Predicting Future Seed Sourcing of Platycladus orientalis (L.) for Future Climates Using Climate Niche Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Zone Delimitation of P. orientalis and Occurrence Data Collection
2.2. Environmental Parameters and Future Climate Scenarios
2.3. Testing for Niche Divergence among Seed Zones
2.4. Development of Climate Niche Models and Projections for Future Climates
2.5. Shifts and Spatial Dynamics of the Suitable Habitats
3. Results
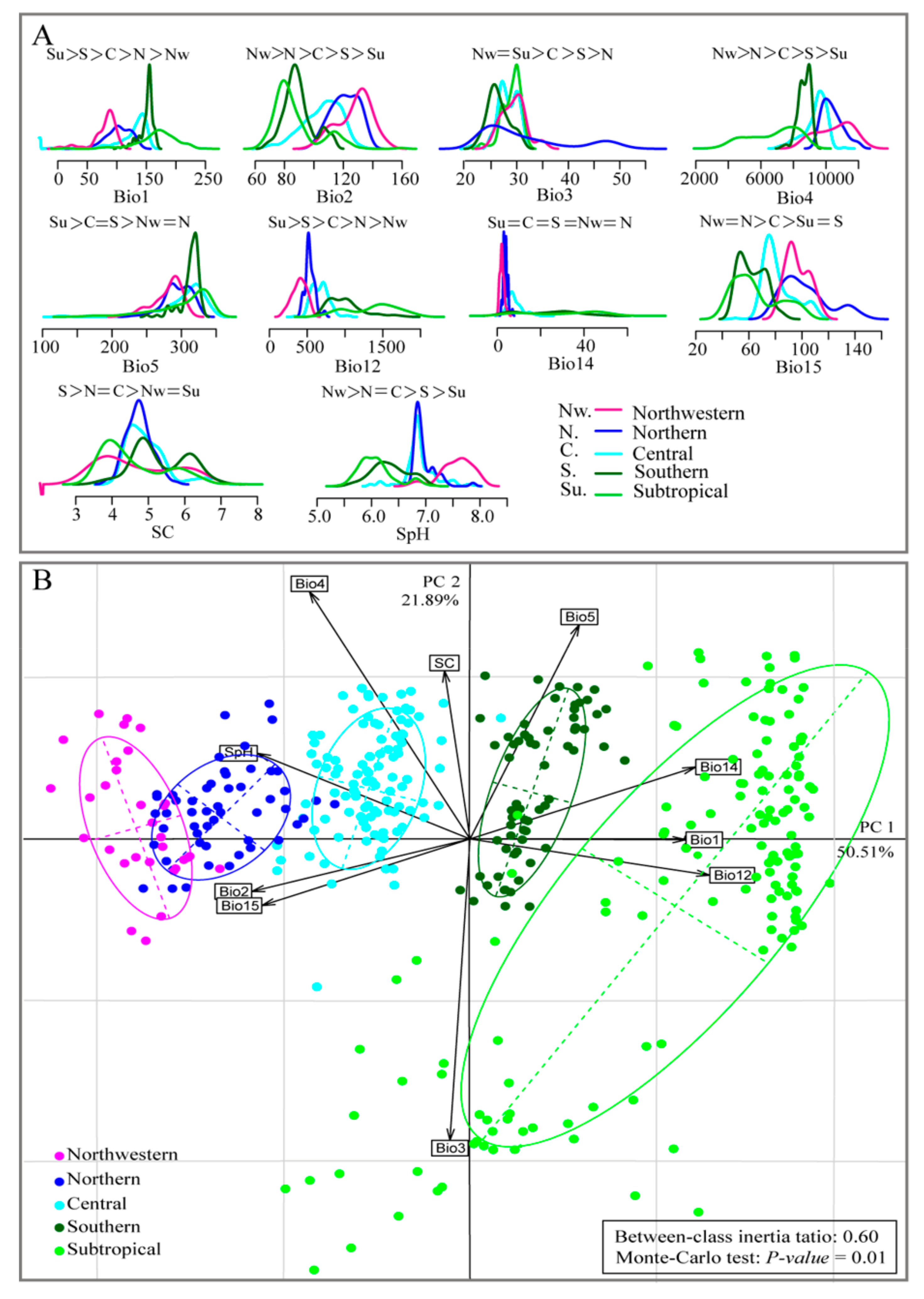
3.1. Testing for Niche Divergence
3.2. Predicted Current Suitable Areas and Important Environmental Factors
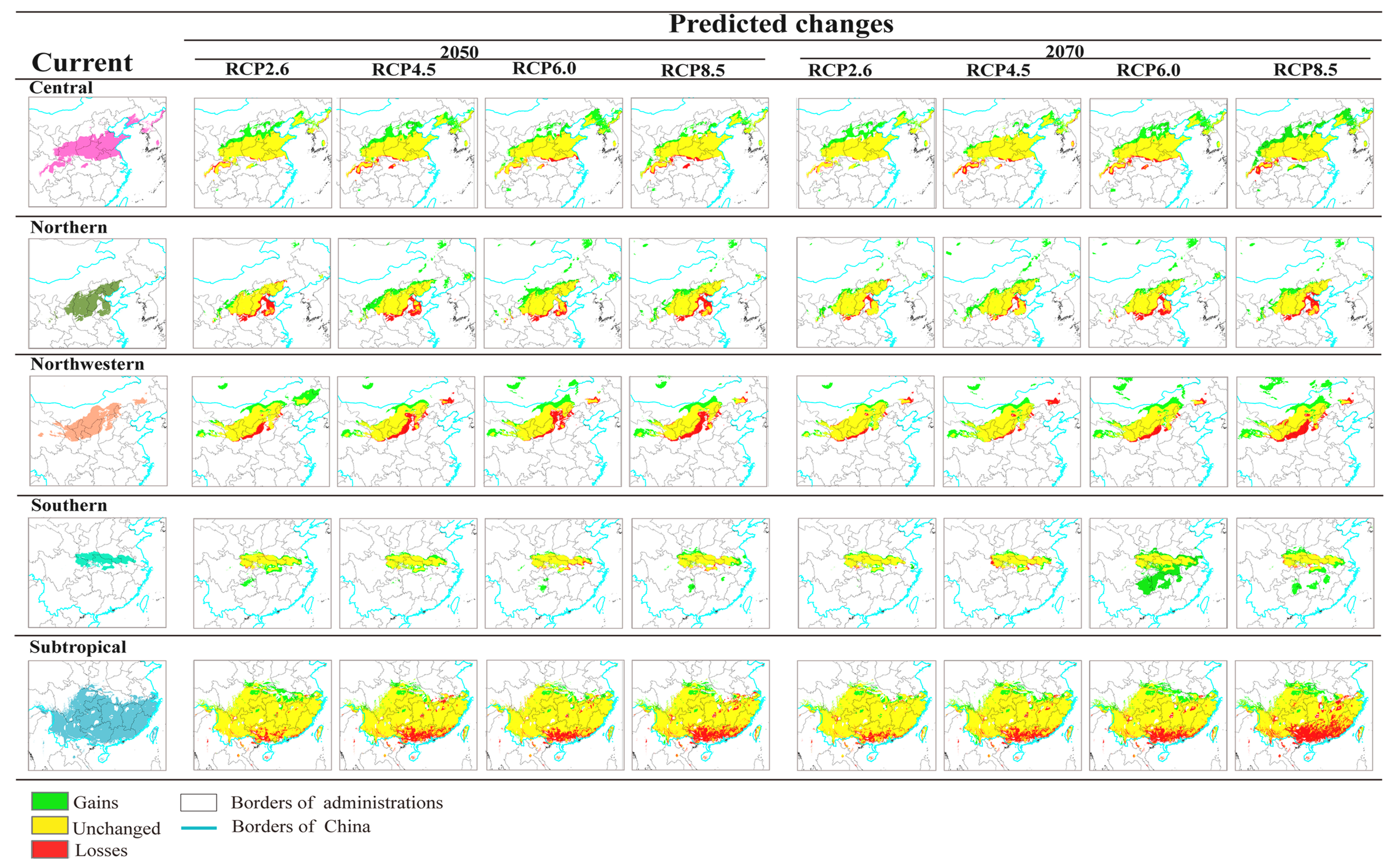
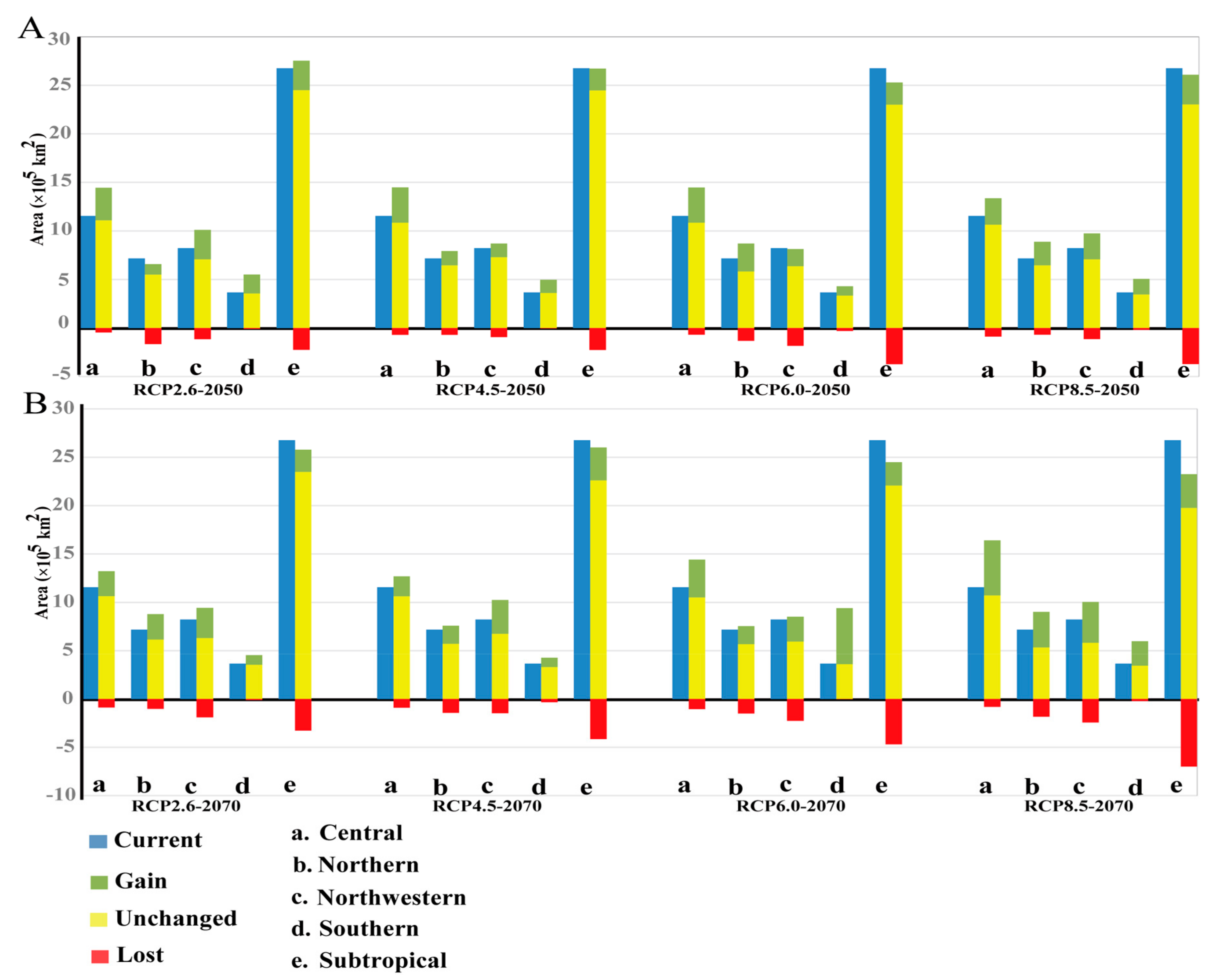
3.3. Projected Suitable Habitat and Habitat Shift under Future Climate Scenarios
3.4. Seed Zones’ Suitable Habitat Centroid Shift in Future Climate
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Woodward, F.I.; Williams, B.G. Climate and plant-distribution at global and local scales. Vegetatio 1987, 69, 189–197. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberon, J.; Sanchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 1999, 285, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Araujo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Allen, S.K.; Plattner, G.-K.; Nauels, A.; Xia, Y.; Stocker, T.F. Climate change 2013: The physical science basis. An overview of the working group 1 contribution to the fifth assessment report of the intergovernmental panel on climate change (IPCC). In Proceedings of the European Geosciences Union (EGU) General Assembly Conference, Vienna, Austria, 7–12 April 2013; EGU General Assembly Conference Abstracts. Cambridge University Press: New York, NY, USA, 2013; Volume 16, pp. 143–151. [Google Scholar]
