1. Introduction
Forest ecosystems contain about 45% of the terrestrial carbon (C), and they interact dynamically with the atmosphere through the sequestration of ecosystem C, deforestation, and forest degradation [
1]. Anthropogenic greenhouse gas (GHG) emissions in 2010 reached 49 ± 4.5 Gt CO
2-eq/year globally. Emissions of CO
2 from land use, land-use change, and forestry processes contributed about 4.1 Gt CO
2-eq for 2016. Most GHG emissions from the forestry sector in Mexico come from deforestation and ecosystem degradation processes. Estimates of deforestation rates in Mexico are inaccurate due to differences in the classification of the forest cover; for example, estimates ranging from 400,000 to 1.5 million ha/year have been reported for 1980–1989 [
2]. However, there is an apparent consensus on rates of around 500,000 ha/year [
3,
4]. It has been reported that the vegetation types with the highest loss of cover area are tropical forests (42% of original area), followed by temperate forests (27%) and scrublands (10%) (
Table 1) [
4]. In general, there is also consensus that the highest deforestation rates have occurred in southeastern Mexico (190,000 ha per year), which are twice as high as those reported in the northwest (96,000 ha per year) and three times those in the west (62,000 ha per year) [
5]. Net carbon emissions to the atmosphere from the deforestation of temperate and tropical forests in Mexico have been estimated at 67 × 10
6 t C/year, with 54.1 × 10
6 t C/year allocated to tropical forests, and the remaining 12.9 × 10
6 t C/year to temperate forests [
2]. Recently, net carbon emissions from changes in land use included 32.4 × 10
6 of CO
2-eq, which accounts for 4.9% of the total emissions. The emissions by forest land converted to pastureland, settlements, and other types of land use, as well as from fires, reached 45.0 × 10
6 CO
2-eq released in 2013. Worldwide total anthropogenic GHG emissions have continued to increase, with larger absolute increases particularly between 2000–2010, despite a growing number of policies aimed at mitigating climate change [
6].
The GHG emission levels from the Mexican forestry sector are relatively low compared with other productive activities (cement industry, energy use, transportation, etc.) [
2]. Mitigation by the forestry sector can make a very significant contribution to a low-cost global mitigation portfolio that provides synergies with adaptation and sustainable development, including extending carbon retention in harvested wood products, product substitution, and biomass production by forest plantations to meet society’s needs for timber, fiber, and energy [
6]. In Mexico, the development of strategies to reduce or mitigate emissions in the forestry sector is imperative not only for the mitigation of climate change per se, but also because of the relationships that emissions have with economic themes associated with the forestry sector of the country (negative trade balance, low industrial development), social matters (poverty and the marginalization of peasants and forest communities), and biological issues (loss of biodiversity) [
7]. These issues have been at the core of Mexican national policies in the forestry sector. For example, recent policies have promoted the further intensification of harvesting of natural forests, the execution of aggressive reforestation programs, and the establishment of commercial plantations, in an attempt to reverse the historical trends of the Mexican forestry sector [
8,
9]. These strategies can be summarized as a transition to planted forests in Mexico, since all of them lead to the establishment of forested and reforested areas at various scales, intensities, and objectives.
The new global scenarios of climate change and the forestry context in Mexico require a rethinking of forest management and development strategies, not only to reverse negative trends, but also to align them with economic, social, and environmental issues [
6]. In this respect, we believe that forest management in its broadest sense—including the management of temperate and tropical forest, afforestation, reforestation, and the establishment of mixed plantations—should constitute the foundation of the new forestry strategy in our country. C sequestration through forest management and forest plantations in Mexico is a real possibility, as it may include and complement all forestry activities as well as mitigate climate change. However, this requires that all efforts aiming to recover and quantify the forest cover area in Mexico be incorporated into management schemes that contribute to sequestering atmospheric carbon and mitigating the effects of climate change, while providing economic options to forest owners through diversifying the use of the country’s forest resources. This paper presents an analysis of the role of forest plantations and the forestry sector as part of a national strategy to face global processes such as climate change and the reduction of GHG, to which the country has made important commitments. Although the paper focuses mainly on the role of forest plantations in carbon sequestration and storage, associated issues e.g., biodiversity and other ecosystem services, are highlighted in the Mexican forestry context.
Planted forests (also refer to as cultivated forests) are generally considered those established with seedlings from forest nurseries, in accordance with particular designs and purposes. This analysis adopts a broad concept of planted forests, which includes not only traditional forest plantations (monocultures), but also forests established through reforestation and afforestation efforts, as well as managed natural forests.
