Fire-Driven Decline of Endemic Allosyncarpia Monsoon Rainforests in Northern Australia
Abstract
:1. Introduction
2. Materials and Methods
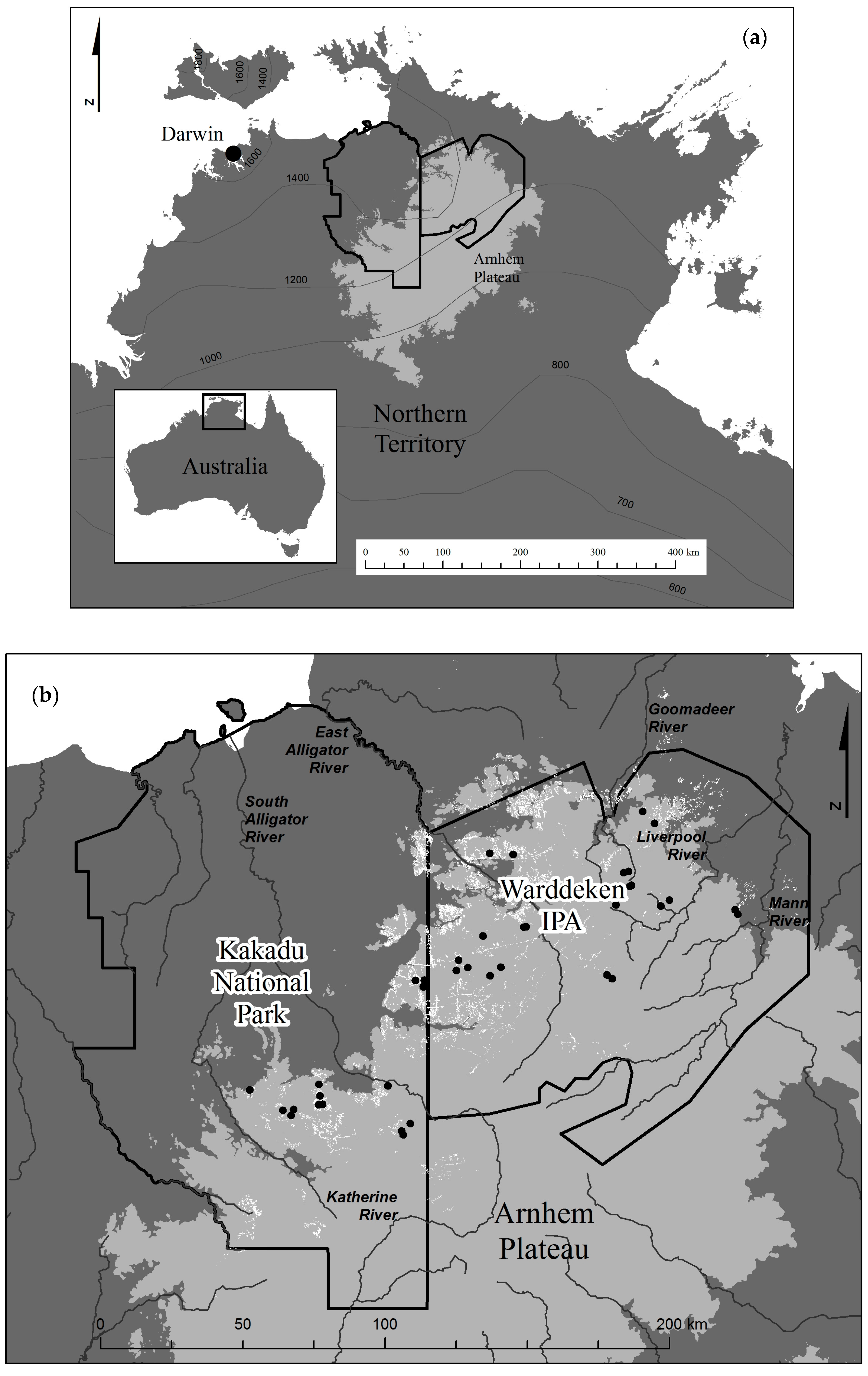
2.1. Study Area
2.2. Allosyncarpia Distribution Mapping
2.3. Canopy Cover Change Assessment
2.4. On-Ground Vegetation Structure Assessment of Canopy Change
3. Results
3.1. Allosyncarpia Forest Distribution
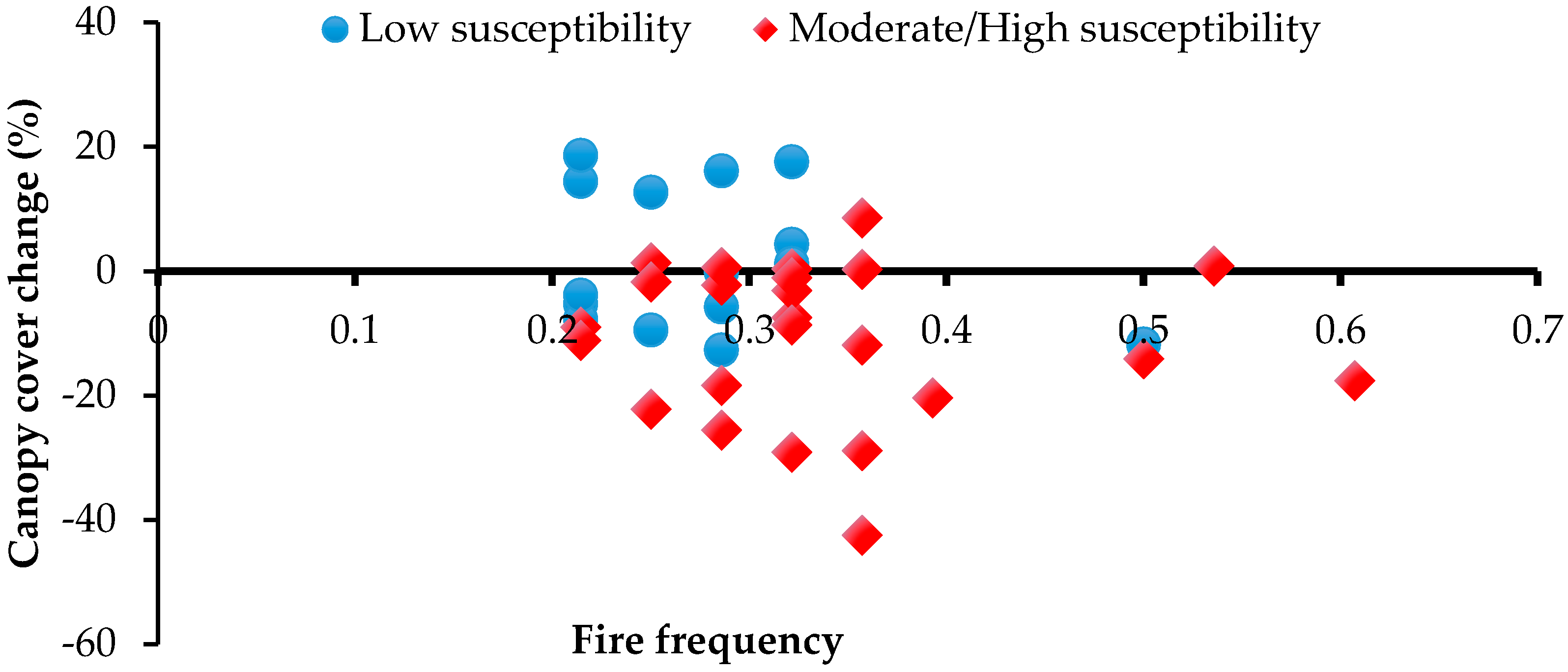
3.2. Canopy Cover Change
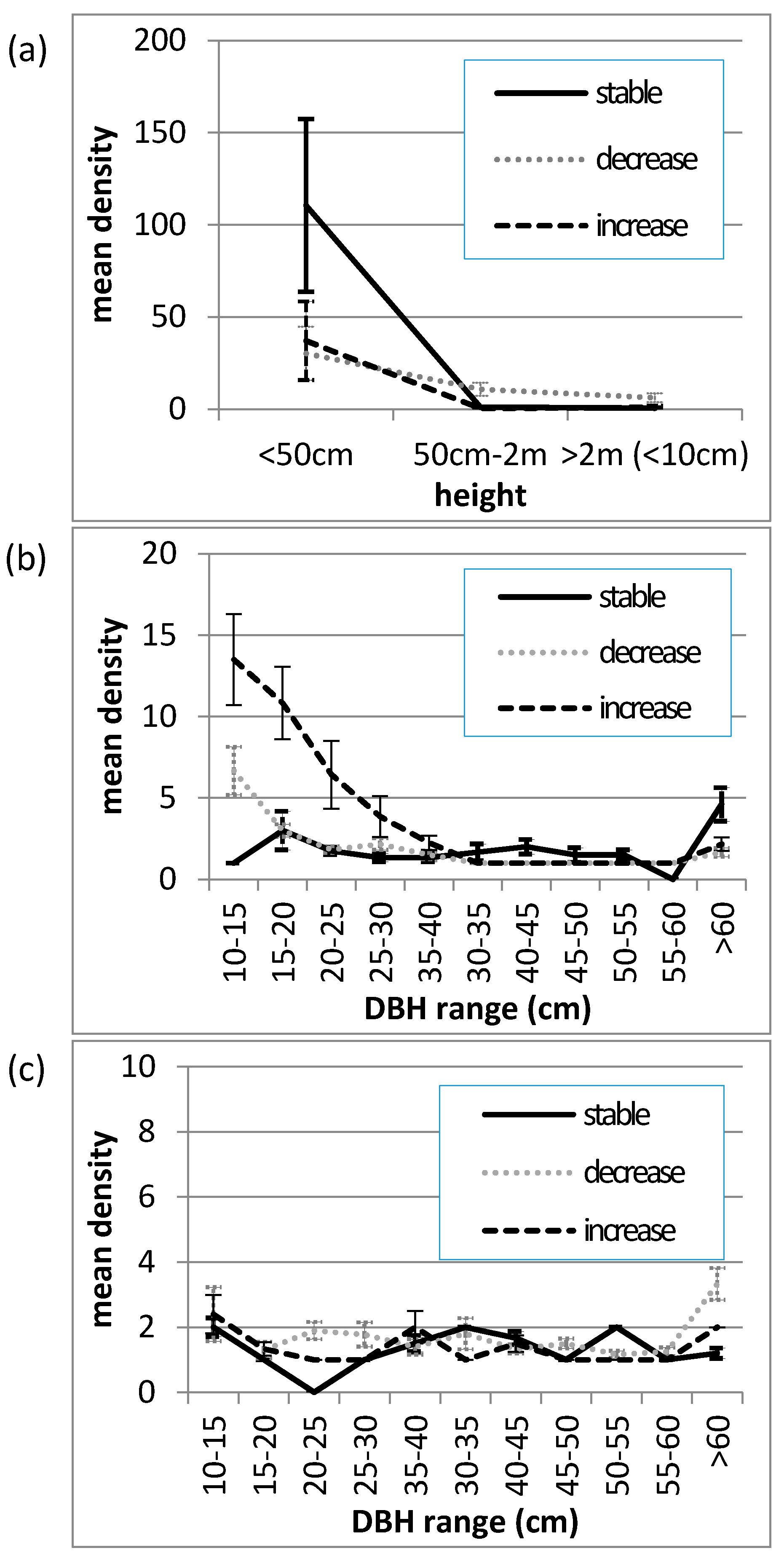
3.3. On-Ground Assessment
4. Discussion
Conservation Management
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| %Change | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site Name | Long. | Lat. | Area in 1950 (ha) | 1950–1980’s | 1980’s–2010 | 1950–2010 | %Fire–Prone | Fire Susceptibility |
| Mimolorrk a | 133.7130 | −12.4673 | 79 | 0% | −12% | −12% | 25 | Low |
| Mimolorrk b | 133.6800 | −12.4266 | 29 | 2% | −18% | −16% | 35 | Moderate |
| Tin camp south a | 133.1920 | −12.5620 | 24 | 3% | 1% | 4% | 40 | Moderate |
| Tin camp south b | 133.1920 | −12.5620 | 32 | −5% | −9% | −14% | 35 | Moderate |
| Korbelak a | 133.6180 | −12.6217 | 27 | −10% | −29% | −36% | 60 | High |
| Korbelak b | 133.6320 | −12.6204 | 48 | −7% | −22% | −28% | 75 | High |
| Kondulgai a | 133.7610 | −12.7112 | 23 | −2% | −20% | −22% | 55 | High |
| Andulgai b | 133.7360 | −12.7289 | 14 | −7% | 13% | 5% | 10 | Low |
| Makkalarl head a | 133.6410 | −12.6631 | 15 | 3% | 0% | 3% | 45 | Moderate |
| Makkalarl tail b | 133.6410 | −12.6631 | 35 | −5% | −42% | −46% | 75 | High |
| Nakarriken b | 133.5880 | −12.7226 | 36 | −10% | −11% | −20% | 80 | High |
| Yidngarremanrneng a | 133.9770 | −12.7566 | 122 | −16% | 9% | −9% | 60 | High |
| Yidngarremanrneng b | 133.9680 | −12.7414 | 35 | −8% | 18% | 9% | 2 | Low |
| East alligator a | 133.3040 | −12.7953 | 14 | 12% | 19% | 32% | 25 | Low |
| East alligator b | 133.3100 | −12.7935 | 22 | −8% | 14% | 5% | 30 | Moderate |
| Maguk a | 132.4050 | −13.3153 | 13 | 2% | −14% | −13% | 40 | Moderate |
| Maguk b | 132.4120 | −13.3102 | 8 | −1% | 1% | 0% | 55 | High |
| Magela creek a | 133.1690 | −12.8224 | 12 | −6% | −2% | −7% | 65 | High |
| Namarrgon creek a | 133.1190 | −12.9210 | 14 | −8% | −6% | −14% | 30 | Moderate |
| Namarrgon creek b | 133.1190 | −12.9210 | 15 | −2% | −5% | −7% | 5 | Low |
| Namarrgon creek d | 133.0990 | −12.8991 | 12 | 0% | 1% | 1% | 25 | Low |
| Hill u662 a | 133.1930 | −12.9500 | 13 | 10% | −13% | −4% | 25 | Low |
| Hill u663 b | 133.2280 | −12.9214 | 32 | −5% | −8% | −12% | 25 | Low |
| Deaf Adder north b | 132.5700 | −13.3727 | 4 | −6% | −2% | −8% | 70 | High |
| Deaf Adder north c | 132.9590 | −12.9667 | 15 | 0% | −4% | −4% | 25 | Low |
| Deaf Adder north d | 132.9850 | −12.9641 | 24 | 5% | −9% | −5% | 5 | Low |
| Deaf Adder north e | 132.9850 | −12.9641 | 10 | −1% | −9% | −9% | 40 | Moderate |
| Kunbambuk a | 133.5820 | −12.9612 | 5 | −5% | −9% | −14% | 60 | High |