- Wang, T.; Wang, G.; Innes, J.; Nitschke, C.; Kang, H. Climatic niche models and their consensus projections for future climates for four major forest tree species in the Asia-Pacific region. For. Ecol. Manag. 2016, 360, 357–366. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Jaquish, B.C.; Lopez-Upton, J.; Saenz-Romero, C.; St Clair, J.B.; Leites, L.P.; Joyce, D.G. Comparative genetic responses to climate for the varieties of Pinus ponderosa and Pseudotsuga menziesii: Realized climate niches. For. Ecol. Manag. 2014, 324, 126–137. [Google Scholar] [CrossRef]
- Booth, T.H. Estimating potential range and hence climatic adaptability in selected tree species. For. Ecol. Manag. 2016, 366, 175–183. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.K.; Gylander, T.; Mbogga, M.S.; Chen, P.Y.; Hamann, A. Assisted migration to address climate change: Recommendations for aspen reforestation in western Canada. Ecol. Appl. 2011, 21, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Kawecki, T.J. Adaptation to marginal habitats. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 321–342. [Google Scholar] [CrossRef]
- Banta, J.A.; Ehrenreich, I.M.; Gerard, S.; Chou, L.; Wilczek, A.; Schmitt, J.; Kover, P.X.; Purugganan, M.D. Climate envelope modelling reveals intraspecific relationships among flowering phenology, niche breadth and potential range size in Arabidopsis thaliana. Ecol. Lett. 2012, 15, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-G.; Jin, Y.; Wang, X.-R.; Mao, J.-F.; Li, Y. Predicting impacts of future climate change on the distribution of the widespread conifer Platycladus orientalis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Khanum, R.; Mumtaz, A.; Kumar, S. Predicting impacts of climate change on medicinal asclepiads of Pakistan using maxent modeling. Acta Oecol. 2013, 49, 23–31. [Google Scholar] [CrossRef]
- O’Neill, G.A.; Hamann, A.; Wang, T. Accounting for population variation improves estimates of the impact of climate change on species’ growth and distribution. J. Appl. Ecol. 2008, 45, 1040–1049. [Google Scholar] [CrossRef]