2. Traditional Forest Plantations
The global area of forest plantations has increased over the past decade, representing ~7% of the global forest area, and the relative rate of annual expansion has been ~2% [
10]. Commercial forest plantations (CFP) are established artificially in suitable land through site preparation and direct planting under highly controlled conditions, and are cultivated and intensively managed for the production of timber or raw materials for industry and trade, thereby strengthening the regional and national economy [
11]. The global coverage of commercial forest plantations has the potential to sequester large amounts of carbon in tree biomass [
12], and it is assumed that they protect the soil, provide environmental benefits, promote sustainable forest development, preserve ecosystems, and protect wildlife [
11,
13]. Most of the reforestation responsible for a gain in forested area in the tropics has been conducted in the form of industrial monocultures involving a limited number of species e.g., fast-growing tropical timber species of genera
Tectona,
Eucalyptus,
Pinus, and
Acacia [
14,
15], which are exotic to most of the areas where they are cultivated.
Currently, some estimates indicate that Mexico has the potential to establish over 10 million hectares of CFP [
11]; since their productivity exceeds that of natural forests by far [
16], commercial forest plantations in Mexico are enormously attractive from a productive point of view. As a consequence, some government incentive programs promote their establishment. Since the 1990s, the establishment of commercial forest plantations has formed part of the strategy to increase timber production and reverse the deficit in the trade balance of forest products in the country [
11,
13]. This trend represents a valuable opportunity to reassess the role of planted forest in the context of new scenarios derived from global change processes such as climate change and biodiversity loss, whose negative effects on forest resources in the mid- and long term have not been clearly identified. For example, ecosystems restoration, the diversification of production, and the delivery of ecosystem services can be included as specific objectives in national programs such as reforestation, payments for environmental services, and the establishment and development of commercial forest plantations. We argue that, with some adaptations, the majority of the objectives mentioned above can be incorporated into the programs of planted forests in Mexico in such a way that two or more objectives are achieved simultaneously, hence contributing to a more efficient use of resources and reinforcement of the country’s capacity to comply with international commitments to the reduction of carbon emissions and the conservation of biodiversity.
3. Forest Management in Mexico
The overall effect of forest management on GHG emissions depends on the type of forest, its management, the type of timber materials produced, and the efficiency of biomass conversion. Therefore, forest management can affect the net C exchange with the atmosphere to a large extent, by both affecting the amount of C stored in the vegetation and soil, and altering the local productivity pathway. Since a large part of the C in harvested products is not readily released to the atmosphere, but rather remains stored in long-lasting products, forest management can contribute to increases in the total amount of carbon sequestration. Some studies have provided estimates of C sequestration in Mexico from sustainable forest management, the conservation of natural protected areas, reforestation, and the restoration of degraded lands [
17,
18]. Also, it has been estimated that ~38 Pg C might be sequestered in 345 × 10
6 ha over a period of 50 years through forestation, reforestation, and agroforestry practices [
19]. In Mexico, silviculture and temperate forest management has followed the inertia driven by other countries (mainly the United States and Canada) to develop its own dynamics in terms of use and the conservation of forest resources [
20,
21,
22]. Hence, similar approaches used in those countries have been applied in Mexico, despite the markedly different circumstances and conditions. From the 1940s, uneven aged silviculture based on the selection silvicultural system was widely used, followed by more intensive systems during the 1980s based on clear-cuts, seed-tree regeneration methods, and thinning, to establish even aged forests [
20].
Under both these silvicultural regimes, albeit more notably in the intensive systems, the forests produced can be considered planted forests, since it is a regulatory requirement [
23] that owners ensure the establishment, survival, and development of seedlings in harvested units. Hence, reforestation after each logging intervention is a widespread practice, and natural regeneration is rather complementary. This practice leads to some level of control of the establishment, composition, and development of the future forest. In some cases, the control of the regeneration of managed forests may involve the reduction of, or changes in, the diversity of tree species, along with the simplification of stand structures where the regeneration and growth of commercial timber species are promoted. For example, studies of the temperate forests of northern Mexico have documented changes in species composition and stand structures as a result of long periods of forest management [
24,
25]. Likewise, under the extensive system, the regeneration of the new stands in harvested areas is also through reforestation. Although efforts are made to maintain structures and a composition of tree species similar to the natural forest, the established seedlings do not always come from natural regeneration. The most extreme cases have occurred when species that are different from those of the original forest are introduced into harvested areas in an attempt to comply with regulatory requirements or to improve the composition of secondary forests. This situation has been common in both tropical areas of Mexico [
26] and temperate forests; for example, from 1962 to 1985, over 36 million seedlings were planted in a forest area of 42,700 hectares in an industrial forest unit in central Mexico [
27]. In summary, natural regeneration occurs to a very low extent in managed forest areas under the two forestry systems applied. Hence, in practice, forest management translates into the management of planted forests within natural forests areas, especially under the current national strategy to increase timber production in Mexico. Under such a strategy, many forested lands are currently shifting from less to more intensive regimes, which might impair the role of the natural forest in climate change mitigation. Intensive silviculture, with shorter harvesting intervals and more intensive logging (i.e., thinning, clear-cuts) generally reduces net carbon storage rates and carbon storage at the stand level, compared with low-intensity silviculture (e.g., the selection system) [
28]. In addition, low-intensity silviculture may create stand structures and a composition more suitable for storing carbon, and disturbance resistance that may prevent catastrophic events such as wildfires. High-severity fires can increase soil erosion, alter nutrient cycling, and decrease post-fire seedling recruitment, thus leading to long-term losses of carbon [
28]. Thus, under climate change projections, natural forest management in Mexico should turn to less intensive methods that allow for some level of timber production while keeping a continuous forest cover to deliver ecosystem services. The intensive management of natural forests should be considered in selected areas where timber production can be maximized to compensate for low levels of timber production under extensive management. Additionally, the management of natural forests in Mexico should induce and maintain some forests as legacies (with minimum management) to provide ecosystem services (long-term carbon storage) and serve as a genetic reserve (i.e., old growth forests).
