Physical Conditions Regulate the Fungal to Bacterial Ratios of a Tropical Suspended Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eden Project Experiments
- 5 ferns were inoculated with 5 individuals of the earthworm Eisenia fetida;
- 5 ferns were dosed with an ammonium nitrate solution, representing 365.7 mg N per kg dry soil;
- 5 ferns received 5 earthworms and ammonium nitrate per fern;
- 5 non-manipulated ferns served as controls.
2.2. Collection of Ferns from Danum Valley
2.3. Hydrolase Enzyme Activity
2.4. Oxidative Enzyme Activity
2.5. Phospholipid Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
3.1. Eden Project Enzyme Activity and Fungal:Bacterial Ratio
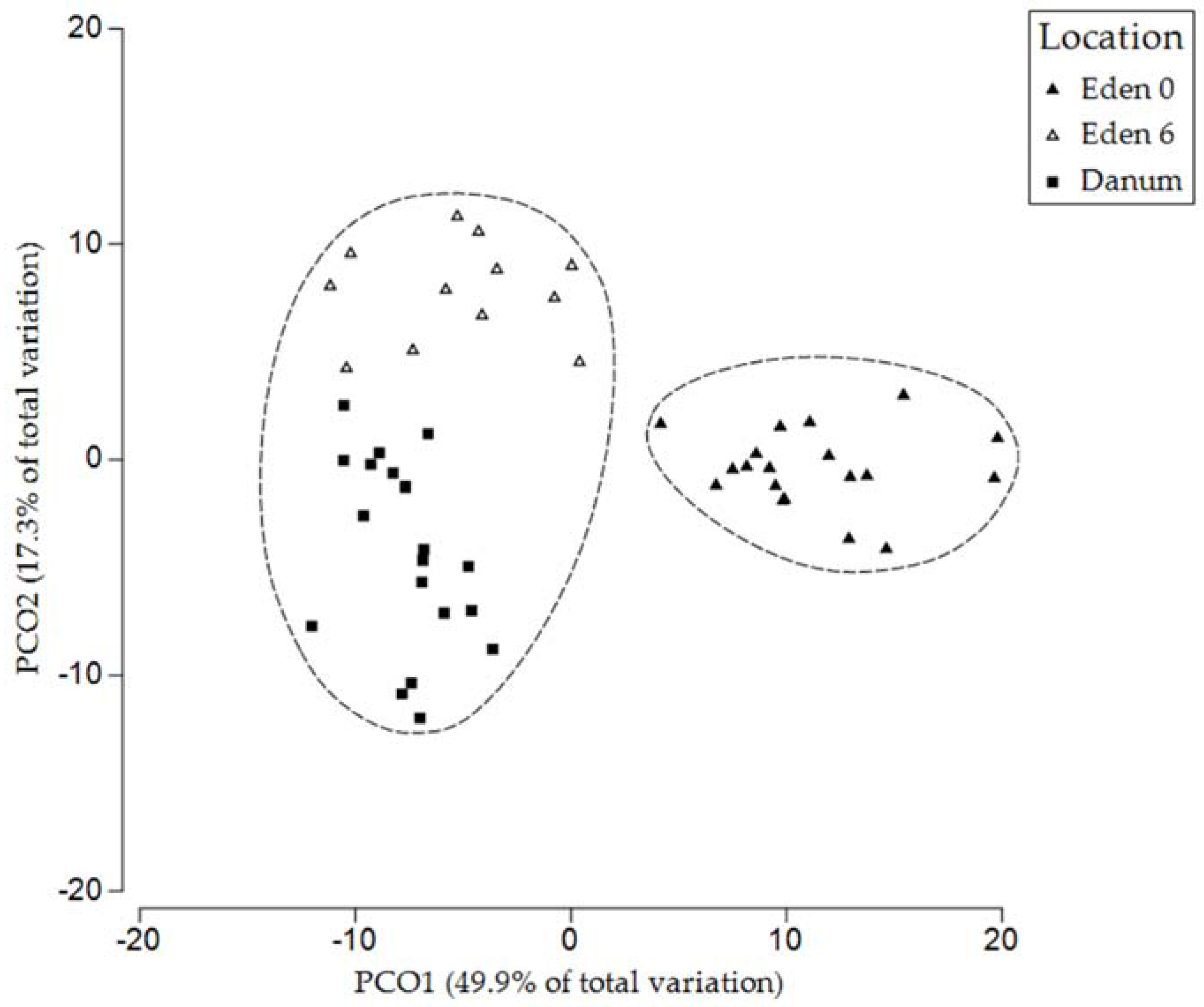
3.2. Fern Soil Microbial Community Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozanne, C.M.; Anhuf, D.; Boulter, S.L.; Keller, M.; Kitching, R.L.; Korner, C.; Meinzer, F.C.; Mitchell, A.W.; Nakashizuka, T.; Dias, P.L.; et al. Biodiversity Meets the Atmosphere: A Global View of Forest Canopies. Science 2003, 301, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Bohlman, S.A.; Matelson, T.J.; Nadkarni, N.M. Moisture and Temperature Patterns of Canopy Humus and Forest Floor Soil of a Montane Cloud Forest, Costa Rica. Biotropica 1995, 27, 13–19. [Google Scholar] [CrossRef]
- Nadkarni, N.M. Factors Affecting the Initiation and Growth of Aboveground Adventitious Roots in a Tropical Cloud Forest Tree: An Experimental Approach. Oecologia 1994, 100, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Hietz, P.; Wanek, W.; Wania, R.; Nadkarni, N.M. Nitrogen-15 Natural Abundance in a Montane Cloud Forest Canopy as an Indicator of Nitrogen Cycling and Epiphyte Nutrition. Oecologia 2002, 131, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, N.M.; Schaefer, D.; Matelson, T.J.; Solano, R. Comparison of Arboreal and Terrestrial Soil Characteristics in a Lower Montane Forest, Monteverde, Costa Rica. Pedobiologia 2002, 46, 24–33. [Google Scholar] [CrossRef]
- Wanek, W.; Arndt, S.K.; Huber, W.; Popp, M. Nitrogen Nutrition during Ontogeny of Hemiepiphytic Clusia Species. Funct. Plant Biol. 2002, 29, 733–740. [Google Scholar] [CrossRef]
- Clark, K.L.; Nadkarni, N.M.; Gholz, H.L. Retention of Inorganic Nitrogen by Epiphytic Bryophytes in a Tropical Montane Forest. Biotropica 2005, 37, 328–336. [Google Scholar] [CrossRef]
