore abundance, and one designed for analysing the spatial dispersal around the infested spot.
2. Material and Methods
2.1. Sampling Area
The sampling was conducted within a 40-year-old forest stand of Pinus radiata D. Don with some Pinus pinaster Aiton close to Ponte Caldelas, Galicia, Spain, 440–480 m a.s.l. (GPS coordinates of the centre: 42.376249°, −8.478177°). The pine stand covers an area of approximately 7.5 ha and it is isolated from other pine forests due to a large forest fire that occurred in 2006. Infection of F. circinatum at this stand was confirmed in 2006 by the Regional Forest Service (Xunta de Galicia). Although not formally checked, F. circinatum appeared to be infecting most of the adult pine trees within the stand. During the experiment, typical symptoms (needle wilting, brown flagging, pitching on the stems, and dieback) were apparent on many trees, especially at the edge of the stand, and particularly in its north-west end, where several trees even died during the course of the experiment.
2.2. Spore Traps
Actively rotating arm spore traps ROTTRAP 120 (Miloň Dvořák, Boršov nad Vltavou, Czech Republic) were used for all the experiments. The construction of this spore trap is based on the description of Perkins and Leighton [
30] and McCartney et al. [
31]. An electric motor rotates 2400 rpm with a 0.8 mm thick U-shaped, square section wire (
Figure 1). The vertical impactors of 50 mm in length distanced at 200 mm were covered for every sampling with a new double-sided non-woven tape (Tesa SE, Norderstadt, Germany). Covering the front side (according to the direction of rotation) of each impactor, the spore trap provided an impaction area of 80 mm
2. According to equations of Noll [
32], the spore traps sample the air at a speed of 120 L·min
−1 with almost a 100% collecting efficiency for particles bigger than 7.18 μm. Each trap was mounted 1.4 m high and powered by a 12 V/19 Ah battery.
2.3. Seasonal Dynamics Sampling
To follow the seasonal dynamics of the F. circinatum inoculum, three spots labelled as A (GPS: 42.378014°, −8.477219°), B (42.376249°, −8.478177°) and C (42.374474°, −8.479641°) were established within the 7.5 ha infested pine stand close to apparently infected trees. In each spot, one spore trap was installed at least two times per month from April 2016 until March 2017, resulting in 30 samplings. In each sampling, the spore traps were running for 48 h.
To monitor the air inoculum splashed by raindrops, each sampling with the spore trap was accompanied by the sampling of the rain water close to the soil surface. For that purpose, a 90 mm Petri dish was left open during the 48-h sampling period. The exposed tapes and 1 mL of rain water were stored in 2-mL microtubes at −20 °C before further processing. The two tapes of each spore trap were mixed and stored together in a single microtube.
2.4. Spatial Spread Sampling
For the analysis of the inoculum spatial spread, 11 spore traps were used. The spore traps were installed following a Latin cross arrangement with the elongated arm following the forecasted wind direction. Eight of the spore traps were established 50 and 500 m from the last tree of the infested forest stand in each of the four orthogonal directions. Additionally, in the direction following the wind, two more spots were established at 100 and 1000 m from the last infected tree. Finally, in the middle of the infested stand, the spot B described in the previous section, was also sampled. For each 48-h sampling period, the forecasted wind direction was previously taken into account to orient the main arm of the experimental design. The wind forecasts were obtained from (
https://www.windguru.com/) and the real values of wind speed and direction were downloaded after the sampling from the same source. Data from Pontevedra, 15 km from the sampling site and the closest continuously measuring spot at these websites, were used. Four samplings (around one per week) were carried out in September–October 2016.
2.5. Meteorological Measurement
Meteorological data were recorded every 15 min throughout the whole sampling year using an automatic climatic station (AMET, Velké Bílovice, Czech Republic) placed in the spot A. The station, established at a height of 1 m, recorded air temperature and humidity, precipitation and leaf wetness. Leaf wetness was measured as an electric resistance of a filter paper placed between two electrodes.