Afforestation and Reforestation
Afforestation and reforestation (tree planting outside of natural forests), including those carried out in urban or peri-urban areas, may also be reasonably considered planted forests to some extent, even when their success in Mexico has been very limited. Some studies indicate survival rates of <40% in reforestations in Mexico [
29]; these are rarely perceived as forests, since many remain as open forests, with low tree densities and trees of different sizes, which are products of the high variability in the quality of seedlings used and poor planting and cultivation practices. The cumulative reforested area in Mexico from 1960 to the mid-1980s has been estimated at more than 400,000 hectares, which amounts to approximately 145,000 hectares established, on the basis of a survival rate of 34% [
2]. This area is likely significantly greater today, given the strong support for reforestation programs in Mexico; hence, a conservative estimate of an additional area totaling more than 500,000 hectares is reasonable, even after maintaining survival rates similar to those reported for 1980. Efforts to offset deforestation in Mexico resulted in approximately 250,000 hectares reforested per year in the past decade [
29]. Therefore, these areas should be considered planted forests for some objectives; for instance, effective reforestation can contribute to mitigating the annual CO
2 emissions from forests [
29]. This is relevant because most reforestation initiatives in Mexico take place on rural land that has been abandoned, degraded, or recovered from other uses, or in urban areas where GHG emissions can be substantial. In addition, since these programs are not implemented for harvesting purposes, they fulfill important ecological functions over long periods of time. However, net increases in soil carbon might be highly variable owing to differences in climate, age, tree type, and soil depth across sites, and only modest gains in soil carbon could be expected in most locations for several decades [
28]. Currently, the role of reforested and afforested areas is not evaluated, either for carbon sequestration or for other ecosystem services, these being considered only as elements of national cover forest recovery.
Reforestations, especially restoration initiatives, have not succeeded in the provision of benefits to forest producers [
2], so there is no longer interest in maintaining them. Therefore, the production of goods and services is an aspect that should be taken care of in reforested areas. Nonetheless, options remain very limited in this regard, since most of the plant production in official and private nurseries dedicated to reforestation programs contemplates only four pine species and two tropical species, with a number of shortcomings in the planning and management of reforested areas. In this context, it is important to consider the strategies proposed by some studies to improve reforestation programs in Mexico [
29,
30], in order to contribute to climate change mitigation.
4. Planted Forests: A Different Panorama
Planting forests is an important practice for climate change mitigation, especially in the tropics, where the carbon sequestration potential is high [
6,
10]. Successful implementation requires knowledge of the roles of species identity and diversity on the carbon accrual of plantations. In Mexico, as in other parts of the world, commercial forest plantations have relied on monocultures to increase productivity, with little or no emphasis on their potential role in the reduction of global warming by acting as carbon sinks [
31], nor in the provision of other ecosystem services. The production of exclusive goods from monocultures (timber, pulp, oils, etc.), rather than ecosystem services, is a trend imposed on the majority of forest plantations comprising fast-growing and exotic species for industrial purposes, for reasons of productivity and predictability in production [
16]. Although the primary aim of industrial plantations is not carbon sequestration or the conservation of biodiversity, several studies argue that with some adjustments, even the most intensive monocultures can contribute greatly in these areas while fulfilling their productive function [
16].