- Cardelús, C.L. Litter Decomposition within the Canopy and Forest Floor of Three Tree Species in a Tropical Lowland Rain Forest, Costa Rica. Biotropica 2010, 42, 300–308. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA, 1979. [Google Scholar]
- Vance, E.D.; Nadkarni, N.M. Microbial Biomass and Activity in Canopy Organic Matter and the Forest Floor of a Tropical Cloud Forest. Soil Biol. Biochem. 1990, 22, 677–684. [Google Scholar] [CrossRef]
- Lambais, M.R.; Crowley, D.E.; Cury, J.C.; Bull, R.C.; Rodrigues, R.R. Bacterial Diversity in Tree Canopies of the Atlantic Forest. Science 2006, 312, 1917. [Google Scholar] [CrossRef] [PubMed]
- Pittl, E.; Innerebner, G.; Wanek, W.; Insam, H. Microbial Communities of Arboreal and Ground Soils in the Esquinas Rainforest, Costa Rica. Plant Soil 2010, 329, 65–74. [Google Scholar] [CrossRef]
- Lambais, M.R.; Lucheta, A.R.; Crowley, D.E. Bacterial Community Assemblages Associated with the Phyllosphere, Dermosphere, and Rhizosphere of Tree Species of the Atlantic Forest are Host Taxon Dependent. Microb. Ecol. 2014, 68, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lowman, M.D.; Schowalter, T.D. Plant Science in Forest Canopies—The First 30 Years of Advances and Challenges (1980–2010). New Phytol. 2012, 194, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kitching, R.L.; Cao, M.; Creedy, T.J.; Fayle, T.M.; Freiberg, M.; Hewitt, C.; Itioka, T.; Koh, L.P.; Ma, K. Forests and their Canopies: Achievements and Horizons in Canopy Science. Trends Ecol. Evol. 2017, 32, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, S.E.; Vitousek, P.M. Nutrient Limitation of Decomposition in Hawaiian Forests. Ecology 2000, 81, 1867–1877. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Extracellular Enzyme Activities and Carbon Chemistry as Drivers of Tropical Plant Litter Decomposition. Biotropica 2004, 36, 285–296. [Google Scholar] [CrossRef]
- Waldrop, M.; Firestone, M. Altered Utilization Patterns of Young and Old Soil C by Microorganisms Caused by Temperature Shifts and N Additions. Biogeochemistry 2004, 67, 235–248. [Google Scholar] [CrossRef]
- Cusack, D.; Chou, W.; Yang, W.; Harmon, M.; Silver, W.; Lidet Team. Controls on Long-Term Root and Leaf Litter Decomposition in Neotropical Forests. Glob. Chang. Biol. 2009, 15, 1339–1355. [Google Scholar] [CrossRef]
- Scullion, J.; Malik, A. Earthworm Activity Affecting Organic Matter, Aggregation and Microbial Activity in Soils Restored After Opencast Mining for Coal. Soil Biol. Biochem. 2000, 32, 119–126. [Google Scholar] [CrossRef]
