Lab and Field Warming Similarly Advance Germination Date and Limit Germination Rate for High and Low Elevation Provenances of Two Widespread Subalpine Conifers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Collection and Processing
2.2. Lab Germination Experiments
2.3. Field Experiment and Germination Observations
2.4. Statistical Analyses
3. Results
3.1. Lab Experimental Effects on Microclimate
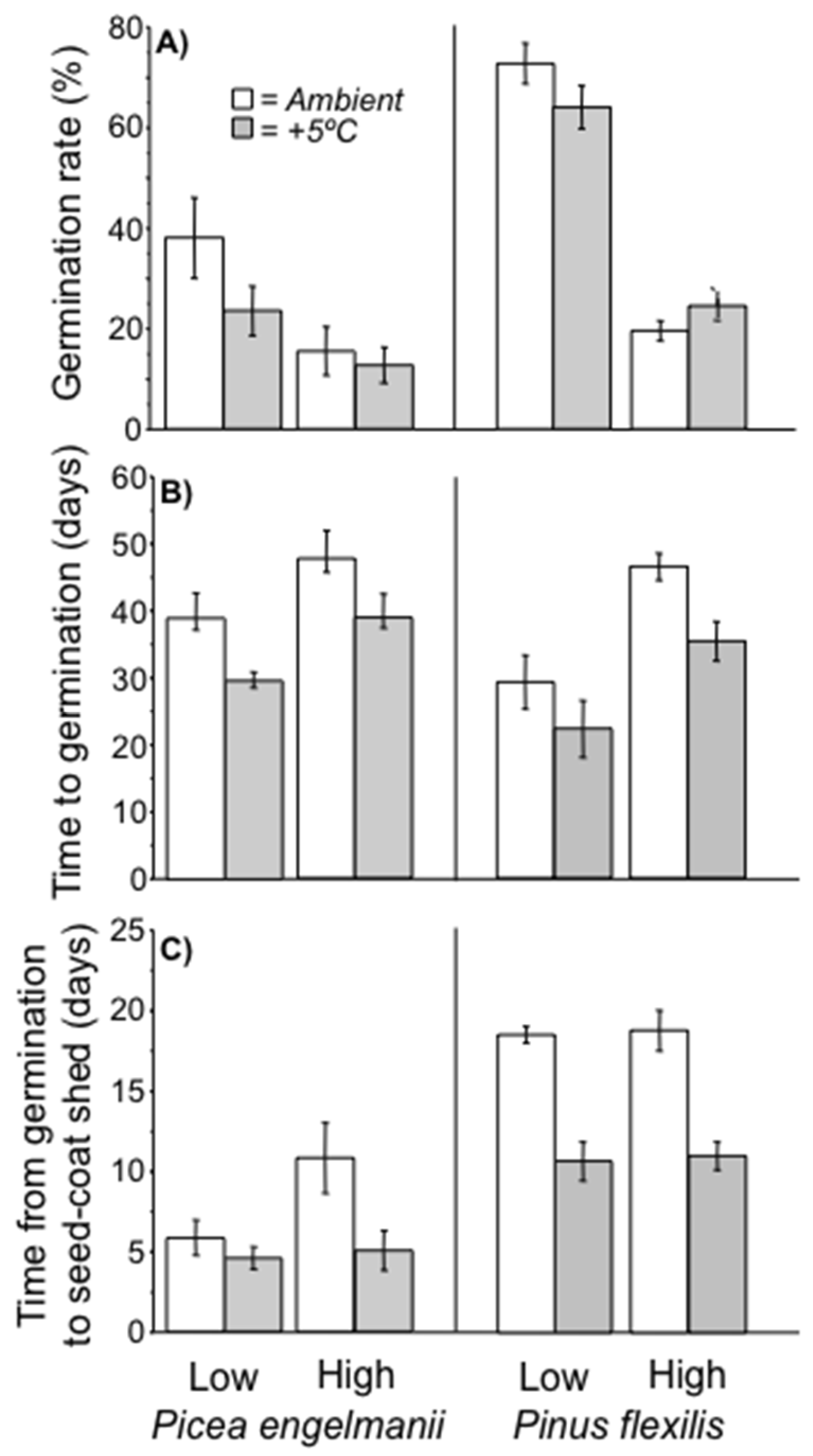
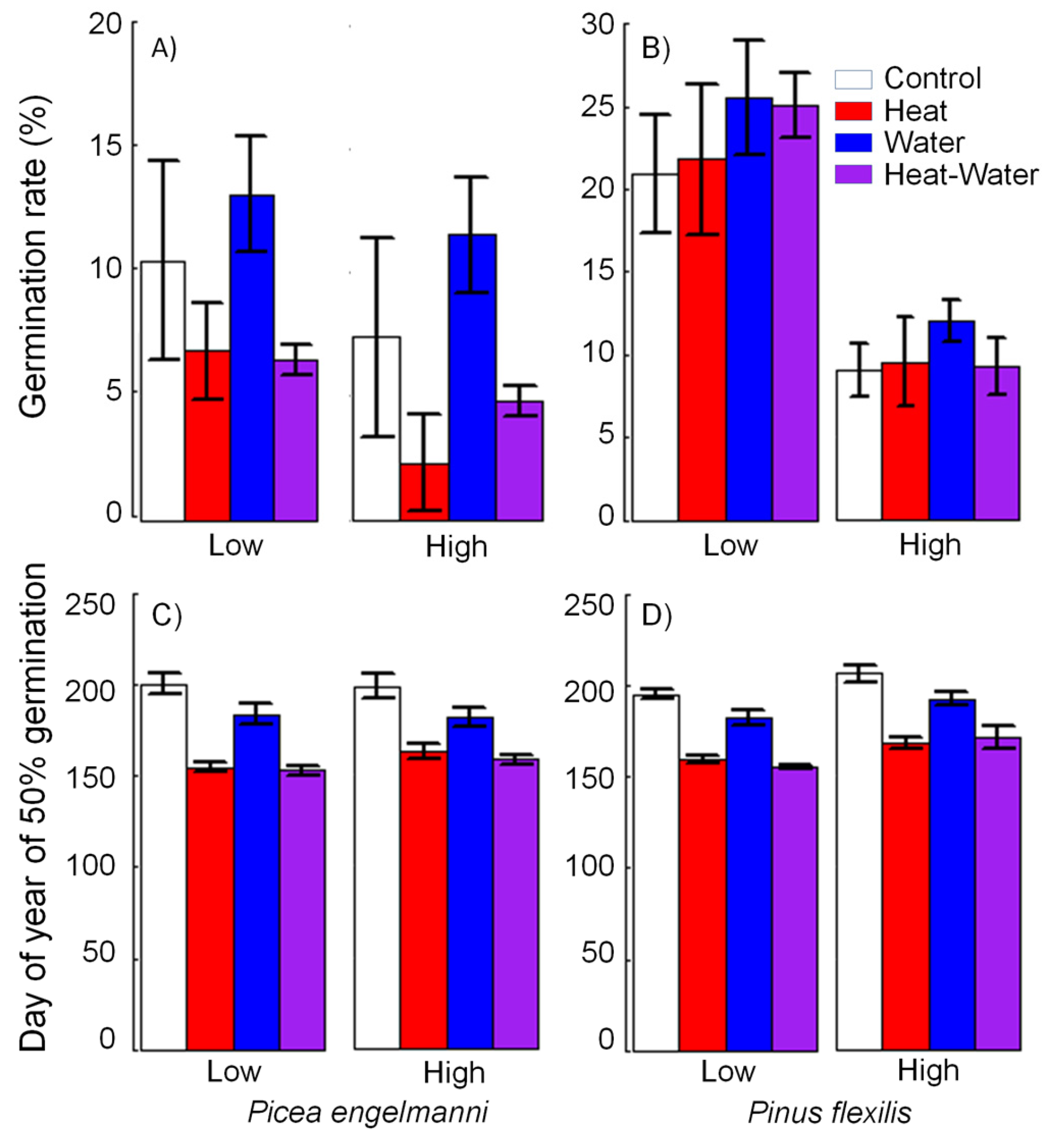
3.2. Lab Germination Percent
3.3. Germination Timing and Rate of Seedling Development
3.4. Seedling Size, Mass Allocation, and Mortality
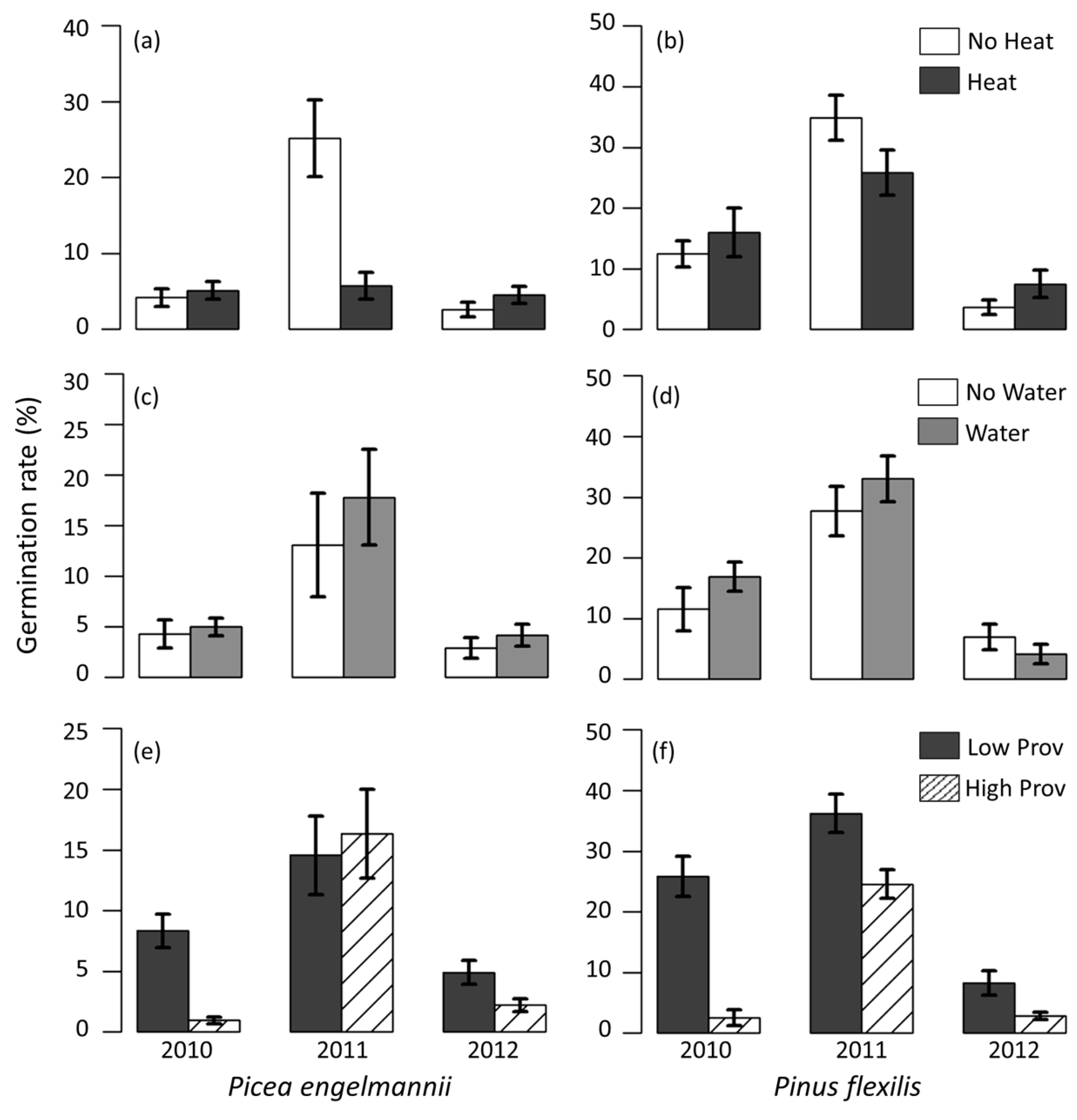
3.5. Field Germination Rate and Timing
4. Discussion
4.1. Climate Sensitivity of Germination and Initial Development
4.2. Provenance Differences in Climate Sensitivity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thuiller, W.; Albert, C.; Araujo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgely, G.F.; Paterson, J.; Schurr, F.M.; et al. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- McLaughlin, B.C.; Zavaleta, E.S. Predicting species responses to climate change: Demography and climate microrefugia in california valley oak (quercus lobata). Glob. Chang. Biol. 2012, 18, 2301–2312. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Dobrowski, S.Z.; Swanson, A.K.; Abatzoglou, J.T.; Holden, Z.A.; Safford, H.D.; Schwartz, M.K.; Gavin, D.G. Forest structure and species traits mediate projected recruitment declines in western us tree species. Glob. Ecol. Biogeogr. 2015, 24, 917–927. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.-C.; Pierrat, J.-C.; Bontemps, J.-D.; Dhôte, J.