- Coops, N.; Waring, R. Assessing forest growth across southwestern Oregon under a range of current and future global change scenarios using a process model, 3-pg. Glob. Chang. Biol. 2001, 7, 15–29. [Google Scholar] [CrossRef]
- Briceno-Elizondo, E.; Garcia-Gonzalo, J.; Peltola, H.; Matala, J.; Kellomäki, S. Sensitivity of growth of Scots pine, Norway spruce and Silver birch to climate change and forest management in boreal conditions. For. Ecol. Manag. 2006, 232, 152–167. [Google Scholar] [CrossRef]
- Howe, G.T.; Aitken, S.N.; Neale, D.B.; Jermstad, K.D.; Wheeler, N.C.; Chen, T.H. From genotype to phenotype: Unraveling the complexities of cold adaptation in forest trees. Can. J. Bot. 2003, 81, 1247–1266. [Google Scholar] [CrossRef]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; Kleunen, M.; Naya, D.E. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, K. Provenance tests as indicators of growth response to climate change in 10 north temperate tree species. Can. J. For. Res. 1996, 26, 1089–1095. [Google Scholar] [CrossRef]
- Mátyás, C. Modeling climate-change effects with provenance test data. Tree Physiol. 1994, 14, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Serra-Varela, M.J.; Alía, R.; Daniels, R.R.; Zimmermann, N.E.; Gonzalo-Jiménez, J.; Grivet, D. Assessing vulnerability of two mediterranean conifers to support genetic conservation management in the face of climate change. Divers. Distrib. 2017, 23, 507–516. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Yanchuk, A.; O’Neill, G.A.; Aitken, S.N. Use of response functions in selecting Lodgepole pine populations for future climates. Glob. Chang. Biol. 2006, 12, 2404–2416. [Google Scholar] [CrossRef]
- Morgenstern, E.K.B. Geographic Variation in Forest Trees: Genetic Basis and Application of Knowledge in Silviculture, 1st ed.; University of British Columbia Press: Vancouver, BC, Canada, 1996; pp. 106–111. [Google Scholar]