- Frouz, J.; Prach, K.; Pižl, V.; Háněl, L.; Starý, J.; Tajovský, K.; Materna, J.; Balík, V.; Kalčík, J.; Řehounková, K. Interactions between Soil Development, Vegetation and Soil Fauna during Spontaneous Succession in Post Mining Sites. Eur. J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Pérès, G.; Tondoh, J. A Review of Earthworm Impact on Soil Function and Ecosystem Services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef] [Green Version]
- Birkhofer, K.; Schöning, I.; Alt, F.; Herold, N.; Klarner, B.; Maraun, M.; Marhan, S.; Oelmann, Y.; Wubet, T.; Yurkov, A. General Relationships between Abiotic Soil Properties and Soil Biota Across Spatial Scales and Different Land-use Types. PLoS ONE 2012, 7, e43292. [Google Scholar] [CrossRef] [PubMed]
- Swallow, M.; Quideau, S.; MacKenzie, M.; Kishchuk, B. Microbial Community Structure and Function: The Effect of Silvicultural Burning and Topographic Variability in Northern Alberta. Soil Biol. Biochem. 2009, 41, 770–777. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol Oxidase, Peroxidase and Organic Matter Dynamics of Soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Hendershot, J.N.; Read, Q.D.; Henning, J.A.; Sanders, N.J.; Classen, A.T. Consistently Inconsistent Drivers of Patterns of Microbial Diversity and Abundance at Macroecological Scales. Ecology 2017, 98, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Moorhead, D.; Linkins, A. The Enzymic Basis of Plant Litter Decomposition: Emergence of an Ecological Process. Appl. Soil Ecol. 1994, 1, 97–111. [Google Scholar] [CrossRef]
- Paterson, E.; Osler, G.; Dawson, L.A.; Gebbing, T.; Sim, A.; Ord, B. Labile and Recalcitrant Plant Fractions are Utilised by Distinct Microbial Communities in Soil: Independent of the Presence of Roots and Mycorrhizal Fungi. Soil Biol. Biochem. 2008, 40, 1103–1113. [Google Scholar] [CrossRef]
- Kramer, S.; Dibbern, D.; Moll, J.; Huenninghaus, M.; Koller, R.; Krueger, D.; Marhan, S.; Urich, T.; Wubet, T.; Bonkowski, M. Resource Partitioning between Bacteria, Fungi, and Protists in the Detritusphere of an Agricultural Soil. Front. Microbiol. 2016, 7, 1524. [Google Scholar] [CrossRef] [PubMed]
- Charteris, A.; Knowles, T.; Michaelides, K.; Evershed, R. Compound-specific Amino Acid 15N Stable Isotope Probing of Nitrogen Assimilation by the Soil Microbial Biomass using Gas Chromatography/Combustion/Isotope Ratio Mass Spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1846–1856. [Google Scholar] [PubMed]