-F. Differences between tree species seedling and adult altitudinal distribution in mountain forests during the recent warm period (1986–2006). Ecography 2009, 32, 765–777. [Google Scholar] [CrossRef]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Chang. Biol. 2012, 18, 1042–1052. [Google Scholar] [CrossRef]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Grubb, P.J. Maintenance of species-richness in plant communities—Importance of regeneration niche. Biol. Rev. Camb. Philos. Soc. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Conlisk, E.; Syphard, A.D.; Franklin, J.; Flint, L.; Flint, A.; Regan, H. Uncertainty in assessing the impacts of global change with coupled dynamic species distribution and population models. Glob. Chang. Biol. 2013, 19, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.A.; Akcakaya, H.R.; Thuiller, W.; Midgley, G.F.; Pearson, R.G.; Phillips, S.J.; Regan, H.M.; Araujo, M.B.; Rebelo, T.G. Predicting extinction risks under climate change: Coupling stochastic population models with dynamic bioclimatic habitat models. Biol. Lett. 2008, 4, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.; Serra-Diaz, J.M.; Syphard, A.D.; Regan, H.M. Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Mitton, J.B. Genetics and the physiological ecology of conifers. In Ecophysiology of Coniferous Forests; Smith, W.K., Hinckley, T.M., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1995; pp. 1–36. [Google Scholar]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evolut. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, O.; Pyhajarvi, T.; Knurr, T. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Reinhardt, K.; Castanha, C.; Germino, M.J.; Kueppers, L.M. Ecophysiological variation in two provenances of pinus flexilis seedlings across an elevation gradient from forest to alpine. Tree Physiol. 2011, 31, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, G.E. Adaptation of Picea engelmannii populations to the heterogeneous environments of the intermountain west. Can. J. Bot. 1994, 72, 1197–1208. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Ying, C.C.; Spittlehouse, D.L.; Hamilton, D.A., Jr. Genetic responses to climate in Pinus contorta: Niche breadth, climate change, and reforestation. Ecol. Monogr. 1999, 69, 375–407. [Google Scholar] [CrossRef]
- St. Clair, J.B. Genetic variation in fall cold hardiness in coastal douglas-fir in western Oregon and Washington. Can. J. Bot. 2006, 84, 1110–1121. [Google Scholar] [CrossRef]
- Wu, H.X.; Ying, C.C. Geographic pattern of local optimality in natural populations of lodgepole pine. For. Ecol. Manag. 2004, 194, 177–198. [Google Scholar] [CrossRef]
- Griesbauer, H.P.; Green, D.S.; O’Neill, G.A. Using a spatiotemporal climate model to assess population-level douglas-fir growth sensitivity to climate change across large climatic gradients in British Columbia, Canada. For. Ecol. Manag. 2011, 261, 589–600. [Google Scholar] [CrossRef]
- McLane, S.C.; Daniels, L.D.; Aitken, S.N. Climate impacts on lodgepole pine (Pinus contorta) radial growth in a provenance experiment. For. Ecol. Manag. 2011, 262, 115–123. [Google Scholar] [CrossRef]
- Wang, T.; O’Neill, G.A.; Aitken, S.N. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol. Appl. 2010, 20, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Conlisk, E.; Castanha, C.; Germino, M.J.; Veblen, T.T.; Smith, J.M.; Moyes, A.B.; Kueppers, L.M. Seed origin and warming constrain lodgepole pine recruitment, slowing the pace of population range shifts. Glob. Chang. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Hamburg, S.P.; Cogbill, C.V. Historical decline of red spruce populations and climatic warming. Nature 1988, 331, 428–431. [Google Scholar] [CrossRef]