- Parker, W.H. Focal point seed zones: Site-specific seed zone delineation using geographic information systems. Can. J. For. Res. 1992, 22, 267–271. [Google Scholar] [CrossRef]
- Hamann, A.; Gylander, T.; Chen, P.Y. Developing seed zones and transfer guidelines with multivariate regression trees. Tree Genet. Genomes 2011, 7, 399–408. [Google Scholar] [CrossRef]
- McKenney, D.; Pedlar, J.; O’Neill, G. Climate change and forest seed zones: Past trends, future prospects and challenges to ponder. For. Chron. 2009, 85, 258–266. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- The Editorial Board of Forest in China. Coniferous forests. In Forest in China, 1st ed; Wu, Z.L. (Ed.) China Forestry Publishing House: Beijing, China, 1998; Volume 2, p. 1065. [Google Scholar]
- Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita. Cupressaceae. In Flora Reipublicae Popularis Sinicae, 1st ed; Science Press: Beijing, China, 1978; Volume 7, pp. 322–325. (In Chinese)
- Dong, T.M.; Chen, X.Y.; Zhang, X.M.; Li, Z.R.; Kong, W.H.B. Platycladus orientalis, 1st ed.; Henan Science and Technology Press: Zhengzhou, China, 1990; pp. 16–59. (In Chinese) [Google Scholar]
- Wu, X.-M. Geographic variation of Platycladus orientalis (L.) franco. J. Beijing For. Univ. 1986, 8, 1–16. (In Chinese) [Google Scholar]
- Shi, W.; Shi, X. Seed Zones of Chinese Forest Trees—Seed zones of Platycladus orientalis (Linn.) Franco. Available online: http://www.spsp.gov.cn/page/P393/71.shtml (accessed on 28 August 2017). (In Chinese)
- Liang, Y.; Chen, Z. Geographical variation trend of Platycladus orientalis provenances. J. Fujian Coll. For. 1989, 9, 134–139. (In Chinese) [Google Scholar]
- Fu, J.; Shen, X. Study on the variation of seed and seedling characters among different Platycladus orientalis provenances. J. Beijing For. Univ. 1987, 2, 133–139. (In Chinese) [Google Scholar]
