Genetic Diversity and Its Spatial Distribution in Self-Regenerating Norway Spruce and Scots Pine Stands
Abstract
:1. Introduction
2. Materials and Methods
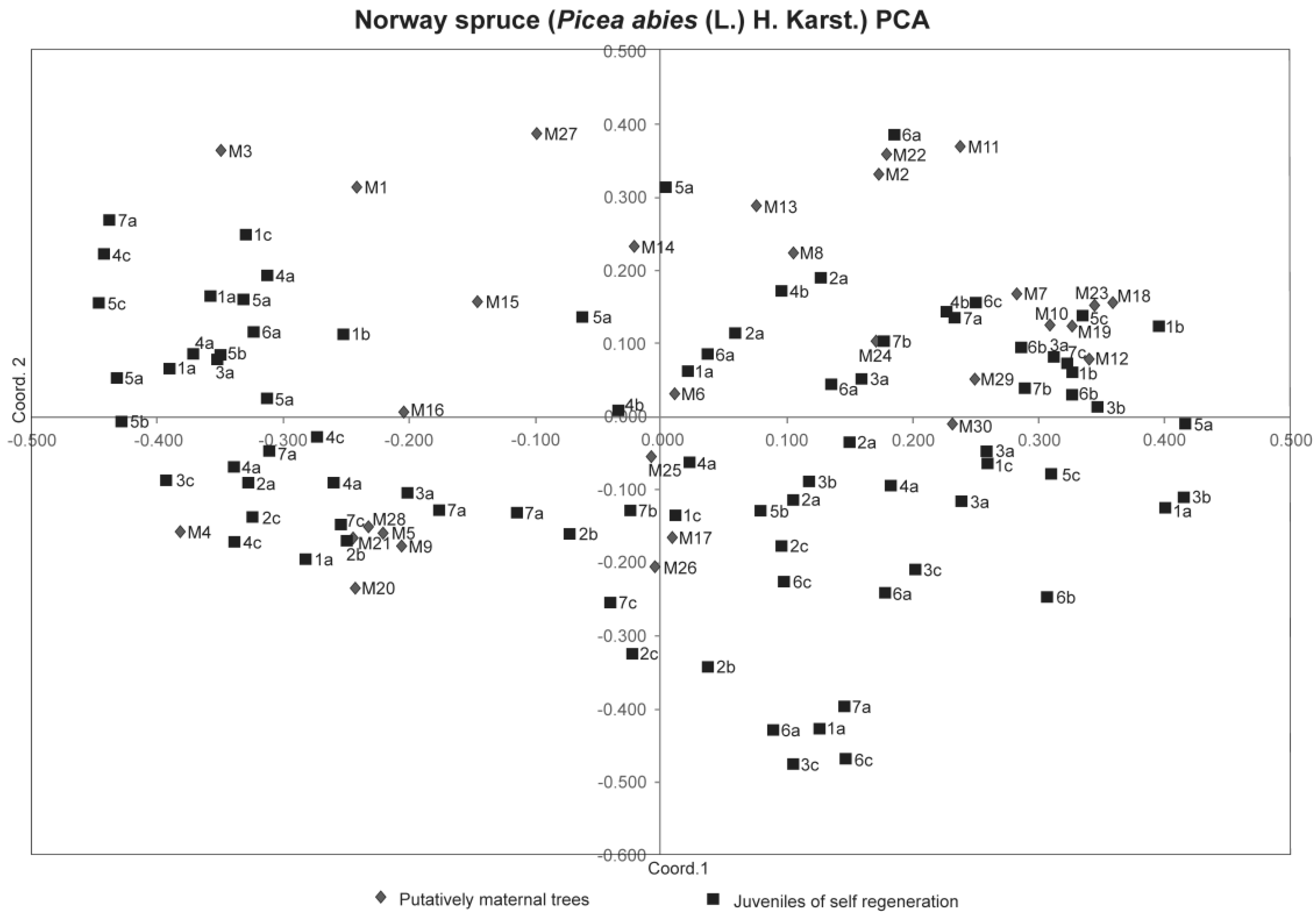
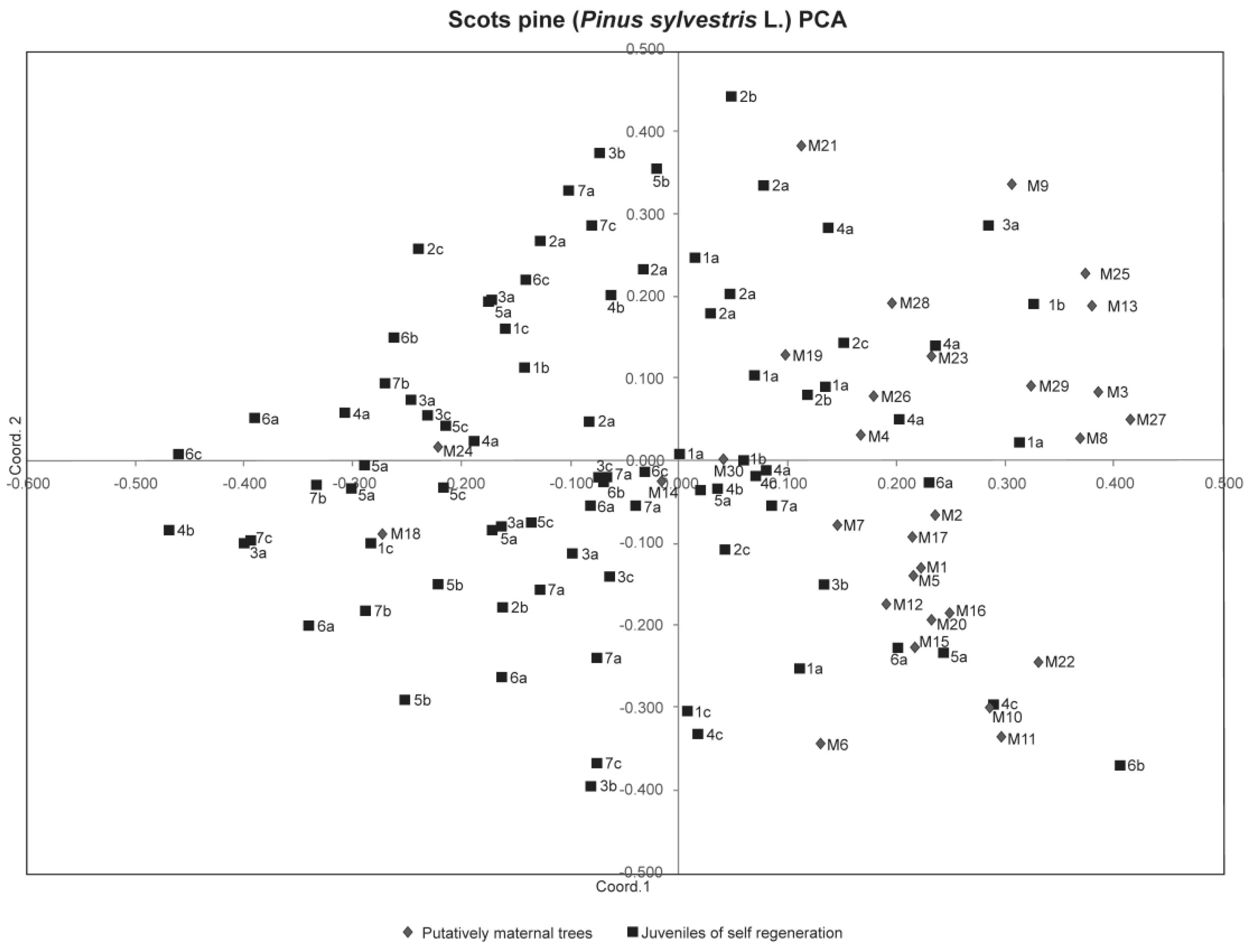
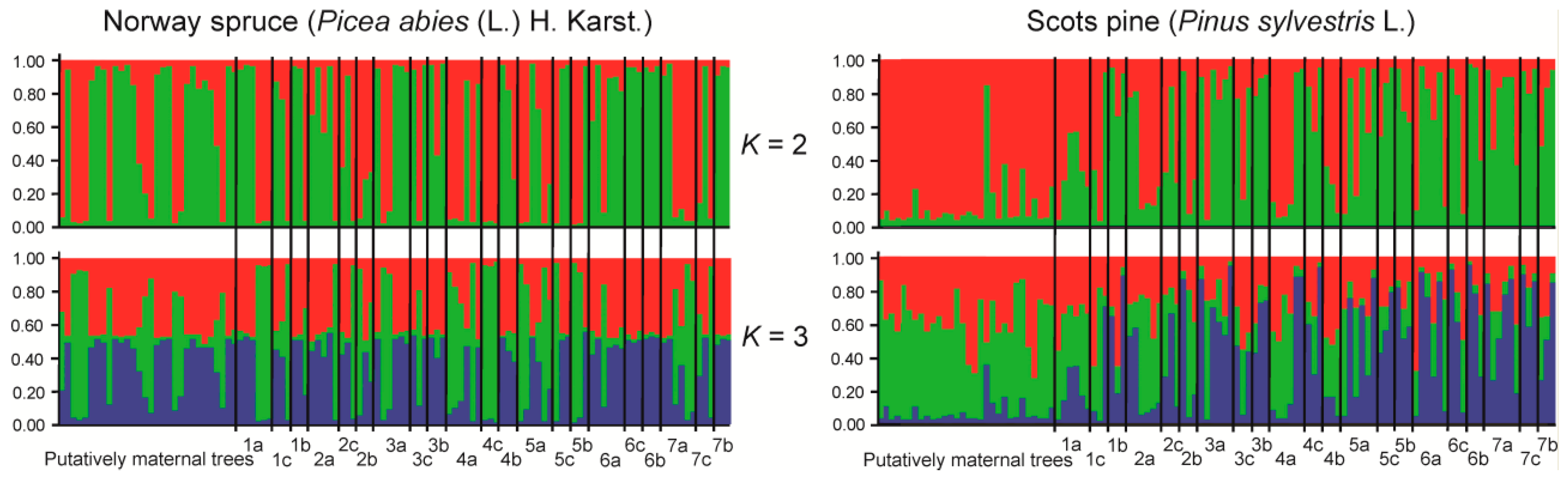
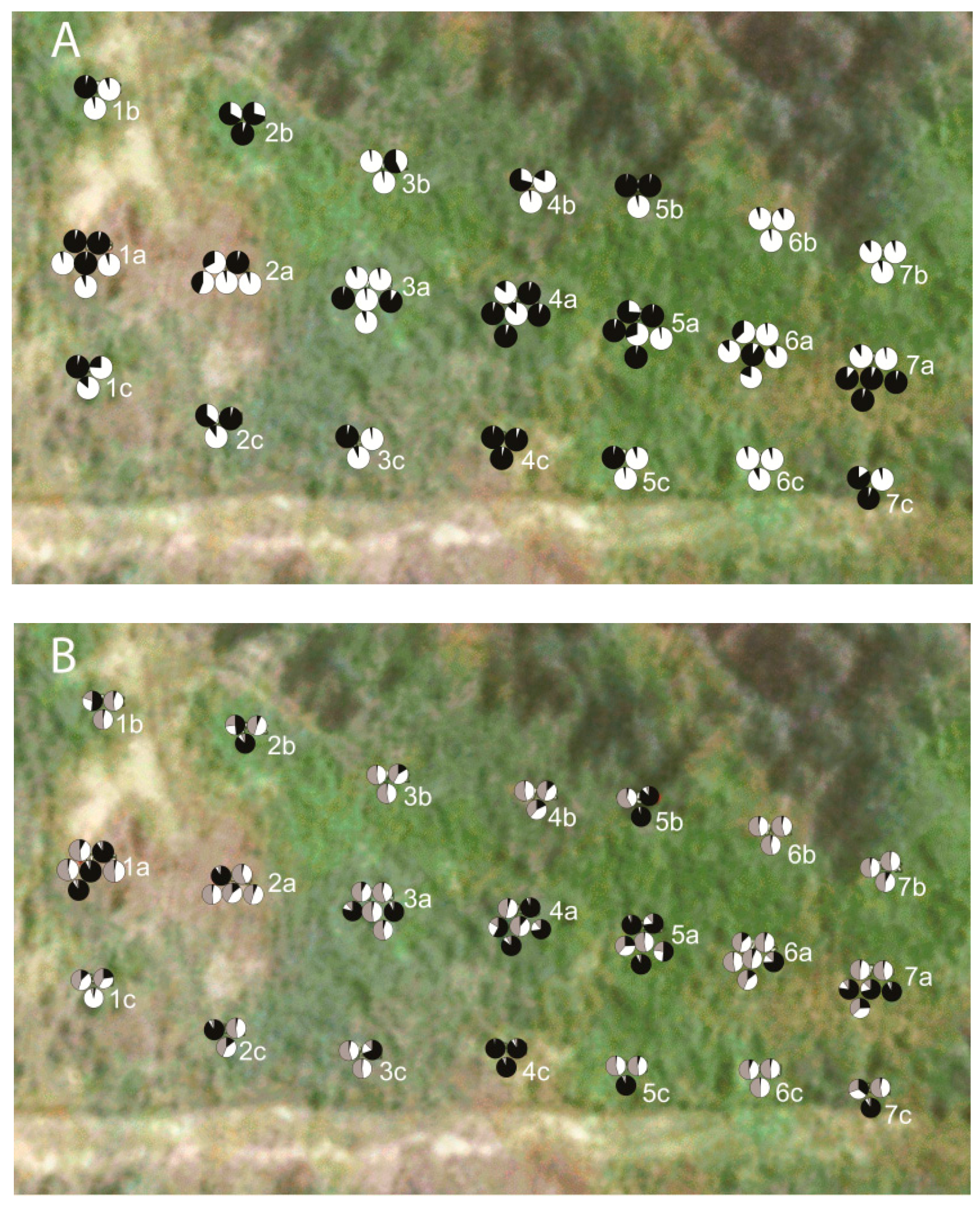
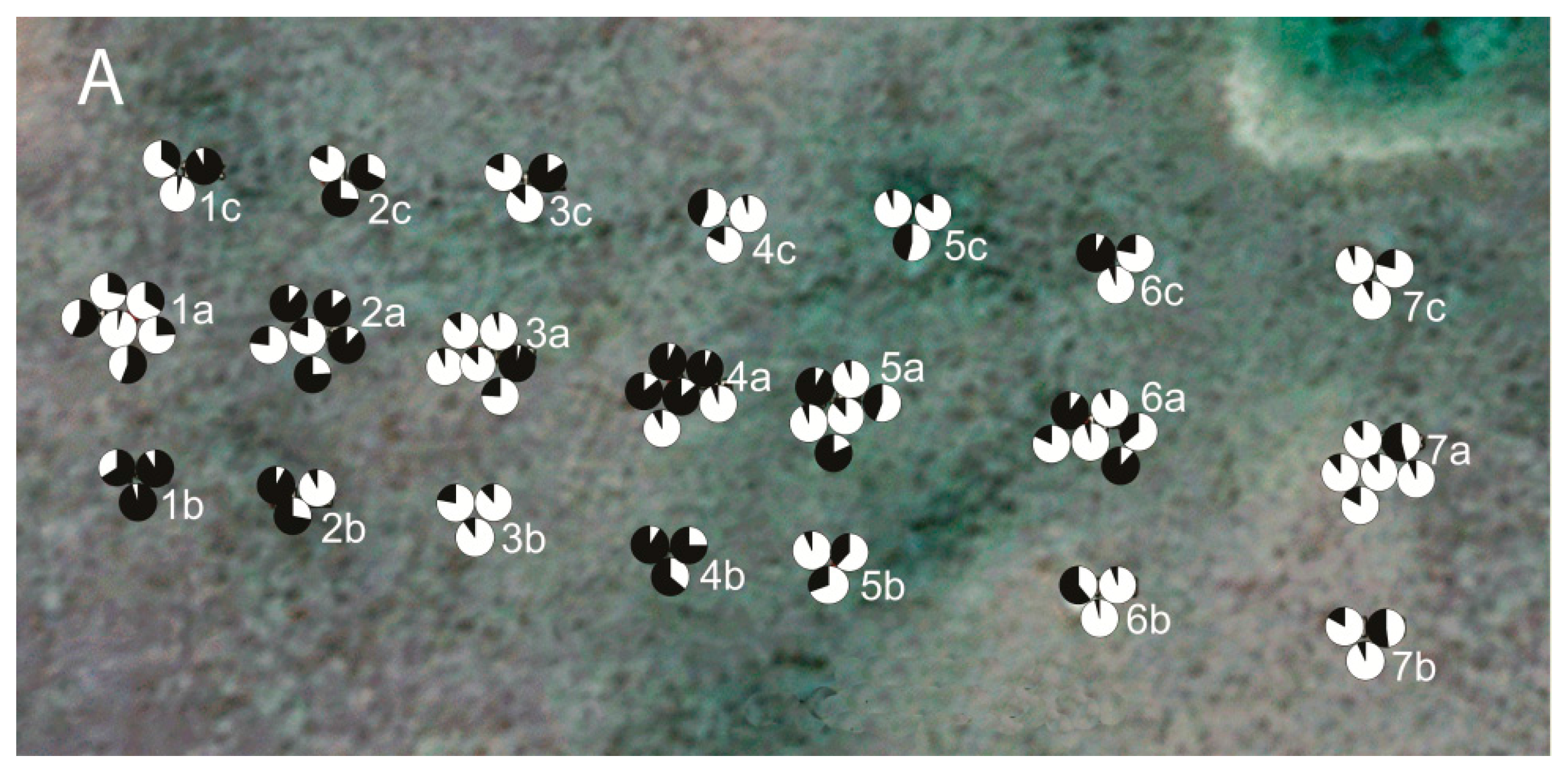
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rajendra, K.C.; Seifert, S.; Prinz, K.; Gailing, O.; Finkeldey, R. Subtle human impacts on neutral genetic diversity and spatial patterns of genetic variation in European beech (Fagus sylvatica). For. Ecol. Manag. 2014, 319, 138–149. [Google Scholar] [CrossRef]