- Murphy, H.T.; VanDerWal, J.; Lovett-Doust, J. Signatures of range expansion and erosion in eastern north american trees. Ecol. Lett. 2010, 13, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Cayan, D.R.; Kammerdiener, S.A.; Dettinger, M.D.; Caprio, J.M.; Peterson, D.H. Changes in the onset of spring in the western United States. Bull. Am. Meteorol. Soc. 2001, 82, 399–415. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the northern hemisphere. Glob. Chang. Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Sacks, W.J.; Schimel, D.S.; Monson, R.K. Coupling between carbon cycling and climate in a high-elevation, subalpine forest: A model-data fusion analysis. Oecologia 2007, 151, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Barnett, T.P.; Adam, J.C.; Lettenmaier, D.P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 2005, 438, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Moyes, A.B.; Castanha, C.; Germino, M.J.; Kueppers, L.M. Warming and the dependence of limber pine (Pinus flexilis) establishment on summer soil moisture within and above its current elevation range. Oecologia 2013, 171, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, M.; Woodward, F.I. A laboratory study of the effect of temperature on red pine seed germination. For. Ecol. Manag. 1993, 62, 145–156. [Google Scholar] [CrossRef]
- Meyer, S.E.; Debaene-Gill, S.B.; Allen, P.S. Using hydrothermal time concepts to model seed germination response to temperature, dormancy loss, and priming effects in elymus elymoides. Seed Sci. Res. 2000, 10, 213–223. [Google Scholar] [CrossRef]
- Rowse, H.R.; Finch-Savage, W.E. Hydrothermal threshold models can describe the germination response of carrot (Daucus carota) and onion (Allium cepa) seed populations across both sub- and supra-optimal temperatures. New Phytol. 2003, 158, 101–108. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, W.K. Factors influencing understory seedling establishment of Engelmann spruce (Picea engelmannii) and subalpine fir (Abies lasiocarpa) in southeast Wyoming. Can. J. Bot. 1982, 60, 2753–2761. [Google Scholar] [CrossRef]