- Fu, J.; Shen, X. Study on the growth rhythm of seedling height in Platycladus orientalis’s provenance tests. J. Beijing For. Univ. 1989, 2, 72–79. (In Chinese) [Google Scholar]
- The National Cooperation Group of Platycladus orientalis. Study on the Seedling Growth and Overwintering Characters of National Platycladus orientalis Provenances. J. Beijing For. Univ. 1986, 2, 72–79. (In Chinese) [Google Scholar]
- Chen, X.; Shen, X.; Shi, W. The growth and overwintering performance of different provenances’ Platycladus orientalis in Beijing. J. Beijing Univ. Agric. 1990, 1, 19–27. (In Chinese) [Google Scholar]
- Chen, X.; Shen, X. Study on the afforestation survival rate and growth variation of young forest of Platycladus orientalis. J. Beijing For. Univ. 1994, 1, 20–29. (In Chinese) [Google Scholar]
- Wu, X.; Ma, J. The differences of photosynthetic characteristics and the responses of seed germination to water stress among different provenances of Platycladus orientalis. Sci. Silvae Sin. 1988, 4, 448–453. (In Chinese) [Google Scholar]
- Luo, W.-X.; He, W.-X.; Zhou, J.-L. Studies on the provenance trial of Platycladus orientalis (L.) at seedling stage. Shaanxi For. Sci.Techonl. 1988, 16, 1–6. (In Chinese) [Google Scholar]
- Shi, X.-B.; Qu, X.-K. Studies on the provenance trial of Platycladus orientalis (L.) at seedling stage. For. Sci. Technol. 1987, 7, 15–16. (In Chinese) [Google Scholar]
- Wang, R.-Z.; Wang, M. Preliminary report on provenance test on Platycladus orientalis (L.). J. Hebei For. Sci. Technol. 1990, 18, 30–34. (In Chinese) [Google Scholar]
- Nunes, L.A.; Pearson, R.G. A null biogeographical test for assessing ecological niche evolution. J. Biogeogr. 2017, 44, 1331–1343. [Google Scholar] [CrossRef]