- Matson, P.; Lohse, K.A.; Hall, S.J. The Globalization of Nitrogen Deposition: Consequences for Terrestrial Ecosystems. AMBIO J. Hum. Environ. 2002, 31, 113–119. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen Addition Reduces Soil Respiration in a Mature Tropical Forest in Southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Yatabe, Y.; Shinohara, W.; Matsumoto, S.; Murakami, N. Patterns of Hybrid Formation among Cryptic Species of Bird-nest Fern, Asplenium nidus Complex (Aspleniaceae), in West Malesia. Bot. J. Linn. Soc. 2009, 160, 42–63. [Google Scholar] [CrossRef]
- Srivastava, D.S.; Kolasa, J.; Bengtsson, J.; Gonzalez, A.; Lawler, S.P.; Miller, T.E.; Munguia, P.; Romanuk, T.; Schneider, D.C.; Trzcinski, M.K. Are Natural Microcosms Useful Model Systems for Ecology? Trends Ecol. Evol. 2004, 19, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.C.; Snaddon, J.L.; Johnson, H.R.; Foster, W.A. The Impact of Bird’s Nest Ferns on Stemflow Nutrient Concentration in a Primary Rain Forest, Sabah, Malaysia. J. Trop. Ecol. 2007, 23, 721–724. [Google Scholar] [CrossRef]
- Ellwood, M.D.F.; Jones, D.T.; Foster, W.A. Canopy Ferns in Lowland Dipterocarp Forest Support a Prolific Abundance of Ants, Termites, and Other Invertebrates. Biotropica 2002, 34, 575–583. [Google Scholar] [CrossRef]
- Ellwood, M.D.F.; Foster, W.A. Doubling the Estimate of Invertebrate Biomass in a Rainforest Canopy. Nature 2004, 429, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, M.D.F.; Manica, A.; Foster, W.A. Stochastic and Deterministic Processes Jointly Structure Tropical Arthropod Communities. Ecol. Lett. 2009, 12, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Fayle, T.M.; Eggleton, P.; Manica, A.; Yusah, K.M.; Foster, W.A. Experimentally Testing and Assessing the Predictive Power of Species Assembly Rules for Tropical Canopy Ants. Ecol. Lett. 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, M.D.F.; Blüthgen, N.; Fayle, T.M.; Foster, W.A.; Menzel, F. Competition can lead to unexpected patterns in tropical ant communities. Acta Oecol. 2016, 75, 24–34. [Google Scholar] [CrossRef]
- Donald, J.; Maxfield, P.; Murray, D.; Ellwood, M.D.F. How Tropical Epiphytes at the Eden Project Contribute to Rainforest Canopy Science. Sibbaldia 2017, 14, 55–68. [Google Scholar]
- Warmington, R.; Eden Project, Cornwall, UK. Personal communication, 2017.