Worldwide, the most commonly planted species are of the genus
Pinus (~20%), followed by
Eucalyptus (10%),
Hevea (5%),
Acacia (4%), and
Tectona (3%). Other broadleaf species account for 18%, and other conifers account for 11% [
32]. In Mexico, commercial forest plantations have been promoted since the 1990s, mainly in the southeast (where 68% of the plantations in the country have been established), in order to take advantage of the subtropical climate of the region and promote the rapid growth of plantations, in addition to the use of exotic fast-growing species such as melina (
Gmelina arborea Roxb
.), teak (
Tectona grandis L.f.), and eucalyptus (
Eucalyptus ssp.), as well as some high-value native species such as the red cedar (
Cedrela odorata L
.) [
11]. Although it has been stated that plantations in Mexico seek to diversify forest production, timber production accounts for the vast majority of plantations (66% of the planted area). From 2010, new plantation schemes qualified for government support, including the production of non-timber forest products (species growing in arid and tropical areas), oil production, and Christmas tree plantations [
11]. Likewise, the list of priority species was expanded to 50 species (12 non-timber and 38 timber species) likely to be supported under the criteria set forth by CONAFOR (the National Forestry Commission). Currently, however, only six species cover more than 86% of the area planted in Mexico, and the total absence of oak species in this program is worth noting.
In terms of productivity, CONAFOR estimates indicate that the mean annual growth of eucalyptus and melina plantations is in the order of 20 m
3/ha/year, while the mean annual growth oscillates around 12 m
3/ha/year for teak, is more than 9 m
3/ha/year for pine species; and finally, for high-value species such as mahogany (
Switenia macrophylla King
.) and red cedar, is approximately 5 m
3/ha/year [
11]. The plantations established with these species are not yet ready for harvesting, but it is expected that with these increment rates and the projected surface to 2025 (over 600,000 ha), the contribution of plantations could reach approximately 4 million m
3/year [
11]. In comparison with other countries, productivity is likely to be lower in Mexico. For example, in general, the growth and mean yield of young teak plantations in Campeche are lower than those reported in other tropical American countries where the species has been established for commercial plantation, which may be attributable to both the germplasm and the management techniques [
33]. This situation might have also influenced the low number of species most commonly used in established plantations (currently less than eight), nearly half of which (45% of the area) are exotic.
From the outset, the commercial forest plantations in Mexico have been conceived as a strategy to increase the productivity of the forestry sector, with an emphasis on seeking financing schemes and economic and legal incentives to promote their establishment [
34]. While these are essential aspects, others factors that are no less important have been neglected, including the development of sources of improved germplasm, new technologies for high-quality seedlings production, and the production of native species with high productive potential. For example, genetic improvement processes in Mexico for native valuable species such as red cedar,
Pinus patula Schiede ex Schltdl. & Cham., and
P. pseudostrobus Lindl. are still in the early development stages, which require at least a decade to pass before the first results are attained. The case of
P. patula illustrates this situation, since other countries (e.g., South Africa) have already developed successful genetic improvement programs for the species [
35], while in Mexico, similar programs are still in progress [
36]. Similarly, technologies for the production of high-quality seedlings in nurseries for commercial plantation and reforestation programs still suffer from shortcomings that should be addressed through specific research at the local level [
29]. Consequently, the establishment of commercial forest plantations has resorted to the importation of germplasm and production technologies that are adapted with limitations to local and regional conditions, leading to the results mentioned above. Such a situation contrasts sharply with the great diversity of tree species that prosper in the forests of Mexico, with >150 different oak species [
37], and >70 taxa of pine [
38], not to mention the tropical species. The above reflects a limited use of the country’s vast forest resources for the establishment of planted forests as part of a strategy to mitigate emissions.