| Kunbambuk b | 133.5780 | −12.9576 | 36 | −21% | −29% | −44% | 60 | High |
| Kunbambuk c | 133.5630 | −12.9465 | 18 | −26% | −18% | −40% | 60 | High |
| Round jungle a | 133.0990 | −12.8991 | 148 | 1% | 1% | 2% | 65 | High |
| Round jungle b | 132.6550 | −13.3294 | 37 | −3% | −7% | −10% | 50 | High |
| Round jungle c | 132.6480 | −13.2931 | 55 | 4% | −1% | 3% | 55 | High |
| Round jungle d | 132.6480 | −13.2931 | 5 | 10% | 0% | 10% | 25 | Low |
| Barramundie gorge a | 132.5620 | −13.3912 | 22 | −5% | −3% | −8% | 50 | High |
| Barramundie gorge c | 132.5700 | −13.3727 | 9 | 6% | −26% | −21% | 33 | Moderate |
| Barramundie gorge d | 132.5700 | −13.3727 | 21 | −8% | 4% | −4% | 15 | Low |
| Gimbat north a | 133.3100 | −12.7935 | 57 | −5% | −12% | −17% | 40 | Moderate |
| Gimbat north b | 132.9190 | −13.4550 | 35 | −3% | 0% | −3% | 40 | Moderate |
| Gimbat north c | 132.9400 | −13.4172 | 26 | −22% | 16% | −9% | 20 | Low |
References
- Trapnell, C.G. Ecological results of woodland burning experiments in northern Rhodesia. J. Ecol. 1959, 47, 129–168. [Google Scholar] [CrossRef]
- Rose-Innes, R. Fire in West African vegetation. In Proceedings of the Tall Timbers Fire Ecology Conference, Tallahassee, FL, USA, 22–23 April 1972. [Google Scholar]
- San Jose, J.J.; Farinas, M.R.; Rosales, J. Spatial patterns of trees and structuring factors in a Trachypogon savana of the Orinoco Llanos. Biotropica 1991, 23, 114–123. [Google Scholar] [CrossRef]
- Swaine, M.D.; Hawthorne, W.D.; Orgle, T.K. The effects of fire exclusion on savanna vegetation at Kpong, Ghana. Biotropica 1992, 24, 166–172. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Price, O.; Whitehead, P.J.; Walsh, A. The “wilderness effect” and the decline of Callitris intratropica on the Arnhem Land Plateau, northern Australia. Aust. J. Bot. 2001, 49, 665–672. [Google Scholar] [CrossRef]
- Bowman, D.M.; Murphy, B.P.; Banfai, D.S. Has global environmental change caused monsoon rainforests to expand in the Australian monsoon tropics? Landsc. Ecol. 2010, 25, 1247–1260. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Stanton, A.C.; Whitehead, P.J. Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: II. Rates of landscape change. J. Biogeogr. 2004, 31, 1305–1316. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Whitehead, P.J.; Cook, G.D.; Hoare, J.L. Response of Eucalyptus-dominated savanna to frequent fires: Lessons from Munmarlary, 1973–1996. Ecol. Monogr. 2003, 73, 349–375. [Google Scholar] [CrossRef]
- Higgins, S.I.; Bond, W.J.; February, E.C.; Bronn, A.; Euston-Brown, D.I.W.; Esline, B.; Govender, N.; Rademan, L.; O’Regan, S.; Potgieter, A.L.F.; et al. Effects of four decades of fire manipulation on woody vegetation structure in savanna. Ecology 2007, 88, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.E.; Prior, L.D.; Bowman, D.M. Decadal dynamics of tree cover in an Australian tropical savanna. Austral Ecol. 2009, 34, 601–612. [Google Scholar] [CrossRef]