- Marsh, C.W.; Greer, A.G. Forest Land-use in Sabah, Malaysia: An Introduction to Danum Valley. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1992, 335, 331–339. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Evans, T.A.; Williams, S.E.; Edwards, D.P. Microhabitats in the Tropics Buffer Temperature in a Globally Coherent Manner. Biol. Lett. 2014, 10, 20140819. [Google Scholar] [CrossRef] [PubMed]
- Donald, J.; Bonnett, S.; Maxfield, P.; Ellwood, M.D.F. The Relative Importance of Invertebrate and Microbial Decomposition in a Rainforest Restoration Project. Restor. Ecol. 2017. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharmacol 1959, 37, 911–917. [Google Scholar]
- Dickson, L.; Bull, I.D.; Gates, P.J.; Evershed, R.P. A Simple Modification of a Silicic Acid Lipid Fractionation Protocol to Eliminate Free Fatty Acids from Glycolipid and Phospholipid Fractions. J. Microbiol. Methods 2009, 78, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, Å.; Bååth, E. The use of Phospholipid Fatty Acid Analysis to Estimate Bacterial and Fungal Biomass in Soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Rubino, M.; Dungait, J.; Evershed, R.; Bertolini, T.; De Angelis, P.; D’Onofrio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F. Carbon Input Belowground is the Major C Flux Contributing to Leaf Litter Mass Loss: Evidences from a 13 C Labelled-Leaf Litter Experiment. Soil Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Wanek, W.; Zotz, G. Are Vascular Epiphytes Nitrogen Or Phosphorus Limited? A Study of Plant 15N Fractionation and Foliar N: P Stoichiometry with the Tank Bromeliad Vriesea sanguinolenta. New Phytol. 