- Farber, O.; Kadmon, R. Assessment of alternative approaches for bioclimatic modeling with special emphasis on the mahalanobis distance. Ecol. Model. 2003, 160, 115–130. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Van Etten, J.R. Geographic Analysis and Modeling with Raster Data: R Package. Available online: https://cran.r-project.org/web/packages/raster/raster.pdf (accessed on 28 August 2017).
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Mao, J.-F.; Wang, X.-R. Distinct niche divergence characterizes the homoploid hybrid speciation of Pinus densata on the Tibetan plateau. Am. Nat. 2011, 177, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Flato, G.; Marotzke, J.; Abiodun, B.; Braconnot, P.; Chou, S.C.; Collins, W.; Cox, P.; Driouech, F.; Emori, S.; Eyring, V.; et al. Evaluation of climate models. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group i to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: London, UK, 2013; Volume 9, pp. 741–866. [Google Scholar]
- Weyant, J.; Azar, C.; Kainuma, M.; Kejun, J.; Nakicenovic, N.; Shukla, P.; La Rovere, E.; Yohe, G. Report of 2.6 VERSUS 2.9 watts/m2 rcpp Evaluation Panel; Technical Report; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2009. [Google Scholar]
- Conover, W. Practical nonparametric statistics. Q. Rev.Biol. 1971, 14, 332–333. [Google Scholar]
- Anderson, M.J.; Robinson, J. Permutation tests for linear models. Aust. N. Z. J. Stat. 2001, 43, 75–88. [Google Scholar] [CrossRef]
- Romesburg, H.C. Exploring, confirming, and randomization tests. Comput. Geosci. 1985, 11, 19–37. [Google Scholar] [CrossRef]
- McCormack, J.E.; Zellmer, A.J.; Knowles, L.L. Does niche divergence accompany allopatric divergence in Aphelocoma jays as predicted under ecological speciation?: Insights from tests with niche models. Evolution 2010, 64, 1231–1244. [Google Scholar] [PubMed]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.0-12. 2015. Available online:http://cran. r-project. org (accessed on 28 August 2017).