- Savolainen, O.; Pyhajarvi, T. Genomic diversity in forest trees. Curr. Opin. Plant Biol. 2007, 10, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wójkiewicz, B.; Litkowiec, M.; Wachowiak, W. Contrasting patterns of genetic variation in core and peripheral populations of highly outcrossing and wind pollinated forest tree species. AoB Plants 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Perini, D.; Binelli, G. The Distribution of Genetic Variation in Norway Spruce (Picea abies Karst.) Populations in the Western Alps. J. Biogeogr. 2007, 34, 929–938. [Google Scholar] [CrossRef]
- Geburek, T.; Mottinger-Kroupa, S.; Morgante, M.; Burg, K. Genetic variation of Norway spruce (Picea abies (L.) Karst.) populations in Austria. II. Micro spatial patterns derived from nuclear sequence tagged microsatellite sites. For. Genet. 1998, 5, 231–237. [Google Scholar]
- Ratnam, W.; Rajora, O.P.; Finkeldey, R.; Aravanopoulos, F.; Bouvet, J.M.; Vaillancourtf, R.E.; Kanashiro, M.; Fady, B.; Tomita, M.; Vinson, C. Genetic effects of forest management practices: Global synthesis and perspectives. For. Ecol. Manag. 2014, 333, 52–65. [Google Scholar] [CrossRef]
- Namkoong, G. Biodiversity issues in genetics, forestry and ethics. For. Chron. 1992, 68, 438–443. [Google Scholar] [CrossRef]
- FOREST EUROPE. State of Europe’s Forests, 2015. Publications Ministerial Conference on the Protection of Forests in Europe (MCPFE), FOREST EUROPE Liaison Unit Madrid. 2015. Available online: http://foresteurope.org/state-europes-forests-2015-report/ (accessed on 10 November 2017).
- FORGER. Project FORGER–Towards the Sustainable Management of Forest Genetic Resources in Europe. 2015. Available online: http://www.fp7-forger.eu/ (accessed on 12 November 2017).