- Walder, T.; Erschbamer, B. Temperature and drought drive differences in germination responses between congeneric species along altitudinal gradients. Plant Ecol. 2015, 216, 1297–1309. [Google Scholar] [CrossRef]
- Allen, P.S.; Meyer, S.E. Ecological aspects of seed dormancy loss. Seed Sci. Res. 1998, 8, 183–192. [Google Scholar] [CrossRef]
- Green, D.S. Adaptive strategies in seedlings of three co-occurring, ecologically distinct northern coniferous tree species across an elevational gradient. Can. J. For. Res. 2005, 35, 910–917. [Google Scholar] [CrossRef]
- Flora of North America Editorial Committee. Flora of North America North of Mexico. Volume 2: Pteridophytes and Gymnosperms; Oxford University Press: New York, NY, USA, 1993; p. 475. [Google Scholar]
- Rehfeldt, G.E.; Crookston, N.L.; Warwell, M.V.; Evans, J.S. Empirical analyses of plant-climate relationships for the western United States. Int. J. Plant Sci. 2006, 167, 1123–1150. [Google Scholar] [CrossRef]
- Monahan, W.B.; Cook, T.; Melton, F.; Connor, J.; Bobowski, B. Forecasting distributional responses of limber pine to climate change at management-relevant scales in Rocky Mountain National Park. PLoS ONE 2013, 8, e83163. [Google Scholar] [CrossRef] [PubMed]
- Kueppers, L.M.; Conlisk, E.; Castanha, C.; Moyes, A.B.; Germino, M.J.; de Valpine, P.; Torn, M.S.; Mitton, J.B. Warming and provenance limit tree recruitment across and beyond the elevation range of subalpine forest. Glob. Chang. Biol. 2017, 23, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Niwot Ridge (Subalpine Forest) AmeriFlux Site Data. Available online: http://urquell.colorado.edu/data_ameriflux (accessed on 14 February 2009).