2.6. DNA Extraction
DNA was extracted from all the samples together with an empty microtube as a negative control of extraction. Spores were disrupted and homogenized directly in a Mixer Mill MM400 (Retsch, Haan, Germany), using a mixture of 0.4 g of 0.1 mm balotina beads and 250 μL of 0.1% Nonidet P40—substitute (AppliChem, Darmstadt, Germany). The homogenization was performed for 10 min at 30 Hz. For further processing, the DNEasy Plant Mini Kit (Qiagen, Valencia, CA, USA) was used according to the manufacturer’s instructions, except the incubation with AP1 buffer, which was prolonged to 60 min. In the last step, DNA of each sample was eluted only once with 100 μL of preheated elution buffer previously incubated for 10 min.
2.7. Quantitative PCR
Direct specific qPCR was performed using a LightCycler
® 480 Instrument II (Roche Diagnostics, Basel, Switzerland), TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and specific primers and a probe [
33] according to the manufacturer’s instructions (pre-incubation: 10 min at 95 °C followed by 45 cycles of denaturation: 10 s at 95 °C; annealing: 30 s at 60 °C; extension: 1 s at 72 °C—single acquisition mode). The reaction mixture was as follows: 0.2 μL of each primer (final concentration 400 nM), 0.2 μL of TaqMan probe (200 nM), 5 μL of TaqMan Universal PCR Master Mix, 1.9 μL of sterile deionized water and 3 μL of template DNA.
Every reaction was performed in three technical repetitions together with a positive control, negative control of isolation and also a negative control of qPCR reaction containing the master mix without template DNA.
The concentrations of F. circinatum DNA in the samples (hereafter CN) were expressed as numbers of copies of the target sequence in 1 μL of template DNA. CNs were determined using a standard curve that was generated by different concentrated aliquots of a plasmid pCR™4-TOPO® vector (Invitrogen, Carlsbad, CA, USA) with an insert of the target gene (fragments were amplified with the primers described above). All reactions were performed in LightCycler® 480 software (Roche Diagnostics).
2.8. Statistical Methods