2011, 192, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.E.; Rundel, P.W.; Cardelús, C.L. The Influence of Life Form on Carbon and Nitrogen Relationships in Tropical Rainforest Ferns. Oecologia 2007, 153, 225. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.A.; McDowell, W.H.; Townsend, A.R.; Vitousek, P.M. The globalization of N deposition: Ecosystem consequences in tropical environments. In New Perspectives on Nitrogen Cycling in the Temperate and Tropical Americas; Townsend, A.R., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 67–83. [Google Scholar]
- Araujo, Y.; Luizão, F.J.; Barros, E. Effect of Earthworm Addition on Soil Nitrogen Availability, Microbial Biomass and Litter Decomposition in Mesocosms. Biol. Fertil. Soils 2004, 39, 146–152. [Google Scholar] [CrossRef]
- Bohlen, P.J.; Edwards, C.A. Earthworm Effects on N Dynamics and Soil Respiration in Microcosms Receiving Organic and Inorganic Nutrients. Soil Biol. Biochem. 1995, 27, 341–348. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Guhr, A.; Marzini, C.; Borken, W.; Poll, C.; Matzner, E. Effect of Water Redistribution by Two Distinct Saprotrophic Fungi on Carbon Mineralization and Nitrogen Translocation in Dry Soil. Soil Biol. Biochem. 2016, 103, 380–387. [Google Scholar] [CrossRef]
- Rousk, J.; Nadkarni, N.M. Growth measurements of saprotrophic fungi and bacteria reveal differences between canopy and forest floor soils. Soil Biol. Biochem. 2009, 41, 862–865. [Google Scholar] [CrossRef]