- Oddou-Muratorio, S.; Klein, E.K.; Demesure-Musch, B. Real-time patterns of pollen flow in the wild-service tree, Sorbus torminalis (Rosaceae). III. Mating patterns and the ecological maternal neighborhood. Am. J. Bot. 2006, 93, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Arnuncio, J.J.; Austerlitz, F. Pollen dispersal in spatially aggregated populations. Am. Nat. 2006, 168, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Restoux, G.; Silva, D.E.; Sagnard, F.; Torre, F.; Klein, E.; Fady, B. Life at the margin: The mating system of Mediterranean conifers. Web Ecol. 2008, 8, 94–102. [Google Scholar] [CrossRef]
- Sagnard, F.; Oddou-Muratorio, S.; Pichot, C.; Vendramin, G.G.; Fady, B. Effect of seed dispersal, adult tree and seedling density on the spatial genetic structure of regeneration at fine temporal and spatial scales. Tree Genet. Genomes 2011, 7, 37–48. [Google Scholar] [CrossRef]
- Vekemans, X.; Hardy, O.J. New insights from fine-scale spatial genetic structure analyses in plant populations. Mol. Ecol. 2004, 13, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Leimu, R.; Mutikainen, P.; Koricheva, J.; Fisher, M. How general are positive relationships between plant population size, fitness and genetic variation? J. Ecol. 2006, 94, 942–952. [Google Scholar] [CrossRef]
- Chomicz-Zegar, E.; Nowakowska, J.A.; Tereb, A. Forest decline has not reduced genetic diversity of naturally regenerated Norway spruce from the Beskids, Poland. Silvae Genet. 2015, 64–5/6, 270–278. [Google Scholar] [CrossRef]
- Buiteveld, J.; Vendramin, G.G.; Leonardi, S.; Kamer, K.; Geburek, T. Genetic diversity and differentiation in European beech (Fagus sylvatica L.) stands varying in management history. For. Ecol. Manag. 2007, 247, 98–106. [Google Scholar] [CrossRef]
- Eriksson, G.; Ekberg, I.; Clapham, D. Genetics Applied to Forestry: An Introduction, 3rd ed.; Elanders Sverige AB: Uppsala, Sweden, 2013; p. 206. [Google Scholar]
- Kosinska, J.; Lewandowski, A.; Chalupka, W. Genetic variability of Scots pine maternal populations and their progenies. Silva Fenn. 2007, 41, 5–12. [Google Scholar] [CrossRef]
- Dering, M.; Chybicki, I. Assessment of genetic diversity in two-species oak seed and their progeny populations. Scan. J. For. Res. 2012, 27, 2–9. [Google Scholar] [CrossRef]
- Müller-Starck, G. Protection of genetic variability in forest trees. For. Genet. 1995, 2, 121–124. Available online: https://stary.tuzvo.sk/files/fg/volumes/1995/FG02-3_121-124.pdf (accessed on 29 November 2017).
- Hu, X.S.; Li, B. Linking evolutionary quantitative genetics to the conservation of genetic resources in natural forest populations. Silvae Genet. 2001, 51, 177–183. [Google Scholar]
- Aravanopoulos, F.A.; Tollefsrud, M.M.; Graudal, L.; Koskela, J.; Kätzel, R.; Soto, A.; Nagy, L.; Pilipovic, A.; Zhelev, P.; Božic, G.; et al. Development of Genetic Monitoring Methods for Genetic Conservation Units of Forest Trees in Europe; Publisher: Rome, Italy, 2015; ISBN 978-92-9255-030-1. [Google Scholar]
- CONGRESS. Conservation Genetic Resources for Effective Species Survival. 2012. Available online: www.congressgenetics.eu (accessed on 10 September 2017).
- Kendall, K.; Stetz, J.; Vojta, C.; Macleod, A. Genetic Monitoring for Managers. U.S. Fish and Wildlife Service. Available online: http://alaska.fws.gov/gem/mainPage_1.htm (accessed on 10 September 2017).