Average CN across the three sampling spots (A, B and C) was calculated for every sampling and used for further analyses. Relationships between CN and meteorological variables (daily averages of air temperature, relative humidity, leaf wetness and precipitation) were tested. Because the analysed variables were not normally distributed even after log-transformation, only non-parametric tests were used. Spearman’s correlation was calculated between CN of each sampling and the average value of each of the meteorological variables measured 3, 7, 14, 21 and 28 days before the end of the sampling. All the statistical analyses were performed in STATISTICA 12 (StatSoft, Tulsa, OK, USA).
4. Discussion
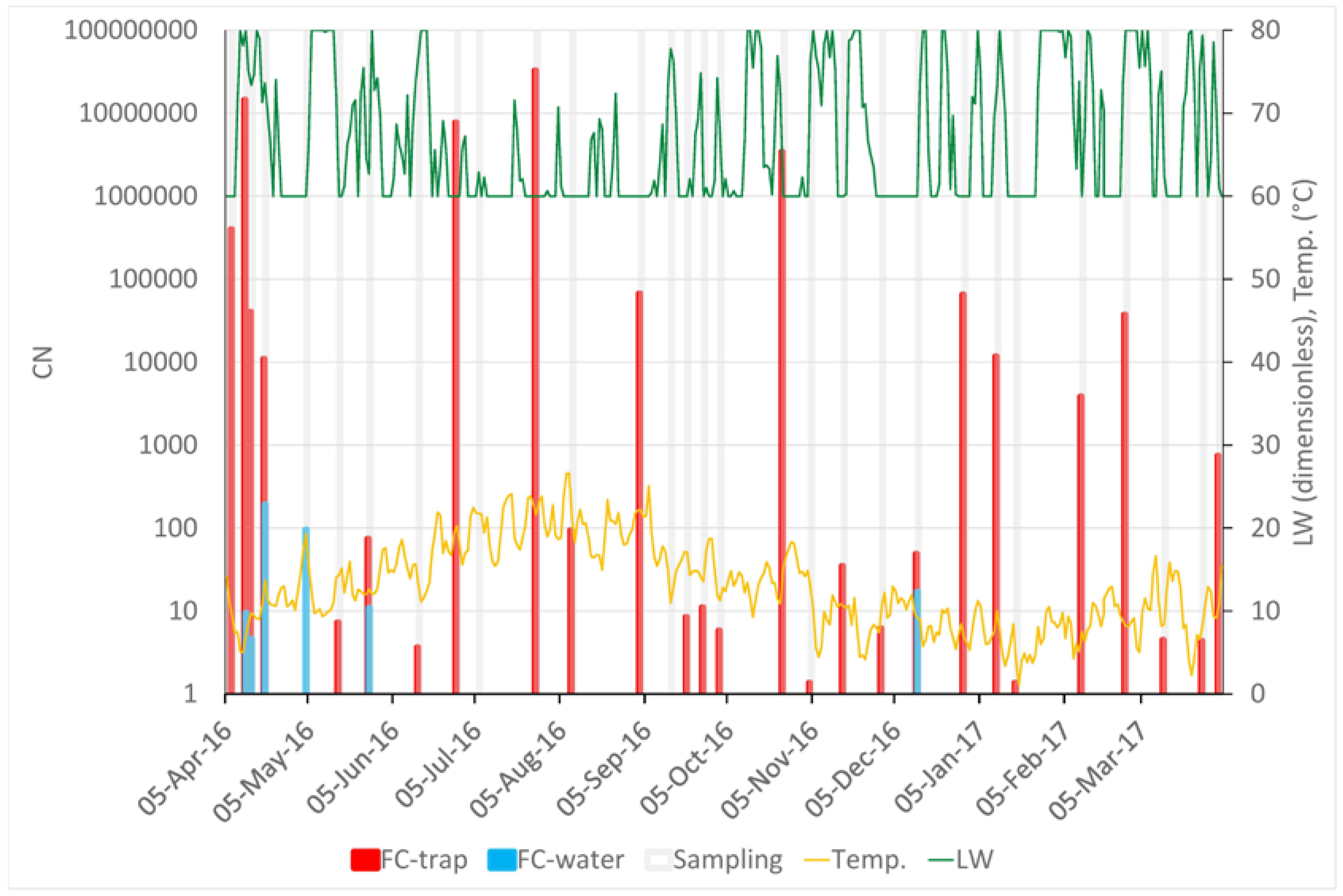
Results from the present paper indicate that F. circinatum can disperse in Galicia (NW Spain) over the whole year. Air sampling by the rotating arm spore traps detected the presence of F. circinatum spores in almost all of the 30 samplings across all seasons, but no clear temporal patterns were observed. Abundance of F. circinatum spores was, however, significantly related to the preceding weather conditions, with mean air temperature and mean leaf wetness negatively affecting the dispersal of the fungus. Spatial patterns of spore dispersal were also poorly defined, with no clear evidence of the effect of the predominating winds. Nevertheless, results presented here indicated that light breezes of just 5 m·s−1 can promote spore dispersal up to distances of at least 1000 m. Altogether, results from the present study shed light on the natural dispersal of F. circinatum from infested stands in Europe and can contribute towards fine tuning appropriate methods to prevent the spread of this important pine disease. Although the results presented here come from just a one-year sampling period, the temperature and precipitation during the course of the experiment were representative of those typically found in the area in the last twelve years. A comparison of climatic data with historical averages indicates, however, that the sampling period included a slightly colder spring and autumn, and a relatively wetter spring. However, deviations from historical averages were within the normal range of the year-to-year variation that is typically observed in the region.