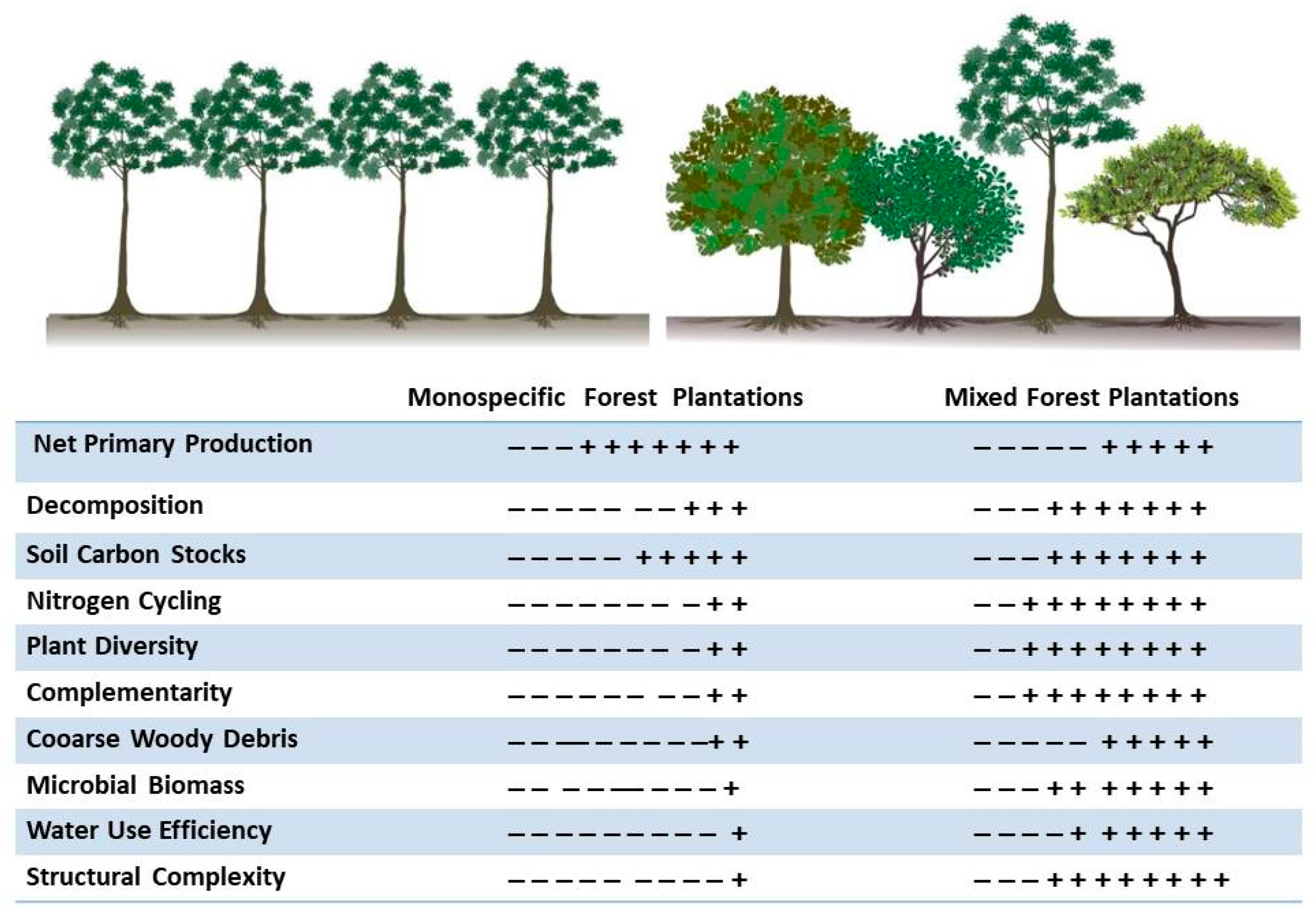
Moreover, ecological and biological aspects such as species diversity, structural diversity, complexity, and complementarity, could be enhanced in plantations but are often ignored. Instead, the role of plantations is limited to biomass production. Currently, most plantations are monospecific with even aged structures, i.e., with a predominance of one or two age groups [
11], indicating the prevalence of simple structures in these plantations. This trend has been maintained for reasons of productivity and costs. Such an approach overlooks the great potential of plantations regarding ecosystem services, which are then neither evaluated nor incorporated in economic and financial assessments. These priorities have limited the accounting of carbon captured or temporarily stored in commercial forest plantations in Mexico.
5. Mixed Forest Plantations in Mexico, Beyond the Mitigation of Climate Change and Productivity
Commercial forest plantations generally rely on a monoculture of exotic species whose selection is based on economic effectiveness criteria rather than biodiversity or ecosystem services. However, plantations of any type can be multifunctional, even when emphasis is given to a particular objective. Multifunctionality does not necessarily imply mixed species (two or more) plantations, but these are more desirable, because they allow the combination and arrangement of species (functions) to achieve diverse objectives in the same place at different times. Single-species plantations can also be multifunctional if biological functions are highlighted through structural diversity involving mosaics of stands of various ages and maintaining permanent buffer strips to reduce the edge effect with other land uses. Hence, the spatial and temporal combination of species is a key aspect in maintaining or improving local biodiversity and increasing the resistance and resilience of plantations [
16]. Well-planned mixed plantations can mimic the structural development of natural forests, making plantations less vulnerable to pests or diseases [
39], and reducing the risk of fire and other disturbances that are likely to increase under climate change scenarios. For this reason, mixed plantations should also give priority, to the extent possible, to the use of native species rather than exclusively exotic species [
40], even when the former are not genetically improved, in order to mimic natural forests. As it has been stated, a consistent mitigation measure is the diversification of forest management units to derive greater structural and/or compositional heterogeneity [
41].