- Meromy, L.; Molotch, N.P.; Williams, M.W.; Musselman, K.N.; Kueppers, L.M. Snowpack-climate manipulation using infrared heaters in subalpine forests of the southern Rocky Mountains, USA. Agric. For. Meteorol. 2015, 203, 142–157. [Google Scholar] [CrossRef]
- Ibanez, I.; Clark, J.S.; LaDeau, S.; HilleRisLambers, J. Exploiting temporal variability to understand tree recruitment response to climate change. Ecol. Monogr. 2007, 77, 163–177. [Google Scholar] [CrossRef]
- Kaufmann, M.R.; Eckard, A.N. Water potential and temperature effects on germination of Engelmann spruce and lodgepole pine seeds. For. Sci. 1977, 23, 27–33. [Google Scholar]
- Loranger, H.; Zotz, G.; Bader, M.Y. Early establishment of trees at the alpine treeline: Idiosyncratic species responses to temperature-moisture interactions. AoB Plants 2016, 8, plw053. [Google Scholar] [CrossRef] [PubMed]
- Conlisk, E.; Castanha, C.; Germino, M.J.; Veblen, T.T.; Smith, J.M.; Kueppers, L.M. Declines in low-elevation subalpine tree populations outpace growth in high-elevation populations with warming. J. Ecol. 2017, 105, 1347–1357. [Google Scholar] [CrossRef]
- Bykova, O.; Chuine, I.; Morin, X.; Higgins, S.I. Temperature dependence of the reproduction niche and its relevance for plant species distributions. J. Biogeogr. 2012. [Google Scholar] [CrossRef]
| Temperature Treatment | Moisture Treatment | |
|---|---|---|
| 0.5 FC | FC | |
| Experiment 1 | ||
| AMB | 0.20 | 0.24 |
| +5C | 0.21 | 0.27 |
| Experiment 2 | ||
| AMB | 0.09 | 0.19 |
| +5C | 0.10 | 0.26 |
| Measure | Factor | Coef. | df | SS | F | p | |
|---|---|---|---|---|---|---|---|
| Picea engelmannii | Germination Rate | Elevation (Low) | 0.595 | 1 | 13.667 | 32.81 | <0.0001 |
| Experiment | −0.903 | 1 | 20.393 | 48.96 | <0.0001 | ||
| Temperature Treatment | −0.358 | 1 | 4.567 | 10.97 | 0.0024 | ||
| H2O content | 7.326 | 1 | 5.512 | 13.23 | 0.0010 | ||
| Elevation * Temp. Treatment | −0.043 | 1 | 0.063 | 0.15 | 0.6991 | ||
| Elevation * H2O content | 2.210 | 1 | 0.782 | 1.88 | 0.1805 | ||
| Temp. Treatment * H2O content | 5.724 | 1 | 4.859 | 11.67 | 0.0018 | ||
| Time to Germination | Elevation (Low) | −0.047 | 1 | 0.087 | 21.38 | <0.0001 | |
| Experiment | 0.088 | 1 | 0.191 | 46.98 | <0.0001 | ||
| Temperature Treatment | −0.052 | 1 | 0.096 | 23.59 | <0.0001 | ||
| H2O content | 0.231 | 1 | 0.005 | 1.35 | 0.2548 | ||
| Elevation * Temp. Treatment | −0.021 | 1 | 0.015 | 3.67 | 0.0646 | ||
| Elevation * H2O content | −0.120 | 1 | 0.002 | 0.56 | 0.4576 | ||
| Temp. Treatment * H2O content | −0.230 | 1 | 0.008 | 1.92 | 0.1750 | ||
| Time from Germination to Seed Coat Shed | Elevation (Low) | −0.057 | 1 | 0.121 | 5.45 | 0.0264 | |
| Experiment | 0.199 | 1 | 0.995 | 44.83 | <0.0001 | ||
| Temperature Treatment | −0.115 | 1 | 0.456 | 20.54 | <0.0001 | ||