- Dray, S.; Dufour, A.; Leeuw, J.D.; Zeileis, A. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Fay, M.P.; Shaw, P.A. Exact and asymptotic weighted logrank tests for interval censored data: The interval R package. J. Stat. Softw. 2010, 36, 1–34. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Jane, E.; Catherine, H.G.; Robert, P.A.; Miroslav, D.; Simon, F.; Antoine, G.; Robert, J.H.; Falk, H.; John, R.L.; Anthony, L.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 94–98. [Google Scholar]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of maxent for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L. Sdmtoolbox: A python-based gis toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Chen, X.; Shen, X. The study on afforestation survival rate of Platycladus orientalis provenances and genetic variation for growth traits of yong forest. J. Beijing For. Univ. 1994, 16, 20–27. (In Chinese) [Google Scholar]
- Mao, A.H.; Li, J.X.; Zhang, C.Y.; Li, Q.J.; Wang, S.; Chen, X.Y.; Li, Y. Geographic variation and provenance selection of Platycladus orientalis in a 19-year-old testing plantation. J. Beijing For. Univ. 2010, 1, 63–68. (In Chinese) [Google Scholar]
- Shi, X.; Zheng, J. Study on provenance classification of Platycladus orientalis. For. Sci. Technol. 1993, 1, 1–5. (In Chinese) [Google Scholar]
- Linhart, Y.B.; Grant, M.C. Evolutionary significance of local genetic differentiation in plants. Annu. Rev. Ecol Syst. 1996, 27, 237–277. [Google Scholar] [CrossRef]
- Alsos, I.G.; Ehrich, D.; Thuiller, W.; Eidesen, P.B.; Tribsch, A.; Schönswetter, P.; Lagaye, C.; Taberlet, P.; Brochmann, C. Genetic consequences of climate change for northern plants. Proc. R. Soc. Lond. B Biol. Sci. 2012, 279, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yin, Y.; Li, B. A new scheme for climate regionalization in China. Acta Geogr. Sin. 2010, 65, 3–12. (In Chinese) [Google Scholar]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, G.E.; Wykoff, W.R.; Ying, C.C. Physiologic plasticity, evolution, and impacts of a changing climate on Pinus contorta. Clim. Chang. 2001, 50, 355–376. [Google Scholar] [CrossRef]
- Ying, C.C.; Yanchuk, A.D. The development of British Columbia’s tree seed transfer guidelines: Purpose, concept, methodology, and implementation. For. Ecol. Manag. 2006, 227, 1–13. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Elam, D.R. Population genetic consequences of small population size: Implications for plant conservation. Annu. Rev. Ecol. Syst. 1993, 24, 217–242. [Google Scholar] [CrossRef]
- Sagarin, R.D.; Gaines, S.D. The ‘abundant centre’ distribution: To what extent is it a biogeographical rule? Ecol. Lett. 2002, 5, 137–147. [Google Scholar] [CrossRef]
- Wagner, V.; Durka, W.; Hensen, I. Increased genetic differentiation but no reduced genetic diversity in peripheral vs. central populations of a steppe grass. Am. J. Bot. 2011, 98, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Garner, T.W.J.; Pearman, P.B.; Angelone, S. Genetic diversity across a vertebrate species’ range: A test of the central-Peripheral hypothesis. Mol. Ecol. 2004, 13, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic variation across species’ geographical ranges: The central-marginal hypothesis and beyond. Mol. Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Hamann, A.; Wang, T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Wang, T.; Campbell, E.M.; O’Neill, G.A.; Aitken, S.N. Projecting future distributions of ecosystem climate niches: Uncertainties and management applications. For. Ecol. Manag. 2012, 279, 128–140. [Google Scholar] [CrossRef]