4.1. Rotating Arm Spore Traps—Efficient Tool for the Detection of Fusarium circinatum
Garbelotto et al. [
28] first applied a passive air sampling system using filter paper to collect deposited spores of
F. circinatum. These authors suggested, however, the use of active air sampling spore traps to obtain more precise results in aerobiological studies of the inoculum of
F. circinatum, especially for the study of its spatial spore dispersal. Here, we used, for the first time, rotating arm spore traps that actively sampled the
F. circinatum spores, increasing the amount of air sampled per unit of time.
According to the amount of positive results from the whole year sampling shown in our study, the rotating arm spore traps combined with qPCR have been proven to be a reliable and efficient detection tool of the
F. circinatum air inoculum. This type of air sampler has been successfully used in a number of studies focused on the detection of fungal pathogens’ airborne inoculum. Recently, it has been used by Chandelier et al. [
34] and Dvořák et al. [
35] for the detection of Ash dieback pathogen, and by Choudhury et al. [
36] for the detection of powdery mildew on spinach. The use of rotating arm spore traps is often limited by their inability to impact very small particles [
37,
38]. Too small particles, being blown by the air pillow generated by the rotation of the impactor, can pass around it [
38]. Using Noll’s equation [
32], ROTTRAP 120 was calculated to be reliably efficient for particles of spherical diameter of at least 7.18 μm. Microconidia of
F. circinatum are known to measure, on average, 9.7 × 3.2 μm and macroconidia 38.2 × 3.6 μm [
1]. Therefore, part of the inoculum might not be sampled. Particularly, those microconidia facing the impactor at its narrow side might be blown around, so ROTTRAP 120 may underestimate the real amount of
F. circinatum spores. Despite this disadvantage, the spore traps were able to catch the inoculum in 90% of the whole year samplings, reaching notable concentrations of the
F. circinatum inoculum (up to CN of 10
7).
4.2. Seasonal Spore Dispersal
The constant occurrence of the inoculum of
F. circinatum (27 positive samplings out of 30) confirms that
F. circinatum persists in the area of Galicia with a notable level throughout the whole year. Similar results with the constant presence of
F. circinatum throughout the whole year were obtained in other areas such as northern California [
28] and South Africa [
39]. This finding means that
F. circinatum in Galicia can develop the infection at any time of the year whenever suitable environmental conditions and susceptible hosts are available for spore germination, as was expected from the calculation of models of spread [
26,
40].
The amount of inoculum expressed as CN varied during the year, oscillating between low and high values, but no clear temporal trends were observed. The most striking difference was noted in July, when the sampling at the beginning of July was negative and the sampling at the end of the month showed a positive result with the highest value of the year. Similar patterns were shown by Garbelotto et al. [
28] in San Francisco in July–August. However, results of the present study are not reliably comparable with those of Garbelotto et al. [
28] due to the length of the sampling time, which was two weeks and continuous in the American study and 48 h twice a month in ours.
4.3. Effect of the Meteorological Conditions
The influence of the weather on the inoculum occurrence was estimated via the correlation between the meteorological variables and the CN. The meteorological variables were included as averages across a certain amount of days before the end of each sampling.
Results indicated neither significant correlations between the CN and the preceding precipitation (3, 7, 14, 21 and 28 days before sampling), nor with the air humidity. These results disagree with those reported by Garbelotto et al. [
28], who found a positive relation between the precipitation and the trapping frequency. A possible explanation of this incongruence may be related to the contrasting spore trapping mechanisms used in the two studies. The spore traps used here sample the particles occurring directly in the air by impaction on the adhesive surface, while the passive spore traps used by Garbelotto et al. [
28] and in other research [
18,
39] sample the spores passively deposited on a filter paper. Passive deposition may be supported by rainfall, as the rain drops trap the air inoculum and deliver it onto the surface of the filter paper. Therefore, that type of air sampling may overestimate the amount of inoculum in the air during rainy periods, favouring the positive correlation between precipitation and trapping frequency. Accordingly, half of our water rain samples were positive, evidencing the ability of the raindrops to collect the spores of