- Stevens, N.; Erasmus, B.; Archibald, S.; Bond, W. Woody encroachment over 70 years in South African savannahs: Overgrazing, global change or extinction aftershock? Philos. Trans. R. Soc. B 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.; José, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Van der Werf, G.; Randerson, J.L.; Collatz, G.; Mu, M.; Kasibhatla, P.; Morton, D.; Defries, R.; Jin, Y.; van Leeuwen, T. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009). Atmos. Chem. Phys. 2010, 10, 11707–11735. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.I.; Scheiter, S. Atmospheric CO2 forces abrupt vegetation shifts locally, but not globally. Nature 2012, 488, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Scheiter, S.; Higgins, S.; Beringer, J.; Hutley, L.B. Climate change and long-term fire management impacts on Australian savannas. New Phytol. 2015, 205, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Beringer, J.; Hutley, L.B.; Abramson, D.; Arndt, S.K.; Briggs, P.; Bristow, M.; Canadell, J.G.; Cernusak, L.A.; Eamus, D.; Edwards, A.C.; et al. Fire in Australian savannas: From leaf to landscape. Glob. Chang. Biol. 2015, 21, 62–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, D.C.; Whitehead, P.J.; Pardon, G.; Matthews, J.; McMahon, P.; McIntyre, D. Geographic patterns and correlates of the decline of granivorous birds in northern Australia. Biol. Conserv. 2005, 90, 53–68. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Legge, S. Rowley Review: The impacts of fire on birds in Australia’s tropical savannas. Emu 2013, 113, 319–352. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Legge, S.; Fitzsimons, J.A.; Traill, B.J.; Burbidge, A.A.; Fisher, A.; Firth, R.S.C.; Gordon, I.J.; Griffith, A.D.; Johnson, C.N.; et al. The disappearing mammal fauna of Australia: Context, cause, and response. Conserv. Lett. 2011, 4, 192–201. [Google Scholar] [CrossRef]
- Ziembicki, M.R.; Woinarski, J.C.Z.; Webb, J.K.; Vanderduys, E.; Tuft, K.; Smith, J.; Ritchie, E.G.; Reardon, T.B.; Radford, I.J.; Preece, N.; et al. Stemming the tide: Progress towards resolving the causes of decline and implementing management responses for the disappearing mammal fauna of northern Australia. Therya 2015, 6, 169–225. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Panton, W.J. Decline of Callitris intratropica in the Northern Territory: Implications for pre- and post-European colonization fire regimes. J. Biogeogr. 1993, 20, 373–381. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Ryan, P.G.; Cheal, D.C. Fire regimes and the conservation of sandstone heath in monsoonal northern Australia: Frequency, interval, patchiness. Biol. Conserv. 2002, 104, 91–106. [Google Scholar] [CrossRef]
- Yates, C.P.; Edwards, A.C.; Russell-Smith, J. Big fires and their ecological impacts in Australian savannas: Size and frequency matters. Int. J. Wildland Fire 2008, 17, 768–781. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S. The impact of Aboriginal landscape burning on the Australian biota. New Phytol. 1998, 140, 385–410. [Google Scholar] [CrossRef]