| H2O content | 0.629 | 1 | 0.040 | 1.79 | 0.1913 | ||
| Elevation * Temp. Treatment | −0.004 | 1 | 0.001 | 0.03 | 0.8675 | ||
| Elevation * H2O content | 0.324 | 1 | 0.016 | 0.71 | 0.4069 | ||
| Temp. Treatment * H2O content | −0.464 | 1 | 0.030 | 1.36 | 0.2520 | ||
| Pinus flexilis | Germination Rate | Elevation (Low) | 1.091 | 1 | 45.373 | 181.39 | <0.0001 |
| Experiment | 0.008 | 1 | 0.002 | 0.01 | 0.9346 | ||
| Temperature Treatment | −0.068 | 1 | 0.164 | 0.65 | 0.4246 | ||
| H2O content | 0.570 | 1 | 0.032 | 0.13 | 0.7218 | ||
| Elevation * Temp. Treatment | −0.167 | 1 | 0.959 | 3.83 | 0.0592 | ||
| Elevation * H2O content | −2.905 | 1 | 1.290 | 5.16 | 0.0303 | ||
| Temp. Treatment * H2O content | 2.794 | 1 | 1.127 | 4.51 | 0.0419 | ||
| Time to Germination | Elevation (Low) | −0.100 | 1 | 0.381 | 118.71 | <0.0001 | |
| Experiment | −0.004 | 1 | 0.000 | 0.13 | 0.7184 | ||
| Temperature Treatment | −0.066 | 1 | 0.153 | 47.59 | <0.0001 | ||
| H2O content | 0.472 | 1 | 0.022 | 6.91 | 0.0132 | ||
| Elevation * Temp. Treatment | −0.005 | 1 | 0.001 | 0.26 | 0.6086 | ||
| Elevation * H2O content | 0.264 | 1 | 0.011 | 3.32 | 0.0780 | ||
| Temp. Treatment * H2O content | −0.255 | 1 | 0.009 | 2.93 | 0.0969 | ||
| Time from Germination to Seed Coat Shed | Elevation (Low) | 0.013 | 1 | 0.006 | 1.09 | 0.3048 | |
| Experiment | 0.012 | 1 | 0.003 | 0.59 | 0.4486 | ||
| Temperature Treatment | −0.109 | 1 | 0.382 | 66.77 | <0.0001 | ||
| H2O content | 0.160 | 1 | 0.002 | 0.42 | 0.5207 | ||
| Elevation * Temp. Treatment | 0.013 | 1 | 0.005 | 0.86 | 0.3610 | ||
| Elevation * H2O content | −0.391 | 1 | 0.020 | 3.50 | 0.0712 | ||
| Temp. Treatment * H2O content | −0.056 | 1 | 0.000 | 0.07 | 0.7942 |
| Picea engelmannii | Pinus flexilis | |||||||
|---|---|---|---|---|---|---|---|---|
| Coef. | SE | LRT (df) | P(χ2) | Coef. | SE | LRT (df) | P(χ2) | |
| Intercept | −3.48 | 0.19 | −2.44 | 0.11 | ||||
| Heat | −0.08 | 0.19 | 0.17 (1) | 0.68 | 0.11 | 0.11 | 1.01 (1) | 0.31 |
| Water | 0.43 | 0.19 | 4.82 (1) | 0.028 | 0.07 | 0.11 | 0.36 (1) | 0.55 |
| Provenance (Low) | 0.53 | 0.09 | 29.09 (1) | <10−5 | 0.82 | 0.08 | 67.11 (1) | <10−5 |
| Cohort | 60.02 (2) | <10−5 | 102.83 (2) | <10−5 | ||||
| 2010 | −0.59 | 0.13 | −0.46 | 0.12 | ||||
| 2011 | 1.12 | 0.12 | 1.50 | 0.12 | ||||
| 2012 | −0.53 | 0.13 | −1.04 | 0.12 | ||||
| Heat * Water | −0.12 | 0.19 | 0.38 (1) | 0.54 | −0.13 | 0.11 | 1.27 (1) | 0.26 |
| Heat * Prov (Low) | 0.03 | 0.09 | 0.08 (1) | 0.78 | −0.05 | 0.08 | 0.33 (1) | 0.57 |
| Water * Prov (Low) | −0.08 | 0.09 | 0.82 (1) | 0.36 | −0.01 | 0.08 | 0.02 (1) | 0.89 |
| Heat * Cohort | 44.11 (2) | <10−5 | 10.09 (2) | 0.0064 | ||||
| 2010 | 0.44 | 0.13 | 0.15 | 0.12 | ||||
| 2011 | −0.93 | 0.12 | −0.37 | 0.11 | ||||
| 2012 | 0.49 | 0.13 | 0.22 | 0.12 | ||||
| Water * Cohort | 0.07 (2) | 0.97 | 5.70 (2) | 0.058 | ||||