F. circinatum.
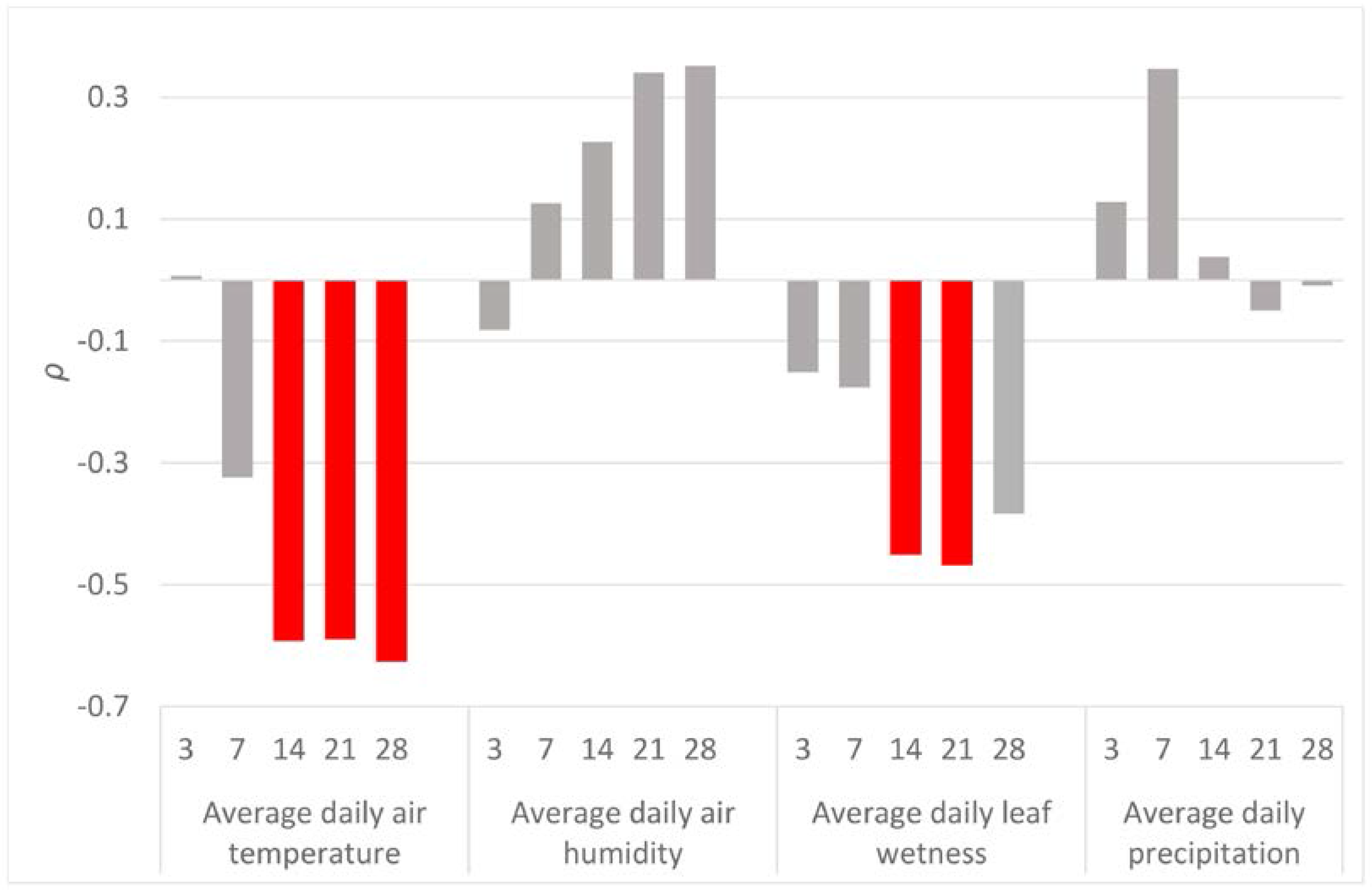
The air temperature was determined as the factor most significantly correlated with
F. circinatum spore abundance. The strongest correlation was found for the average of temperatures of the previous 28 days (
ρ = −0.63,
N = 30,
p < 0.05), although the last 14 days (
ρ = −0.59,
N = 30,
p < 0.05) and 21 days (
ρ = −0.59,
N = 30,
p < 0.05) were also significant. These negative correlation coefficients may be due to the pathogen’s demand of lower temperatures or/and the limiting effect of extremely high temperatures for developing fruiting structures (phialides and sporodochia), followed by conidial production in the next 14–28 days. The preferences of the
F. circinatum’s inoculum for lower temperatures were also pointed out by Garbelotto et al. [
28], who observed a higher amount of inoculum during the coldest period of the year from November to March. These authors also suggested that the minimum temperature of sporulation is around 0 °C. Such temperatures are rare in the region of Galicia. During our monitoring, the minimal temperature dropped below zero for only a few hours for five days. The lowest recorded temperature during the whole year was −3.4 °C, which was recorded on 19 January during one of the samplings with a positive but extremely low CN = 1.36. This value was the lowest CN detected throughout the whole year, apart from the three zero values. Therefore, our results also confirm that too low temperatures are a limiting factor of
F. circinatum sporulation. The negative influence of temperature on spore production is apparently opposite to the ability of spores to germinate. Inman et al. [
41] revealed that, at 10 °C, the germination of spores is limited to around 10%, being more than 70% at 15 °C and more than 90% at 20 °C.
Leaf wetness (LW) was calculated as the second most correlated meteorological variable with
F. circinatum spore abundance. Leaf wetness is an important variable which specifically affects the fungal growth and fructification of
F. circinatum on the plant surfaces [
35,
42,
43]. This variable was significantly correlated with precipitation (
ρ = 0.83,
N = 337,
p < 0.05) and apart from the wetness caused by rainfall, it also records the occurrence of dew, condensation from fog, etc. As occurred with the air temperature, its effect was negative and delayed two (
ρ = −0.45,
N = 30,
p < 0.05) to three (
ρ = −0.47,
N = 30,