- Ritchie, D. Things Fall Apart: The End of an Era of Systematic Indigenous Fire Management. In Culture, Ecology and Economy of Savanna Fire Management in Northern Australia: Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P.J., Cooke, P.M., Eds.; CSIRO Publications: Melbourne, Australia, 2009. [Google Scholar]
- Yibarbuk, D.; Whitehead, P.J.; Russell-Smith, J.; Jackson, D.; Godjuwa, C.; Fisher, A.; Cooke, P.; Choquenot, D.; Bowman, D. Fire ecology and Aboriginal land management in central Arnhem Land, Northern Australia: A tradition of ecosystem management. J. Biogeogr. 2001, 28, 325–343. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Yates, C.P.; Whitehead, P.J.; Smith, R.; Craig, R.; Allan, G.E.; Thackway, R.; Frakes, I.; Cridland, S.; Meyer, M.C.P.; et al. Bushfires ‘down under’: Patterns and implications of contemporary Australian landscape burning. Int. J. Wildland Fire 2007, 16, 361–377. [Google Scholar] [CrossRef]
- Edwards, A.; Russell-Smith, J.; Meyer, M. Contemporary fire regime risks to key ecological assets and processes in north Australian savannas. Int. J. Wildland Fire 2015, 24, 857–870. [Google Scholar] [CrossRef]
- Murphy, B.P.; Lehmann, C.E.R.; Russell-Smith, J.; Lawes, M.J. Fire regimes and woody biomass dynamics in northern Australian savannas. J. Biogeogr. 2014, 41, 133–144. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Stanton, P.J.; Whitehead, P.J.; Edwards, A.C. Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: I. Successional Processes. J. Biogeogr. 2004, 31, 1293–1303. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Risler, J.; Kean, L. Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecol. 2004, 29, 156–176. [Google Scholar] [CrossRef]
- Banfai, D.; Bowman, D. Forty years of lowland monsoon rainforest expansion in Kakadu National Park, Northern Australia. Biol. Conserv. 2006, 131, 553–565. [Google Scholar] [CrossRef]
- Bowman, D.M.; Dingle, J.K. Late 20th century landscape-wide expansion of Allosyncarpia ternata (Myrtaceae) forests in Kakadu National Park, northern Australia. Aust. J. Bot. 2006, 54, 707–715. [Google Scholar] [CrossRef]
- Brook, B.W.; Bowman, D.M. Postcards from the past: Charting the landscape-scale conversion of tropical Australian savanna to closed forest during the 20th century. Landsc. Ecol. 2006, 21, 1253–1266. [Google Scholar] [CrossRef]