- Fussi, B.; Westergren, M.; Aravanopoulos, A.; Baier, R.; Kavaliauskas, D.; Finzgar, D.; Alizoti, P.; Bozic, G.; Avramidou, E.; Konnert, M.; et al. Forest genetic monitoring: An overview of concepts and definitions. Environ. Monit. Assess 2016, 188, 493. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, G.; Boyle, T.; El-Kassaby, Y.A.; Palmberg-Lerche, C.; Eriksson, G.; Gregorius, H.R.; Joly, H.; Kremer, A.; Savolainen, O.; Wickneswari, R.; et al. Criteria and Indicators for Sustainable Forest Management: Assessment and Monitoring of Genetic Variation; FAO: Rome, Italy, 2002. [Google Scholar]
- Aravanopoulos, F.A. Genetic monitoring in natural perennial plant populations. Botany 2011, 89, 75–81. [Google Scholar] [CrossRef]
- Konnert, M.; Maurer, W.; Degen, B.; Kätzel, R. Genetic monitoring in forests-early warning and controlling system for ecosystemic changes. iForest 2010, 4, 77–81. [Google Scholar] [CrossRef]
- Yazdani, R.; Muona, O.; Rudin, D.; Szmidt, A.E. Genetic structure of Pinus sylvestris seed tree stand and naturally regenerated understory. For. Sci. 1985, 31, 430–436. [Google Scholar]
- Nowakowska, A.N.; Zachara, T.; Konecka, A. Genetic variability of Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies L. Karst.) natural regeneration compared with their maternal stands. Lesne Prace Badawcze (For. Res. Pap.) 2014, 75, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Kashi, Y.; Soller, M. Functional roles of microsatellites and minisatellites. In Microsatellite Evolution and Application; Goldstein, D.D., Schlotterer, C., Eds.; Oxford University Press: Oxford, UK, 1998; pp. 10–23. [Google Scholar]
- Dering, M.; Misiorny, A.; Chalupka, W. Inter-year variation in selfing, background pollination, and paternal contribution in a Norway spruce clonal seed orchard. Can. J. For. Res. 2014, 44, 760–767. [Google Scholar] [CrossRef]
- Funda, T.; Wennström, U.; Almqvist, C.; Torimaru, T.; Gull, B.; Wang, X. Low rates of pollen contamination in a scots pine seed orchard in Sweden: The exception or the norm? Scand. J. For. Res. 2015, 30, 573–586. [Google Scholar] [CrossRef]
- Wojnicka-Półtorak, A.; Wachowiak, W.; Prus-Głowacki, W.; Celiński, K.; Korczyk, A. Genetic heterogeneity in age classes of naturally regenerated old growth forest of Picea abies (L.) Karst. Silvae Genet. 2014, 63, 185–190. [Google Scholar] [CrossRef]
- Wojnicka-Półtorak, A.; Celiński, K.; Chudzińska, E. Temporal dynamics in the genetic structure of a natural population of Picea abies. Biologia 2016, 71, 875–884. [Google Scholar] [CrossRef]
- Wojnicka-Półtorak, A.; Celiński, K.; Chudzińska, E. Genetic diversity among age classes of a Pinus sylvestris (L.) population from Białowieża primeval forest, Poland. Forests 2017, 8, 227. [Google Scholar] [CrossRef]
- Butkus, A.; Dumčienė, V.; Eigirdas, M.; Kuliešis, A.; Vižlenskas, D. (Eds.) Lithuanian Statistical Year Book of Forestry; State Forest Service: Kaunas, Lithuania, 2016; p. 184. ISBN 1648-8008. [Google Scholar]
- Soranzo, N.; Alia, R.; Provan, J.; Powell, W. Patterns of variation at a mitochondrial sequence-tagged-site locus provides new insights into the postglacial history of European Pinus sylvestris populations. Mol. Ecol. 2000, 9, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Belletti, P.; Ferrazzini, D.; Piotti, A.; Monteleone, I.; Ducci, F. Genetic variation and divergence in Scots pine (Pinus sylvestris L.) within its natural range in Italy. Eur. J. For. Res. 2012, 131, 1127–1138. [Google Scholar] [CrossRef]
- Putman, A.I.; Carbone, I. Challenges in analysis and interpretation of microsatellite data for population genetic studies. Ecol. Evol. 2014, 4, 4399–4428. [Google Scholar] [CrossRef] [PubMed]
- Väli, Ü.; Saag, P.; Dombrovski, V.; Meyburg, B.U.; Maciorowski, G.; Mizera, T.; Treinys, M.; Fagerberg, S. Microsatellites and single nucleotide polymorphisms in avian hybrid identification: A comparative case study. J. Avian Biol. 2010, 41, 34–49. [Google Scholar] [CrossRef]