In Mexico, it is possible to proactively design a scheme for the establishment of mixed plantations that produce various materials (timber and non-timber forest products), as well as deliver ecosystem services that provide social and economic benefits through a greater tree species diversity in both temperate and tropical regions. However, the successful implementation of this type of plantation requires knowledge of the role of species identity and diversity in carbon buildup in plantations, because a high variability in underground C stocks has been observed in multispecies forest plantations. In addition to species identity, the proportion of species planted within the total area and the species richness should both be considered composition parameters that affect the significance of the carbon dioxide capture. For example, the inclusion of species with different growth rates between deciduous and evergreen may allow differential harvest times in mixed plantations. This would enable a functional complementarity that is suitable for production purposes as well as for the generation of environmental services, while also maintaining a permanent forest cover as well as above- and belowground carbon pools. Also, mixed species stands must combine species that are complementary in characteristics such as shade tolerance, rate of height growth, crown structure, foliar and root phenology, and root depth. Understanding the interdependence of ecological processes that occur in forest plantations is essential in order to ensure the future provision of goods and services by both managed and planted forests [
42,
43] (
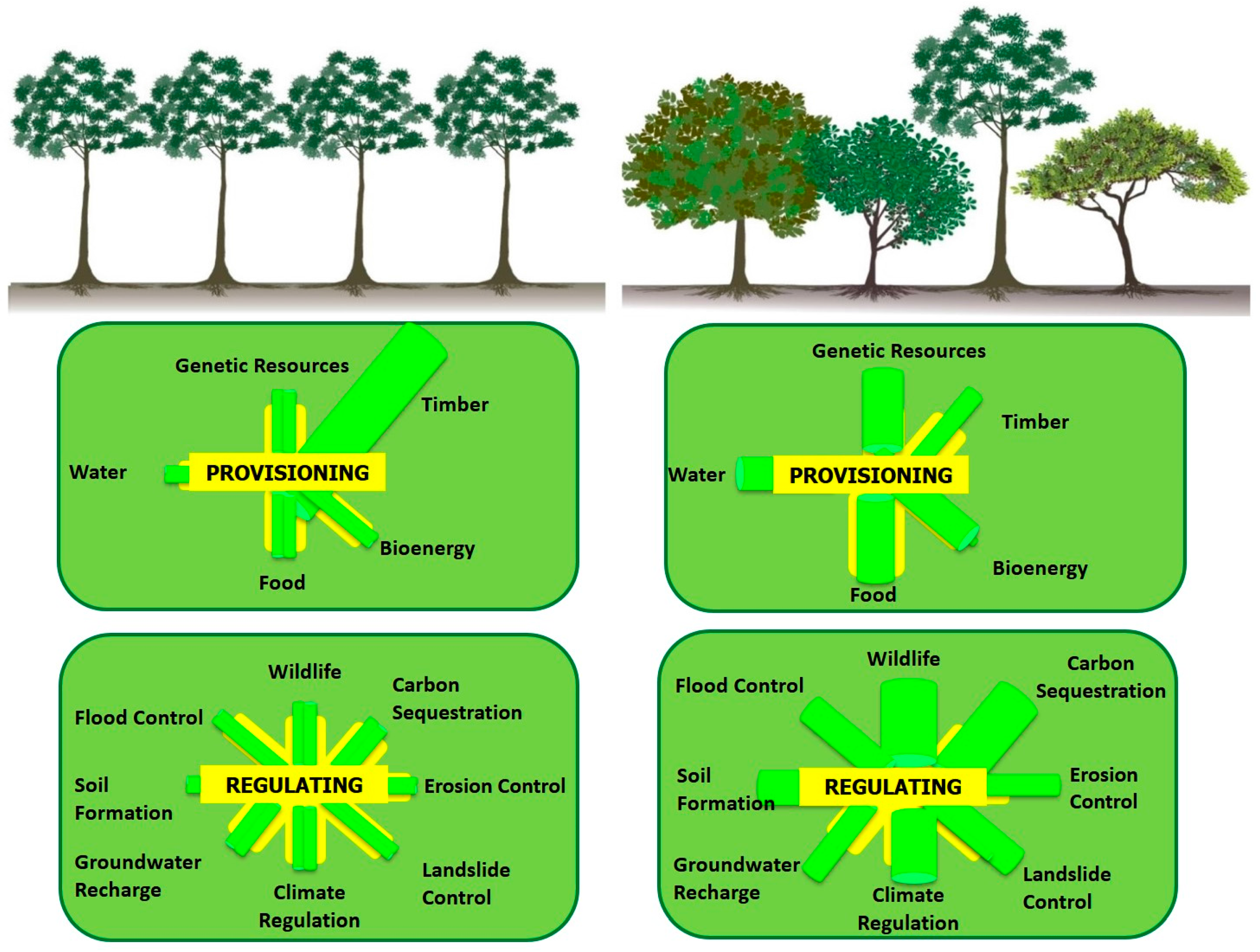
Figure 1). In Mexico, knowledge should also be sought regarding the impact of various plant species and their response to environmental conditions [
44]. Currently, very little is known in Mexico about the variability in primary productivity related to the composition of tree species and the effect of specific mixtures, which opens up an area of opportunity for the study of the primary productivity of planted forests.