- Ondei, S.; Prior, L.D.; Vigilante, T.; Bowman, D.M. Fire and cattle disturbance affects vegetation structure and rain forest expansion into savanna in the Australian monsoon tropics. J. Biogeogr. 2017, 44, 2331–2342. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Stanton, P. Fire Regimes and Fire Management of Rainforest Communities across Northern Australia: A Review. In Flammable Australia: The Fire Regimes and Biodiversity of a Continent; Bradstock, R.A., Williams, J.E., Gill, A.M., Eds.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Banfai, D.S.; Bowman, D.M.J.S. Drivers of rain-forest boundary dynamics in Kakadu National Park, northern Australia: A field assessment. J. Trop. Ecol. 2007, 23, 73–86. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Lucas, D.E.; Brock, J.; Bowman, D.M.J.S. Allosyncarpia-dominated rain forest in monsoonal northern Australia. J. Veg. Sci. 1993, 4, 67–82. [Google Scholar] [CrossRef]
- Haynes, C.D. The pattern and ecology of munwag: Traditional aboriginal fire regimes in north-central Arnhem Land. In Ecology of the Wet-Dry Tropics, Proceedings of the Ecological Society of Australia 13, 1985; CSIRO: Melbourne, Australia, 1985. [Google Scholar]
- Bowman, D.; Prior, L. Impact of Aboriginal landscape burning on woody vegetation in Eucalyptus tetrodonta savanna in Arnhem Land, northern Australia. J. Biogeogr. 2004, 31, 807–817. [Google Scholar] [CrossRef]
- Prior, L.D.; Bowman, D.M.J.S.; Brook, B.W. Growth and survival of two north Australian relictual tree species, Allosyncarpia ternata (Myrtaceae) and Callitris intratropica (Cupressaceae). Ecol. Res. 2007, 22, 228–236. [Google Scholar] [CrossRef]
- Edwards, A.C.; Russell-Smith, J. Ecological thresholds and the status of fire-sensitive vegetation in western Arnhem Land, northern Australia: Implications for management. Int. J. Wildland Fire 2009, 18, 127–146. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Edwards, A.C.; Woinarski, J.C.Z.; McCartney, J.; Kerin, S.; Winderlich, S.; Murphy, B.P.; Watt, F. An assessment of the first ten years of the three parks (Kakadu, Litchfield, Nitmiluk) fire regime and biodiversity monitoring. In Culture, Ecology and Economy of Fire Management in North Australian Savannas: Rekindling the WURRK Tradition; Russell-Smith, J., Whitehead, P., Cooke, P., Eds.; CSIRO Publishing: Melbourne, Australia, 2009. [Google Scholar]
- Woinarski, J.C.; Russell-Smith, J.; Andersen, A.N.; Brennan, K. Fire management and biodiversity of the western Arnhem Land Plateau. In Culture, Ecology and Economy of Fire Management in North Australian Savannas: Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P.J., Cooke, P.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2009; pp. 201–228. [Google Scholar]
- Commonwealth of Australia. The Nationally Protected Arnhem Plateau Sandstone Shrubland Complex; Department of Sustainability, Environment, Water, Population and Communities, Australian Government: Canberra, Australia, 2012. Available online: http://www.environment.gov.au/resource/nationally-protected-arnhem-plateau-sandstone-shrubland-complex (accessed on 10 April 2015).
- Thackway, R.; Cresswell, I. An Interim Biogeographic Regionalisation for Australia: A Framework for Establishing the National System of Reserves; Version 4.0; Australian Nature Conservation Agency: Canberra, Australia, 1995; p. 88.