- Santure, A.W.; Stapley, J.; Ball, A.D.; Birkhead, T.R.; Burke, T.; Slate, J. On the use of large marker panels to estimate inbreeding and relatedness: Empirical and simulation studies of a pedigreed zebra finch population typed at 771 SNPs. Mol. Ecol. 2010, 19, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Hauser, L.; Baird, M.; Hilborn, R.; Seeb, L.W.; Seeb, J.E. An empirical comparison of SNPs and microsatellites for parentage and kinship assignment in a wild sockeye salmon (Oncorhynchus nerka) population. Mol. Ecol. Resour. 2011, 11 (Suppl. 1), 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.T.; Weise, J.A.; Bonnar, S.; Nolin, D.; Satkoski Trask, J.; Smith, D.G.; Ferguson, B.; Ha, J.; Kubisch, H.M.; Vinson, A.; et al. An empirical comparison of short tandem repeats (STRs) and single nucleotide polymorphisms (SNPs) for relatedness estimation in Chinese rhesus macaques (Macaca mulatta). Am. J. Primatol. 2014, 76, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Buschiazzo, E.; Gemmell, N.J. Conservation of human microsatellites across 450 million years of evolution. Genome Biol. Evol. 2010, 2, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Karazija, S. Lietuvos Miškų Tipai [Forest Types of Lithuania]; Mokslas: Vilnius, Lithuania, 1988; p. 213. ISBN 5-420-00421-6. (In Lithuanian) [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from plant tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Ryman, N.; Palm, S.; Andre, C.; Carvalho, G.R.; Dahlgren, T.G.; Jorde, P.E.; Laikre, L.; Larsson, C.; Palme, A.; Ruzzante, D.E. Power for detecting genetic divergence: Differences between statistical methods and marker loci. Mol. Ecol. 2006, 15, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Haasl, R.J.; Payseur, B.A. Multi-locus inference of population structure: A comparison between single nucleotide polymorphisms and microsatellites. Heredity 2011, 106, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Fluch, S.; Burg, A.; Kopecky, D.; Homolka, A.; Spiess, N.; Vendramin, G.G. Characterization of variable EST SSR markers for Norway spruce (Picea abies L.). BMC Res. Notes 2011, 4, 401. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, F.; Pinzauti, F.; Kujala, S.T.; González-Martínez, S.C.; Vendramin, G.G. Novel polymorphic nuclear microsatellite markers for Pinus sylvestris L. Conserv. Genet. Resour. 2012, 4, 231. [Google Scholar] [CrossRef]
- Elsik, C.G.; Minihan, V.T.; Hall, S.E.; Scarpa, A.M.; Williams, C.G. Low-copy microsatellite markers for Pinus taeda L. Genome 2000, 43, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population-structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987; ISBN 0-231-06320-2. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of Population Structure Using Multilocus Genotype data: Linked Loci and Correlated Allele Frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [PubMed]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007. [Google Scholar] [CrossRef] [PubMed]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Res. 2009, 9, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; von Holdt, B.M. Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M.; Barton, N.H. A comparison of three indirect methods for estimating average levels of gene flow. Evolution 1989, 43, 1349–1368. [Google Scholar] [CrossRef] [PubMed]
- Kerpauskaitė, V.; Danusevičius, D. Giminystės grupių išsidėstymo dėsningumai natūralios kilmės paprastosios pušies medyne. Miškininkystė 2015, 2, 40–47. (In Lithuanian) [Google Scholar]
- Zimmer, K.; Sønstebø, J.H. A preliminary study on the genetic structure of Northern European Pinus sylvestris L. by means of neutral microsatellite markers. Scand. J. For. Res. 2017. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Mitton, J.B.; Sturgeon, K.B.; Davis, M.L. Genetic variation in space and time in a population of ponderosa pine. Heredity 1981, 46, 407–426. [Google Scholar] [CrossRef]
- Sproule, A.T.; Dancik, B.P. The mating system of black spruce in north-central Alberta, Canada. Silvae Genet. 1996, 45, 159–164. [Google Scholar]
- Epperson, B.K. Spatial patterns of genetic variation within plant populations. In Plant Population Genetics, Breeding, and Genetic Resources; Brown, A.H.D., Clegg, M.T., Kahler, A.L., Weir, B.S., Eds.; Sinauer: Sunderland, MA, USA, 1990; pp. 254–278. [Google Scholar]
- Knowles, P. Spatial genetic structure within two natural stands of black spruce (Picea mariana (Mill.) B.S.P.). Silvae Genet. 1991, 40, 13–19. [Google Scholar]