The benefits of mixed-species plantations include improved soil fertility and nutrient cycling, reduced soil erosion, greater resilience against pests, aesthetic improvement of the landscape, the provision of wildlife habitats or biological corridors, and the restoration of biodiversity in degraded land [
45] (
Figure 2). The assessment of primary productivity has gained special importance from the point of view of climate change mitigation and the sustainable use of forest resources. In China, for example, emphasis has been given to support forest productivity and growth through mixtures of species (conifer and broad-leaved). Their findings indicate that these mixtures provide the maximum ecological benefits for conservation, protection, and restoration relative to monospecific pine-planted forests, although the latter have a higher commercial value. Ecological processes must be understood so that proposals of mixed plantations consider the functional characteristics of each species, thereby enhancing forest productivity. For example, regarding the biomass production of
Eucalyptus spp. in tropical ecosystems, mixed plantations that included nitrogen-fixing species (i.e.,
Albizia falcataria (
L.),
Acacia mearnsii De Wild.) increased
Eucalyptus biomass production and the storage of C in soil, and had greater nutrient availability than monospecific plantations [
46,
47]. However, mixtures of
A. falcataria and
E. saligna Sm., in Hawaii had no effect on soil P (phosphorus) transformation processes in comparison with pure stands. The effects of mixed plantations on soil biogeochemistry have had a variety of responses, ranging from a linear influence on soil N (nitrogen) and C, to antagonistic dynamics between N and P availability [
46]. The creation of more diverse structures and associated vegetation in plantations will also contribute to a better carbon balance through increased primary productivity, as well as highlight their role in the mitigation of CO
2 emissions. For example, conifer species tend to sequester a little more C (<5–10%) than deciduous species in the same region, although this difference is less important than the differences resulting from management practices at a regional level [
48]. However, rather than a simple linear relationship between species composition and soil C storage, belowground species interactions may be more complex when coarse and fine root biomass are measured.
Forests can sequester C, but this leads to other important biophysical changes, such as the exchange of energy and mass between the land surface and the atmosphere, and the amount of water recycled to the atmosphere through evapotranspiration [
49]. A permanent forest cover is essential in order to mitigate the effects of climate change (higher temperatures and less precipitation). Here, both planted and managed natural forests can make a contribution, not only through harvest practices and schedules [
50], but also by using combinations of species with diverse functional properties. For example, deciduous trees have two features that may make them more effective for local cooling: first, they have a summer albedo of up to 0.1 (on a 0 to 1 reflexivity scale) higher than coniferous forests, depending on the region [
51]. Second, in midsummer, they have a higher canopy conductance (the ease with which plants transpire water) and an evaporative fraction that is approximately twofold that of coniferous forests [
52]. The additional transpiration of deciduous canopies results in local cooling and the potential formation of clouds that could lead to an increased albedo and lower temperatures when the incoming solar radiation approaches its peak annual level [
53]. Hence, deciduous species can provide more biophysical cooling than conifer species. Tropical forests cool the atmosphere at local and regional scales [
49,
54] because their high rates of transpiration contribute to cloud formation, considerably reducing both surface temperatures and the amount of sunlight reaching the Earth’s surface [
55]. The rapid growth that characterizes exotic plantation trees suggests that these species are also high water users; that exotic timber stands can use much more water than native forest has significant implications for the management of forested areas for hydrologic services in addition to other ecological services and objectives [
56].
Worldwide, plantations constitute a significant proportion of the total forest area, resulting in a strong anthropogenic influence on the composition of forest stands. The composition, structure, and function of plantations are often highly simplified, because they are composed of a single tree species and a single age cohort [
57]; therefore, they can hardly be fully “friendly” for wildlife [
58]. Hence, structural diversity is a generally accepted means of enhancing levels of biodiversity through the provision of a greater diversity of microhabitats [
59] (
Figure 2). Some studies have demonstrated that non-managed forests provide better conditions for flora and fauna than plantations [
60,
61] as a result of the positive effect of diversity on productivity. Additionally, practices such as a greater tolerance to the associated vegetation, especially once the plantation has been established, can be incorporated to promote greater diversity and complexity in the shrub and herb strata that favor the presence of wildlife [
58]. Other studies comparing ground vegetation in conifer monocultures with deciduous broadleaf monocultures/semi-natural broadleaf forests have also found that shrub species and forest specialist herbs occur more commonly in broadleaf stands (e.g.,
Fraxinus excelsior L.,
Quercus robur L
./
Q.
petraea (Matt
.) Liebl.) than in conifer monocultures, especially heavy-shading conifer species (e.g.,
Picea sitchensis (Bong.) Carr.,
Picea abies (L.) H. Karst
.) [
62]. However, other studies found no differences in animal abundance between the controls and the various types of retention islands, although significantly lower animal numbers were found in clear-felled areas [
63]. Similarly, there were no significant differences in species richness, diversity, or evenness when comparing species present in the ground vegetation of mixtures and monocultures of oak and Scots pine in three different geographical regions.