- Ingwersen, F. Kakadu-Alligator Rivers Region, Northern Territory. In Centres of Plant Diversity: A Guide and Strategy for Their Conservation. Vol. 2. Asia, Australasia and the Pacific; Davis, S.D., Hamilton, A.C., Eds.; WWF and IUCN: Cambridge, UK, 1995; pp. 471–475. [Google Scholar]
- Crisp, M.D.; Laffan, S.; Linder, H.P.; Monro, A. Endemism in the Australian flora. J. Biogeogr. 2001, 28, 183–198. [Google Scholar] [CrossRef]
- Emerson, D.; Mills, K.; Miyakawa, K.; Hallett, M.L.; Cao, L. The petrophysics, geophysics and structure of the Koongarra site, Northern Territory. Explor. Geophys. 1993, 24, 1–71. [Google Scholar] [CrossRef]
- McAlpine, J.R. Climate and water balance. In Land Systems of the Alligator Rivers Area, Northern Territory; Land Research Series No. 38; Story, R., Ed.; CSIRO: Melbourne, Australia, 1976; pp. 35–49. [Google Scholar]
- Finlayson, C.M.; von Oertzen, I. Landscape and Vegetation Ecology of the Kakadu Region, Northern Australia; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 23. [Google Scholar]
- Russell-Smith, J.; Setterfield, S.A. Monsoon rain forest seedling dynamics, northern Australia: Contrasts with regeneration in eucalypt-dominated savannas. J. Biogeogr. 2006, 33, 1597–1614. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Ryan, P.G.; Klessa, D.; Waight, G.; Harwood, R. Fire regimes, fire-sensitive vegetation, and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia. J. Appl. Ecol. 1998, 35, 829–846. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Whitehead, P.; Cooke, P. Managing Fire Regimes in North Australian Savannas—Ecology, Culture, Economy. Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P., Cooke, P., Eds.; CSIRO Publishing: Canberra, Australia, 2009. [Google Scholar]
- Russell-Smith, J.; Edwards, A.C. Seasonality and fire severity in savanna landscapes of monsoonal northern Australia. Int. J. Wildland Fire 2006, 15, 541–550. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Edwards, A.C.; Price, O.F. Simplifying the savanna: The trajectory of fire-sensitive vegetation mosaics in northern Australia. J. Biogeogr. 2012, 39, 1303–1317. [Google Scholar] [CrossRef]
- Trauernicht, C.; Murphy, B.P.; Tangalin, N.; Bowman, D.M. Cultural legacies, fire ecology, and environmental change in the Stone Country of Arnhem Land and Kakadu National Park, Australia. Ecol. Evol. 2013, 3, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.G.; Jones, R.; Smith, M.A. Thermoluminescence dating of a 50,000-year-old human occupation site in northern Australia. Nature 1990, 345, 153–156. [Google Scholar] [CrossRef]
- Clarkson, C.; Jacobs, Z.; Marwick, B.; Fullagar, R.; Wallis, L.; Smith, M.; Roberts, R.G.; Hayes, E.; Lowe, K.; Carah, X. Human occupation of northern Australia by 65,000 years ago. Nature 2017, 547, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Russell-Smith, J.; Lucas, D.; Gapindi, M.; Gunbunuka, B.; Kapirigi, N.; Namingum, G.; Lucas, K.; Giuliani, P.; Chaloupka, G. Aboriginal resource utilization and fire management practice in western Arnhem Land, monsoonal northern Australia: Notes for prehistory, lessons for the future. Hum. Ecol. 1997, 25, 159–196. [Google Scholar] [CrossRef]
- Garde, M.; Nadjamerrek, L.B.; Kolkkiwarra, M.; Kalarriya, J.; Djandjomerr, J.; Birriyabirriya, B.; Bilindja, R.; Kubarkku, M.; Biless, P. The Language of Fire: Seasonality, Resources and Landscape Burning on the Arnhem Land Plateau. In Managing Fire Regimes in North Australian Savannas—Ecology, Culture, Economy; Russell-Smith, J., Whitehead, P., Eds.; CSIRO Publishing: Canberra, Australia, 2009; pp. 86–164. [Google Scholar]
- Cooke, P.M. Buffalo and Tin, Baki and Jesus: The Creation of a Modern Wilderness. In Culture, Ecology and Economy of Savanna Fire Management in Northern Australia: Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P.J., Cooke, P.M., Eds.; CSIRO Publications: Melbourne, Australia, 2009. [Google Scholar]
- Geoscience Australia. 1 Arc Second—Digital Elevation Model; US National Geospatial-Intelligence Agency (NGA), US National Aeronautics and Space Administration (NASA), Eds.; Geoscience Australia: Canberra, Australia, 2000.
- Geoscience Australia. Australia 1:250,000 Geological Series; Geoscience Australia: Canberra, Australia, 1963–1998.
- Morgan, J.L.; Gergel, S.E.; Coops, N.C. Aerial photography: A rapidly evolving tool for ecological management. BioScience 2010, 60, 47–59. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Ryan, P.G.; Durieu, R. A LANDSAT MSS-derived fire history of Kakadu National Park, monsoonal northern Australia, 1980–94: Seasonal extent, frequency and patchiness. J. Appl. Ecol. 1997, 34, 748–766. [Google Scholar] [CrossRef]
- Williams, R.J.; Gill, A.M.; Moore, P.H.R. Fire Behaviour. In Fire in Tropical Savannas: The Kapalga Experiment; Andersen, A., Cook, G., Williams, R., Eds.; Springer: New York, NY, USA, 2003; pp. 33–46. [Google Scholar]
- Fensham, R.; Fairfax, R. Assessing woody vegetation cover change in north-west Australian savanna using aerial photography. Int. J. Wildland Fire 2003, 12, 359–367. [Google Scholar] [CrossRef]
- Fensham, R.; Fairfax, R.; Ward, D. Drought-induced tree death in savanna. Glob. Chang. Biol. 2009, 15, 380–387. [Google Scholar] [CrossRef]
- Smith, I. An assessment of recent trends in Australian rainfall. Aust. Meteorol. Mag. 2004, 53, 163–173. [Google Scholar]
- Bureau of Meteorology. Climate Change Trend Maps. Available online: http://www.bom.gov.au/climate/change/index.shtml#tabs=Tracker&tracker=trend-maps (accessed on 5 August 2016).