| 2010 | 0.03 | 0.13 | 0.18 | 0.12 | ||||
| 2011 | −0.02 | 0.12 | 0.11 | 0.11 | ||||
| 2012 | −0.01 | 0.13 | −0.29 | 0.12 | ||||
| Prov (Low) * Cohort | 26.52 (2) | <10−5 | 42.25 (2) | <10−5 | ||||
| 2010 | 0.66 | 0.13 | 0.84 | 0.12 | ||||
| 2011 | −0.56 | 0.12 | −0.50 | 0.11 | ||||
| 2012 | −0.09 | 0.13 | −0.34 | 0.12 | ||||
| Picea engelmannii | Pinus flexilis | |||||||
|---|---|---|---|---|---|---|---|---|
| Coef. | SE | LRT (df) | P(χ2) | Coef. | SE | LRT (df) | P(χ2) | |
| Intercept | 173.97 | 2.15 | 178.45 | 1.35 | ||||
| Heat | −17.08 | 2.15 | 31.56 (1) | <10−5 | −16.38 | 1.35 | 46.11 (1) | <10−5 |
| Water | −5.48 | 2.14 | 6.80 (1) | 0.009 | −4.09 | 1.35 | 9.12 (1) | 0.003 |
| Provenance (Low) | −0.04 | 1.16 | 0.00 (1) | 0.97 | −5.04 | 1.16 | 19.64 (1) | <10−5 |
| Cohort | 69.79 (2) | <10−5 | 64.14 (2) | <10−5 | ||||
| 2010 | −3.66 | 1.70 | −0.47 | 1.67 | ||||
| 2011 | 15.00 | 1.59 | 12.66 | 1.61 | ||||
| 2012 | −11.34 | 1.69 | −12.19 | 1.65 | ||||
| Heat *Water | 3.37 | 2.14 | 2.88 (1) | 0.09 | 2.69 | 1.35 | 4.46 (1) | 0.035 |
| Heat * Prov (Low) | −1.59 | 1.15 | 2.20 (1) | 0.14 | 0.70 | 1.16 | 0.43 (1) | 0.51 |
| Water * Prov (Low) | 0.10 | 1.15 | 0.01 (1) | 0.93 | −0.27 | 1.16 | 0.06 (1) | 0.81 |
| Heat *Cohort | 44.11 (2) | <10−5 | 41.51 (2) | <10−5 | ||||
| 2010 | −4.46 | 1.68 | −1.40 | 1.66 | ||||
| 2011 | 10.64 | 1.59 | 10.07 | 1.61 | ||||
| 2012 | −6.19 | 1.67 | −8.66 | 1.65 | ||||
| Water * Cohort | 15.27 (2) | 0.0005 | 18.76 (2) | <10−5 | ||||
| 2010 | 2.98 | 1.68 | 3.90 | 1.67 | ||||
| 2011 | 3.41 | 1.59 | 3.14 | 1.62 | ||||
| 2012 | −6.39 | 1.67 | −7.04 | 1.65 | ||||
| Prov (Low) * Cohort | 0.11 (2) | 0.95 | 8.17 (2) | 0.017 | ||||
| 2010 | 0.12 | 1.65 | 1.70 | 1.67 | ||||
| 2011 | 0.38 | 1.57 | 2.73 | 1.61 | ||||
| 2012 | −0.49 | 1.57 | −4.42 | 1.65 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kueppers, L.M.; Faist, A.; Ferrenberg, S.; Castanha, C.; Conlisk, E.; Wolf, J. Lab and Field Warming Similarly Advance Germination Date and Limit Germination Rate for High and Low Elevation Provenances of Two Widespread Subalpine Conifers. Forests 2017, 8, 433. https://doi.org/10.3390/f8110433
Kueppers LM, Faist A, Ferrenberg S, Castanha C, Conlisk E, Wolf J. Lab and Field Warming Similarly Advance Germination Date and Limit Germination Rate for High and Low Elevation Provenances of Two Widespread Subalpine Conifers. Forests. 2017; 8(11):433. https://doi.org/10.3390/f8110433
Chicago/Turabian StyleKueppers, Lara M., Akasha Faist, Scott Ferrenberg, Cristina Castanha, Erin Conlisk, and Jennifer Wolf. 2017. "Lab and Field Warming Similarly Advance Germination Date and Limit Germination Rate for High and Low Elevation Provenances of Two Widespread Subalpine Conifers" Forests 8, no. 11: 433. https://doi.org/10.3390/f8110433