- Johnson, G.; Sorensen, F.C.; St Clair, J.B.; Cronn, R.C. Pacific northwest forest tree seed zones a template for native plants? Nativ. Plants J. 2004, 5, 131–140. [Google Scholar] [CrossRef]

| Code | Name | Resolution | Unit | Source |
|---|---|---|---|---|
| Bio1 | Annual mean air temperature | 30″ × 30″ | °C × 10 | http://www.worldclim.org/ |
| Bio2 | Mean diurnal air temperature range | 30″ × 30″ | °C × 10 | http://www.worldclim.org/ |
| Bio3 | Isothermality | 30″ × 30″ | ×100 | http://www.worldclim.org/ |
| Bio4 | Air temperature seasonality | 30″ × 30″ | ×100 | http://www.worldclim.org/ |
| Bio5 | Max air temperature of the warmest month | 30″ × 30″ | °C × 10 | http://www.worldclim.org/ |
| Bio12 | Annual precipitation | 30″ × 30″ | mm | http://www.worldclim.org/ |
| Bio14 | Precipitation of the driest month | 30″ × 30″ | mm | http://www.worldclim.org/ |
| Bio15 | Precipitation seasonality (coefficient of variation) | 30″ × 30″ | mm | http://www.worldclim.org/ |
| SC | Soil organic carbon | 0.5° × 0.5° | http://www.sage.wisc.edu/atlas/index.php | |
| SpH | Soil pH | 0.5° × 0.5° | http://www.sage.wisc.edu/atlas/index.php |
| Bioclimate Variables | Contributions (%) | ||||
|---|---|---|---|---|---|
| Central | Northern | Northwestern | Southern | Subtropical | |
| Annual mean air temperature (Bio1) | 22.6 | 18.6 | 34.2 | 26.6 | 18.8 |
| Mean diurnal air temperature range (Bio2) | - | - | - | - | - |
| Isothermality (Bio3) | - | 18.0 | - | - | - |
| Air temperature seasonality (Bio4) | 19.2 | 22.0 | - | 11.6 | 17.9 |
| Max air temperature of the warmest month (Bio5) | - | - | - | - | - |
| Annual precipitation (Bio12) | 12.3 | 19.6 | 13.4 | 31.5 | 32.2 |
| Precipitation of the driest month (Bio14) | 17.6 | - | - | 11.8 | 13.0 |
| Precipitation seasonality (Bio15) | 12.1 | - | 15.4 | - | - |
| Soil organic carbon (SC) | - | - | - | - | - |
| Soil pH (SpH) | 14.8 | - | 23.0 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.-G.; Wang, T.; Liu, S.-S.; Jiao, S.-Q.; Jia, K.-H.; Zhou, S.-S.; Jin, Y.; Li, Y.; El-Kassaby, Y.A.; Mao, J.-F. Predicting Future Seed Sourcing of Platycladus orientalis (L.) for Future Climates Using Climate Niche Models. Forests 2017, 8, 471. https://doi.org/10.3390/f8120471
Hu X-G, Wang T, Liu S-S, Jiao S-Q, Jia K-H, Zhou S-S, Jin Y, Li Y, El-Kassaby YA, Mao J-F. Predicting Future Seed Sourcing of Platycladus orientalis (L.) for Future Climates Using Climate Niche Models. Forests. 2017; 8(12):471. https://doi.org/10.3390/f8120471
Chicago/Turabian StyleHu, Xian-Ge, Tongli Wang, Si-Si Liu, Si-Qian Jiao, Kai-Hua Jia, Shan-Shan Zhou, Yuqing Jin, Yue Li, Yousry A. El-Kassaby, and Jian-Feng Mao. 2017. "Predicting Future Seed Sourcing of Platycladus orientalis (L.) for Future Climates Using Climate Niche Models" Forests 8, no. 12: 471. https://doi.org/10.3390/f8120471