- Roderick, M.L.; Farquhar, G.D. Changes in Australian pan evaporation from 1970 to 2002. Int. J. Climatol. 2004, 24, 1077–1090. [Google Scholar] [CrossRef]
- Lewis, S.L.; Lloyd, J.; Sitch, S.; Mitchard, E.T.; Laurance, W.F. Changing ecology of tropical forests: Evidence and drivers. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 529–549. [Google Scholar] [CrossRef]
- Van Der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.; Boom, A.; Bongers, F.; Pons, T.L.; Terburg, G.; Zuidema, P.A. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2015, 8, 24–28. [Google Scholar] [CrossRef]
- Stevens, N.; Lehmann, C.E.; Murphy, B.P.; Durigan, G. Savanna woody encroachment is widespread across three continents. Glob. Chang. Biol. 2017, 23, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Russell-Smith, J.; Price, O.; Murphy, B.P. Managing the matrix: Decadal responses of eucalypt-dominated mesic savanna to ambient fire regimes. Ecol. Appl. 2010, 20, 1615–1632. [Google Scholar] [CrossRef] [PubMed]
- Trauernicht, C.; Murphy, B.P.; Prior, L.D.; Lawes, M.J.; Bowman, D.M. Human-imposed, fine-grained patch burning explains the population stability of a fire-sensitive conifer in a frequently burnt northern Australia savanna. Ecosystems 2016, 19, 896–909. [Google Scholar] [CrossRef]
- Kennedy, R.E.; Townsend, P.A.; Gross, J.E.; Cohen, W.B.; Bolstad, P.; Wang, Y.Q.; Adams, P. Remote sensing change detection tools for natural resource managers: Understanding concepts and tradeoffs in the design of landscape monitoring projects. Remote Sens. Environ. 2009, 113, 1382–1396. [Google Scholar] [CrossRef]
- Russell-Smith, J. The Forest in Motion: Exploratory Studies in Western Arnhem Land, Northern Australia. In The Forest in Motion: Exploratory Studies in Western Arnhem Land, Northern Australia; Australian National University: Canberra, Australia, 1986. [Google Scholar]
- Bowman, D.M.J.S. Environmental determinants of Allosyncarpia ternata forests that are endemic to western Arnhem Land, northern Australia. Aust. J. Bot. 1991, 39, 575–589. [Google Scholar] [CrossRef]
- Fordyce, I.R.; Eamus, D.; Duff, G.A.; Williams, R.J. The role of seedling age and size in the recovery of Allosyncarpia ternata following fire. Aust. J. Ecol. 1997, 22, 262–269. [Google Scholar] [CrossRef]
- Fordyce, I.R.; Eamus, D.; Duff, G.A. Episodic seedling growth in Allosyncarpia ternata, a lignotuberous monsoon rainforest tree of tropical Australia. Austral Ecol. 2000, 25, 25–35. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S. Preliminary observations on the mortality of Allosyncarpia ternata stems on the Arnhem Land plateau, northern Australia. In Australian Forestry; Institute of Foresters of Australia: Perth, Australia, 1994; pp. 62–64. [Google Scholar]
- Wilson, B.A.; Bowman, D.M.J.S. Fire, storm, flood and drought: The vegetation ecology of Howards Peninsula, Northern Territory, Australia. Aust. J. Ecol. 1987, 12, 165–174. [Google Scholar] [CrossRef]
- Hutley, L.; Evans, B.; Beringer, J.; Cook, G.; Maier, S.; Razon, E. Impacts of an extreme cyclone event on landscape-scale savanna fire, productivity and greenhouse gas emissions. Environ. Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Cook, G.D.; Goyens, C.M. The impact of wind on trees in Australian tropical savannas: Lessons from Cyclone Monica. Austral Ecol. 2008, 33, 462–470. [Google Scholar] [CrossRef]
- Russell-Smith, J. The Status and Condition of Monsoon Vine-Forests in the Kakadu Region: A Management Report. In Unpublished Report to Australian National Parks and Wildlife Service, Canberra; Australian National Parks and Wildlife Service: Canberra, Australia, 1984. [Google Scholar]
- Petty, A.; Werner, P.; Lehmann, C.; Riley, J.; Banfai, D.; Elliott, L. Savanna responses to feral buffalo in Kakadu National Park, Australia. Ecol. Monogr. 2007, 77, 441–463. [Google Scholar] [CrossRef]
- Archer, S.; Schimel, D.S.; Holland, E.A. Mechanisms of shrubland expansion: Land use, climate or CO2? Clim. Chang. 1995, 29, 91–99. [Google Scholar] [CrossRef]
- Scholes, R.J.; Archer, S.R. Tree-grass interactions in savannas. Annu. Rev. Ecol. Syst. 1997, 28, 517–544. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, G.F. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob. Chang. Biol. 2000, 6, 865–869. [Google Scholar] [CrossRef]