p < 0.05) weeks before sampling. The presence of water on the host surface seems, thus, to be unfavourable for the fructification structures’ development. Again, conditions for sporulation appear to be opposite to those needed for spore germination [
44], creating a putative obstacle for the disease spread.
The correlation analysis carried out in the present study can be biased by some other disturbing factors related to the construction of the spore trap. Particularly, the impactors with the adhesive tape are not protected from the rain and, therefore, some particles that are already trapped may be washed out by rain showers. However, according to the results of the correlation analyses, this does not seem to be happening, as there was not a significant correlation between the CN and the precipitations over the past 3 days (including the duration of the sampling).
4.4. Spatial Spore Dispersal
Unfortunately, the inoculum levels in the air during the period of this survey were very low in comparison with the rest of the samplings over the whole year. Nevertheless, results allowed some conclusions to be inferred about the spatial spread of the inoculum.
Although the relationship between spore occurrence and the predominant wind was not very clear, the influence of the wind speed was apparent. Light breezes of just 5 m·s
−1 can promote spore dispersal up to distances of at least 1000 m·s
−1. In the case of such winds (three samplings out of four), the spore trap distanced at 1000 m detected amounts of inoculum similar to those at 50 m distance. A similar result was found by Garbelotto et al. [
28], who did not find significant differences between trapping frequencies 100, 200 and 300 m from the putative source of the inoculum. These authors did not confirm any differences in the inoculum levels due to the wind direction. They explained this lack of relationship by the long duration of the sampling period (two weeks per sampling), which makes the result insensitive to short-term wind direction changes. In our work, we are also unable to provide any statistical support for our observations due to the changing wind direction, the low amount of inoculum detected during the sampling periods, and the low wind speed in some of the samplings. Additionally, the geomorphology of the sampled area is very diverse, which leads to particular local air currents spreading the air inoculum, and the wind direction data and speed were downloaded for a locality 15 km from the sampled area. Results regarding the influence of wind direction and speed on
F. circinatum spore dispersal should be, thus, managed with care.