- Watkins, J.E.; Mack, M.C.; Sinclair, T.R.; Mulkey, S.S. Ecological and Evolutionary Consequences of Desiccation Tolerance in Tropical Fern Gametophytes. New Phytol. 2007, 176, 708–717. [Google Scholar] [CrossRef] [PubMed]


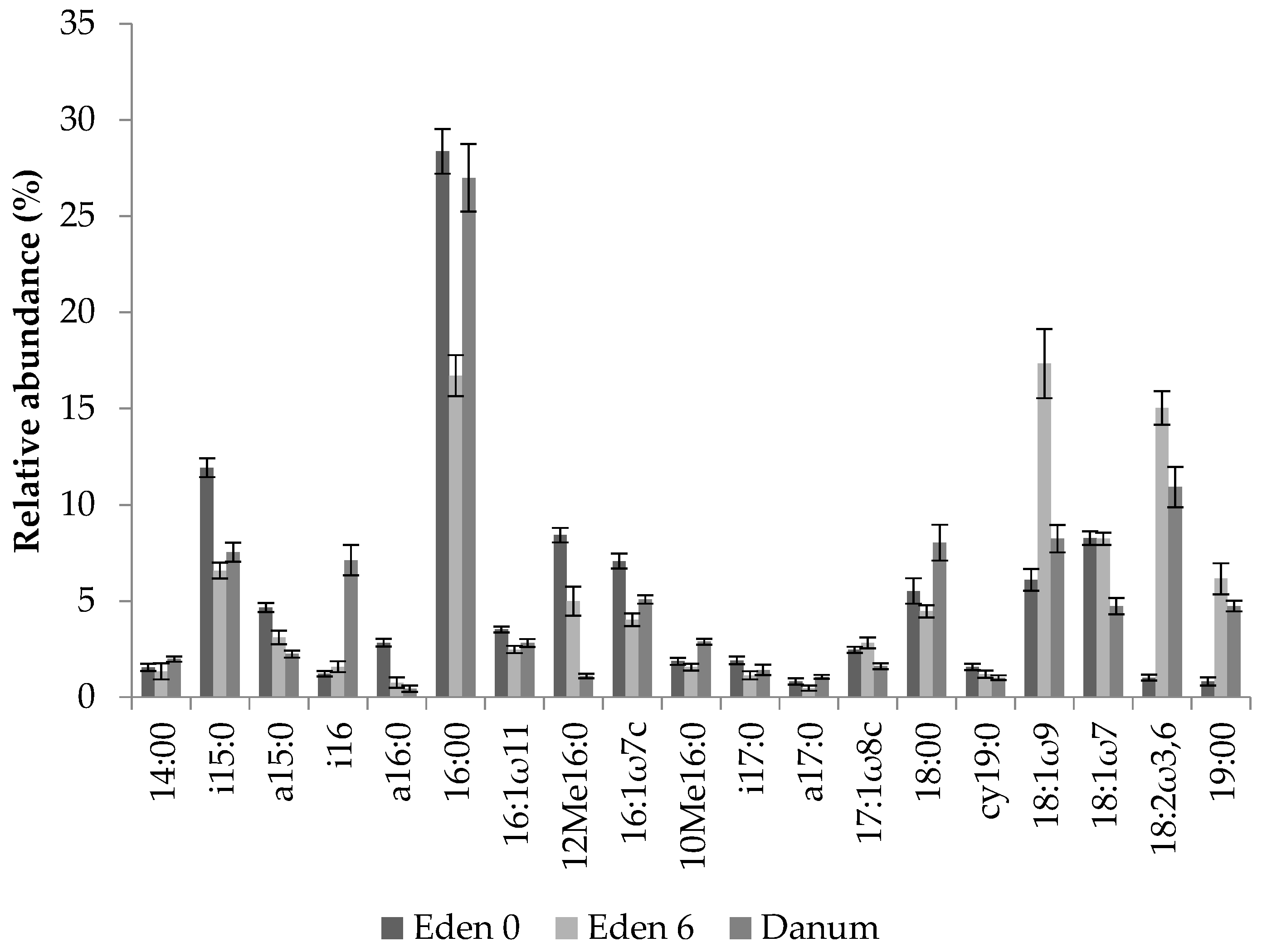
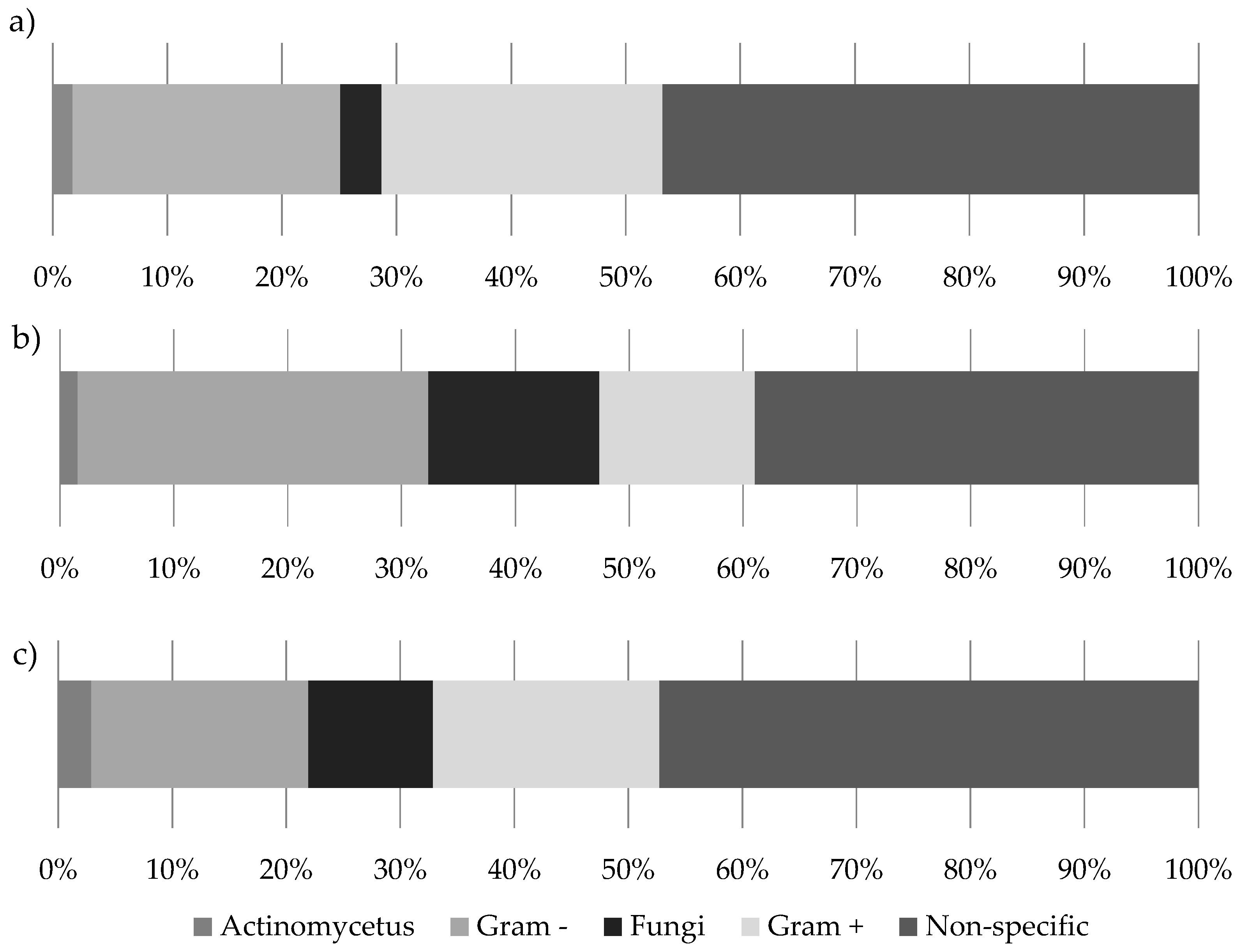
| Microbial Group | PLFA Peaks |
|---|---|
| Gram + bacteria | i15:0, a15:0, i16:0, a16:0, i17:0, a17:0 |
| Gram − bacteria | 16:1ω7c, cy19:0, 18:1ω9, 18:1ω7 |
| Actinomycetes | 10Me16:0 |
| Fungi | 18:2ω3,6 |
| N-a-glucosaminidase | β-glucosidase | Phenol oxidase | Fungi:Bacteria | |
|---|---|---|---|---|
| Time | F1,52 = 42.2 p < 0.001 | F1,49 = 46.0 p < 0.001 | F1,52 = 55.0 p < 0.001 | F1,43 = 29.1 p < 0.001 |
| Treatment | F3,52 = 0.918 p = 0.439 | F3,49 = 0.754 ns p = 0.526 | F3,52 = 0.508 p = 0.678 | F3,43 = 2.30 p = 0.090 |
| Ti x Tr | F3,52 = 0.910 ns p = 0.443 | F3,49 = 0.288 ns p = 0.834 | F3,52 = 0.666 ns p = 0.577 | F3,43 = 0.455 ns p = 0.722 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donald, J.; Bonnett, S.; Cutler, M.; Majalap, N.; Maxfield, P.; Ellwood, M.D.F. Physical Conditions Regulate the Fungal to Bacterial Ratios of a Tropical Suspended Soil. Forests 2017, 8, 474. https://doi.org/10.3390/f8120474
Donald J, Bonnett S, Cutler M, Majalap N, Maxfield P, Ellwood MDF. Physical Conditions Regulate the Fungal to Bacterial Ratios of a Tropical Suspended Soil. Forests. 2017; 8(12):474. https://doi.org/10.3390/f8120474
Chicago/Turabian StyleDonald, Julian, Sam Bonnett, Michael Cutler, Noreen Majalap, Pete Maxfield, and M. D. Farnon Ellwood. 2017. "Physical Conditions Regulate the Fungal to Bacterial Ratios of a Tropical Suspended Soil" Forests 8, no. 12: 474. https://doi.org/10.3390/f8120474