- Murphy, B.P.; Cochrane, M.A.; Russell-Smith, J. Prescribed burning protects endangered tropical heathlands of the Arnhem Plateau, northern Australia. J. Appl. Ecol. 2015, 52, 980–991. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Evans, J.; Edwards, A.C.; Simms, A. Assessing ecological performance thresholds in fire-prone Kakadu National Park, northern Australia. Ecosphere 2017, 8, e01856. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Yates, C.P.; Edwards, A.C.; Whitehead, P.J.; Murphy, B.P. Deriving multiple benefits from carbon market-based savanna fire management: An Australian example. PLoS ONE 2015, 10, e0143426. [Google Scholar] [CrossRef] [PubMed]


| Selection Criteria | Metric |
|---|---|
| Functional analysis size | Area 10–150 ha |
| Distinct, isolated, forest patch | Minimum 100 m distance from another patch >10 ha |
| Functional shape | Patch perimeter (km): area (ha) ratio within parameters, as below: Patch 10–39 ha: <15 Patch 40–79 ha: <12.5 Patch 80–150 ha: <10 |
| Broad geographic distribution | Patches from western, eastern, southern regions, as defined by contiguous 1:250,000 topographic mapping covering the majority of Allosyncarpia’s range |
| Not associated with major river | Exclude patches associated with stream order >5 |
| Not in path of major cyclone, especially very damaging Cyclone Monica in 2006 | Exclude patches <50 km from coast |
| Topographic variation | Include patches both in relatively subdued sandstone, and open sandy terrain, but exclude patches in steep (>20°) terrain to ensure assessment reliability |
| Image availability | Available historical aerial photos at consistent scales, and 2010 high resolution satellite imagery |
| Image quality | Amenable for visual interpretation |
| Cost efficiency | If possible, select more than one patch meeting above criteria per imagery sample |
| Geographic Context | Arnhem Plateau Study Area (%) | Allosyncarpia Distribution (%) | Sampled Forest Patches (%; No. of Sites Given in Parentheses) |
|---|---|---|---|
| Mean annual rainfall (mm) | |||
| 1200–1300 | 23.0% | 0 | 0 |
| 1300–1400 | 42.0% | 34.0% | 35.0% (14) |
| 1400–1500 | 25.0% | 32.0% | 37.5% (15) |
| 1500–1600 | 10.0% | 33.0% | 27.5% (11) |
| Proximity to Drainage | |||
| <250 m from major river | 1.0% | 0 | |
| <25 m from stream | 8.0% | 8 sites | |
| Terrain | |||
| steep: >20% | 3.0% | 14.0% | 0% |
| rugged: 5–20% | 23.0% | 59.0% | 37.5% (15) |
| flat: <5% | 74.0% | 27.0% | 62.5% (25) |
| Surface type | |||
| Rocky | 51.5% | 90.0% | 60.0% (23) |
| Sandy | 48.5% | 10.0% | 40.0% (17) |
| Interaction between terrain and surface | |||
| Steep rocky | 2.0% | 12.0% | 0% |
| Steep x sandy | 1.0% | 2.0% | 0% |
| Rugged x rocky | 17.0% | 54.5% | 37.5% (15) |
| Rugged x sandy | 5.0% | 4.5% | 0.0% |
| Flat x rocky | 32.5% | 23.0% | 22.5% (9) |
| Flat x sandy | 42.5% | 4.0% | 40.0% (16) |
| Canopy Cover Change | ||||
|---|---|---|---|---|
| Geographic Context | No. of Patches | 1950–1982/87 | 1982/87–2010 | 1950–2010 |
| Patch trend summary | ||||
| Mean trend | 40 | (−3.7 ± 1.3) | (−6.1 ± 2.1) | (−9.5 ± 2.4) |
| No. declining patches | 22 | 23 | 29 | |
| No. increasing patches | 8 | 7 | 8 | |
| No. stable patches | 10 | 10 | 3 | |
| Warddeken IPA 1 | 24 | (−4.9 ± 1.5) | (−8.4 ± 2.4) | (−8.4 ± 2.2) |
| Kakadu National Park | 16 | (−1.6 ± 0.5) | (−4.1 ± 1.4) | (−6.0 ± 1.9) |
| Rainfall zone | ||||
| 1300–1400 mm | 14 | −8.1 ± 2.7 | −12.2 ± 3.9 | −19.2 ± 6.1 |
| 1400–1500 mm | 15 | −2.3 ± 0.5 | −2.0 ± 0.7 | −4.6 ± 1.5 |
| 1500–1600 mm | 11 | 0.0 ± 0.2 | −3.8 ± 1.2 | −3.8 ± 1.6 |
| Terrain | ||||
| Steep: >20° | 0 | |||
| Rugged: 5–20° | 10 | (−2.2 ± 0.8) | (−1.7 ± 0.6) | (−4.1 ± 1.4) |
| Flat: <5° | 19 | (−7.5 ± 2.3) | (−7.7 ± 2.3) | (−14.3 ± 4.3) |
| Mixed (Flat and Rugged) | 11 | (1.4 ± 0.5) | (−7.2 ± 2.5) | (−6.1 ± 2.1) |
| Surface type | ||||
| Sandstone/Rocky | 25 | (−3.8 ± 1.0) | (−5.3 ± 1.4) | (−10.3 ± 0.5) |
| Sandy plain | 15 | (−3.5 ± 1.6) | (−7.3 ± 2.5) | (−9 ± 3.0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeman, J.; Edwards, A.C.; Russell-Smith, J. Fire-Driven Decline of Endemic Allosyncarpia Monsoon Rainforests in Northern Australia. Forests 2017, 8, 481. https://doi.org/10.3390/f8120481
Freeman J, Edwards AC, Russell-Smith J. Fire-Driven Decline of Endemic Allosyncarpia Monsoon Rainforests in Northern Australia. Forests. 2017; 8(12):481. https://doi.org/10.3390/f8120481
Chicago/Turabian StyleFreeman, Jeremy, Andrew C. Edwards, and Jeremy Russell-Smith. 2017. "Fire-Driven Decline of Endemic Allosyncarpia Monsoon Rainforests in Northern Australia" Forests 8, no. 12: 481. https://doi.org/10.3390/f8120481