Barriers and Possible Tradeoffs in Mexico
The establishment of forestry-related projects in Mexico to mitigate the effects of climate change would involve barriers and tradeoffs. As it has been pointed out: “it is impossible to make changes to the forest and forest products system that are large enough to have an impact on atmospheric CO
2 concentration without also having large effects on other ecosystems or ecosystem services” [
28]. Uncertainties, co-benefits, and tradeoffs for carbon mitigation strategies have been outlined elsewhere (such as Table 2 in McKinley et al. [
28]), some of these observations (those related to forest management and afforestation) could be applicable to the Mexican situation. Additionally, most of the tradeoffs and barriers for the carbon strategy of mixed forest plantations in Mexico relate to socio-economic aspects, such as the structure of land tenure and differences in ecological, economic, and social characteristics among regions. For instance, studies in northern Mexico have shown the importance of the relationship between property regimes and the extension of property, and the effectiveness of strategies to conserve biodiversity [
64]. In Mexico, there are regions (i.e., Durango, Puebla, Oaxaca, and Michoacán) with a long tradition of forest management and detailed information on timber harvesting and management systems, which ideally could function as trial areas. Therefore, considering most collectively-owned lands, it is recommended that actions be planned and implemented at the regional or landscape level, such as for example by leveraging the management by Forest Management Units established by CONAFOR in order to incorporate mixed or multifunctional plantations to already existing production schemes. This would incorporate CONAFOR in the process in order to provide economic incentives for the strategy, since forest plantation projects involve high initial costs [
18], which forest owners can hardly afford. The establishment costs of carbon-related forestry projects in Mexico are highly variable, which suggest that forest plantations for carbon mitigation might not be an efficient land use in some regions [
18]. Moreover, the monitoring costs of carbon storage rates could be large, as well as constrained by the low technical capacities in most forest regions, in addition to lacunae in the knowledge on the biological performance of mixed tree species. Therefore, each project should be carefully assessed, since the opportunity costs of alternative land uses vary depending on the technical capacities, associated risks, and the availability of forest resources for conservation or production, all of which affect the feasibility for carbon sequestration strategies [
18].
6. Conclusions
In Mexico, planted forests should add an ecological perspective to forestry in order to increase productivity, reduce forest degradation, and ensure the maintenance of ecological integrity by preserving ecosystem processes such as changes in forest biomass and carbon storage. Although the underlying basis of the effects of forest management on net carbon emissions to the atmosphere are well understood, the incorporation of planted forests into carbon management schemes is a complex issue. For example, no baseline data are available on the initial stocks of organic carbon in soil, and hence the actual changes in this stock cannot be quantified.
Mixed forest plantations in Mexico offer a potentially successful alternative means of climate change mitigation and the provision of goods and ecosystem services, if their design incorporates the following criteria: (1) a proper combination of specific functions (biophysical effects) through the composition of species that can influence the regulation of local climate and carbon sequestration, etc., and (2) simultaneous or sequential delivery of commodities and ecosystem services that improve owner´s quality of life, thus ensuring incentives for sustainable management. A few efforts have been made to estimate the cost efficiency of some carbon-related forestry projects in Mexico [
18], which provide insight about the feasibility of these kinds of alternatives. The contemplation of not only production, but also the provision of ecosystem services and economic, social, and environmental synergies is required in order for the forestry sector in Mexico to better address the challenges of the new global environment. The encouragement of planted forest in Mexico as a long-term activity needs to be based on the proper management of the country´s forest richness. As seen in this review, most forestry strategies still suffer from gaps and flaws that must be addressed as a previous step in order to outline a forest carbon policy in Mexico. It is clear that mixed forest plantations still require a better understanding of the biophysical and ecological processes involved in carbon sequestration in order to recommend efficient management practices that address both timber production and other ecosystem services. It is evident that research should also be addressed at local or regional levels in order to make these kind of plantations more likely to succeed at operational levels.
The current state of the forestry sector in Mexico represents an ideal scenario for running long-term experiments on tropical and temperate mixed forest plantations well replicated in time and space, as well as demonstration sites at the operational level to collect economic, social, and ecological data. Such experiments would make it possible to strengthen the national technical capacities to provide forest resource managers with the most appropriate decision-making elements in view of possible future climate change scenarios.