- Bush, R.M.; Smouse, P.E. Evidence for the adaptive significance of allozymes in forest trees. New For. 1992, 6, 179–196. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between fitness and genetic diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Tollefsrud, M.M.; Sønstebø, J.H.; Brochmann, C.; Johnsen, Ø.; Skrøppa, T.; Vendramin, G.G. Combined analysis of nuclear and mitochondrial markers provide new insight into the genetic structure of North European Picea abies. Heredity 2009, 102, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Scotti, I.; Paglia, G.; Magni, F.; Morgante, M. Population genetics of Norway spruce (Picea abies Karst.) at regional scale: Sensitivity of different microsatellite motif classes in detecting differentiation. Ann. For. Sci. 2006, 63, 458–491. [Google Scholar] [CrossRef]
- Gömöry, D. Effect of stand origin on the genetic diversity of Norway spruce (Picea abies Karst.) populations. For. Ecol. Manag. 1992, 54, 215–223. [Google Scholar] [CrossRef]



| Locus | Primer Sequences (5′-3′) | Repeat Motifs | Fluorescent Label Dyes | Ta |
|---|---|---|---|---|
| P. abies PA28 | GGCCGAAAGTGCTACTGCTA | (TCG)n | 6-FAM | 57.3 °C |
| TGCTCCAGAAGAACACTCACA | ||||
| P. abies PA33 | GGTCGAGGAGGAGGAGGTAG | (CGG)n | 6-FAM | 59.5 °C |
| CACCGCTAGTGCAGTCTCTG | ||||
| P. abies PA25 | TGATTGAAATGATGGCTGCT | (CTG)n | NED | 59.4 °C |
| CATGTACGGTGCTCCTCCTC | ||||
| P. abies PA60 | CGCCGTATCCATTCCCAAGC | (GGCTG)n | NED | 61.4 °C |
| CCCAGCCCAGTTCAGTTTGC | ||||
| P. abies PA56 | ATCGTCTGCATTGCATTCAC | (AGGTG)n | PET | 55.8 °C |
| CTTCGTTCCTTCCTGATCCA | ||||
| P. abies PA22 | TCACTGGCCACAGTTTATCG | (TTC)n | PET | 58.7 °C |
| ATGAGGCCCAAGAGGAAGAC | ||||
| P. abies PA48 | ATTGCACAAGAGCGAACCTT | (CGG)n | VIC | 55.5 °C |
| CCAGCACCAAAATCACCAG | ||||
| P. abies PA44 | AAGGCAGCCAAAGTGAAGAA | (GGA)n | VIC | 57.6 °C |
| CTTGGCATTCCCTAGTGAGC | ||||
| P. sylvestris Spac12.5 | CTTCTTCACTAGTTTCCTTTGG | (GT)20(GA)10 | PET | 51.0 °C |
| TTGGTTATAGGCATAGATTGC | ||||
| P. sylvestris Spac11.4 | TCACAAAACACGTGATTCACA | (AT)5(GT)19 | VIC | 51.0 °C |
| GAAAATAGCCCTGTGTGAGACA | ||||
| P. sylvestris Spac7.14 | TTCGTAGGACTAAAAATGTGTG | (TG)17(AG)21 | 6-FAM | 49.8 °C |
| CAAAGTGGATTTTGACCG | ||||
| P. sylvestris Psyl16 | GCTCTGCCCATGCTATCACT | (AT)7 | VIC | 51.0 °C |
| TGATGCTACCCAATGAGGTG | ||||
| P. sylvestris Psyl57 | CCCCACATCTCTACAGTCCAA | (ACC)7 | NED | 55.0 °C |
| TGCTCTTGGATTTGTTGCTG | ||||
| P. sylvestris Psyl18 | ACTACCTGGCATTCGTCCTG | (GCA)7 | NED | 55.0 °C |
| GGATCTGGTCCATTTCGTGT | ||||
| P. sylvestris PtTX4001 | CTATTTGAGTTAAGAAGGGAGTC | (GT)15 | 6-FAM | 51.0 °C |
| CTGTGGGTAGCATCATC | ||||
| P. sylvestris PtTX4011 | GGTAACATTGGGAAAACACTCA | (CA)20 | PET | 51.0 °C |
| TTAACCATCTATGCCAATCACTT |
| Sample Origin | n | Na | Ne | I | Ho | He | F |
|---|---|---|---|---|---|---|---|
| Norway spruce old a | 30 | 7.13 ± 1.09 | 3.49 ± 0.21 | 1.45 ± 0.09 | 0.875 ± 0.051 | 0.705 ± 0.018 | −0.254 ± 0.092 |
| Norway spruce young b | 83 | 8.50 ± 1.77 | 3.83 ± 0.43 | 1.51 ± 0.14 | 0.877 ± 0.041 | 0.718 ± 0.027 | −0.239 ± 0.083 |
| Scots pine old a | 30 | 10.50 ± 1.65 | 6.38 ± 1.26 | 1.92 ± 0.20 | 0.861 ± 0.035 | 0.793 ± 0.042 | −0.120 ± 0.099 |
| Scots pine young b | 84 | 11.75 ± 1.87 | 5.23 ± 1.18 | 1.83 ± 0.17 | 0.798 ± 0.060 | 0.759 ± 0.034 | −0.080 ± 0.113 |
| P. abies Loci | Fis | Fst | P. Sylvestris Loci | Fis | Fst |
|---|---|---|---|---|---|
| PA28 | −0.191 | 0.012 | Spac12.5 | −0.008 | 0.079 |
| PA33 | −0.269 | 0.008 | PtTX4011 | 0.002 | 0.019 |
| PA25 | −0.338 | 0.022 | Psyl18 | −0.640 | 0.004 |
| PA48 | 0.286 | 0.028 | Spac7.14 | 0.252 | 0.018 |
| PA60 | −0.515 | 0.015 | Spac11.4 | 0.178 | 0.008 |
| PA44 | −0.367 | 0.024 | Psyl16 | −0.259 | 0.031 |
| PA22 | −0.322 | 0.001 | PtTX4001 | −0.236 | 0.003 |
| PA56 | −0.240 | 0.003 | Psyl57 | −0.070 | 0.002 |
| Mean | −0.245 ± 0.083 | 0.014 ± 0.004 | Mean | −0.098 ± 0.100 | 0.020 ± 0.009 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbylaitė, R.; Pliūra, A.; Lygis, V.; Suchockas, V.; Jankauskienė, J.; Labokas, J. Genetic Diversity and Its Spatial Distribution in Self-Regenerating Norway Spruce and Scots Pine Stands. Forests 2017, 8, 470. https://doi.org/10.3390/f8120470
Verbylaitė R, Pliūra A, Lygis V, Suchockas V, Jankauskienė J, Labokas J. Genetic Diversity and Its Spatial Distribution in Self-Regenerating Norway Spruce and Scots Pine Stands. Forests. 2017; 8(12):470. https://doi.org/10.3390/f8120470
Chicago/Turabian StyleVerbylaitė, Rita, Alfas Pliūra, Vaidotas Lygis, Vytautas Suchockas, Jurga Jankauskienė, and Juozas Labokas. 2017. "Genetic Diversity and Its Spatial Distribution in Self-Regenerating Norway Spruce and Scots Pine Stands" Forests 8, no. 12: 470. https://doi.org/10.3390/f8120470





