Regeneration Responses to Management for Old-Growth Characteristics in Northern Hardwood-Conifer Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Objective 1: Regeneration Density and Diversity Responses
3.2. Objective 2: Competitive Interactions Among Species
3.3. Objective 3: Interactions with Herbivory, Substrate and Climate
4. Discussion
4.1. Regeneration Response to Old-Growth Management
4.2. Sources of Variability in Regeneration Dynamics
4.3. Effects of Climate Variability and Drought
5. Management Implications
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Site | JRF | MMSF | |||||
|---|---|---|---|---|---|---|---|
| Treatment | Control | SCE | Control | Group | SCE | Single-Tree | |
| Seedling | |||||||
| Striped maple | 727 ± 421 | 1471 ± 424 | 885 ± 220 | 269 ± 82 | 617 ± 267 | 422 ± 115 | |
| Red maple | 9899 ± 3826 | 22,359 ± 9863 | 32 ± 32 | 95 ± 63 | 1439 ± 1184 | 53 ± 37 | |
| Sugar maple | 3052 ± 1278 | 4206 ± 2084 | 15,591 ± 4930 | 4096 ± 2357 | 18,770 ± 9065 | 19,889 ± 8417 | |
| Mountain maple | 0 ± 0 | 0 ± 0 | 395 ± 195 | 127 ± 66 | 300 ± 166 | 158 ± 83 | |
| Yellow birch | 4254 ± 2298 | 1407 ± 838 | 6120 ± 1301 | 7005 ± 1390 | 9614 ± 1953 | 6378 ± 1665 | |
| Sweet birch | 0 ± 0 | 0 ± 0 | 0 ± 0 | 47 ± 47 | 0 ± 0 | 0 ± 0 | |
| Paper birch | 0 ± 0 | 0 ± 0 | 63 ± 35 | 79 ± 26 | 79 ± 49 | 88 ± 60 | |
| Bitternut hickory | 0 ± 0 | 0 ± 0 | 364 ± 313 | 237 ± 237 | 1059 ± 621 | 35 ± 35 | |
| American beech | 3004 ± 1382 | 1850 ± 464 | 1265 ± 294 | 3447 ± 553 | 6578 ± 1983 | 1125 ± 289 | |
| White ash | 901 ± 329 | 2325 ± 720 | 32 ± 21 | 348 ± 196 | 680 ± 236 | 141 ± 81 | |
| Hophornbeam | 1344 ± 680 | 2625 ± 1078 | 16 ± 16 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Red spruce | 0 ± 0 | 0 ± 0 | 411 ± 177 | 174 ± 60 | 158 ± 91 | 193 ± 51 | |
| White pine | 32 ± 21 | 79 ± 35 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Pin cherry | 0 ± 0 | 16 ± 16 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Black cherry | 0 ± 0 | 0 ± 0 | 32 ± 21 | 16 ± 16 | 32 ± 32 | 105 ± 46 | |
| Red oak | 63 ± 35 | 1502 ± 346 | 0 ± 0 | 0 ± 0 | 47 ± 34 | 0 ± 0 | |
| Eastern hemlock | 411 ± 283 | 885 ± 385 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Sapling | |||||||
| Striped maple | 20 ± 14 | 296 ± 84 | 80 ± 36 | 176 ± 75 | 396 ± 90 | 720 ± 127 | |
| Sugar maple | 80 ± 58 | 4 ± 4 | 56 ± 25 | 4 ± 4 | 116 ± 58 | 98 ± 46 | |
| Mountain maple | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 18 ± 18 | |
| Yellow birch | 12 ± 12 | 12 ± 9 | 236 ± 93 | 648 ± 205 | 360 ± 160 | 1564 ± 559 | |
| Sweet birch | 0 ± 0 | 0 ± 0 | 0 ± 0 | 8 ± 5 | 0 ± 0 | 0 ± 0 | |
| American beech | 768 ± 238 | 1596 ± 501 | 736 ± 144 | 4788 ± 1831 | 2240 ± 433 | 964 ± 117 | |
| White pine | 8 ± 8 | 12 ± 12 | 0 ± 0 | 8 ± 5 | 16 ± 11 | 0 ± 0 | |
| Hophornbeam | 8 ± 8 | 244 ± 192 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Red spruce | 0 ± 0 | 0 ± 0 | 96 ± 36 | 272 ± 68 | 100 ± 26 | 244 ± 74 | |
| Black cherry | 0 ± 0 | 0 ± 0 | 0 ± 0 | 8 ± 8 | 0 ± 0 | 0 ± 0 | |
| Eastern hemlock | 140 ± 68 | 96 ± 46 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
References
- Nunery, J.S.; Keeton, W.S. Forest Carbon Storage in the Northeastern United States: Net Effects of Harvesting Frequency, Post-Harvest Retention, and Wood Products. For. Ecol. Manag. 2010, 259, 1363–1375. [Google Scholar] [CrossRef]
- Burrascano, S.; Keeton, W.S.; Sabatini, F.M.; Blasi, C. Commonality and Variability in the Structural Attributes of Moist Temperate Old-Growth Forests: A Global Review. For. Ecol. Manag. 2013, 291, 458–479. [Google Scholar] [CrossRef]
- Franklin, J.F.; Lindenmayer, D.B.; MacMahon, J.A.; McKee, A.; Magnuson, J.; Perry, D.A.; Waide, R.; Foster, D. Threads of Continuity. Conserv. Pract. 2000, 1, 8–17. [Google Scholar] [CrossRef]
- North, M.P.; Keeton, W.S. Emulating Natural Disturbance Regimes: An Emerging Approach for Sustainable Forest Management. In Patterns and Processes in Forest Landscapes; Lafortezza, R., Sanesi, G., Chen, J., Crow, T.R., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 341–372. [Google Scholar]
- Cogbill, C.V.; Burk, J.; Motzkin, G. The Forests of Presettlement New England, USA: Spatial and Compositional Patterns Based on Town Proprietor Surveys. J. Biogeogr. 2002, 29, 1279–1304. [Google Scholar] [CrossRef]
- Kern, C.; Burton, J.I.; Raymond, P.; D’Amato, A.W.; Keeton, W.S.; Royo, A.A.; Walters, M.B.; Webster, C.R.; Willis, J.L. Challenges Facing Gap-Based Silviculture and Possible Solutions for Mesic Northern Forests in North America. Forestry 2017, 90, 4–17. [Google Scholar] [CrossRef]
- Lorimer, C.G.; White, A.S. Scale and Frequency of Natural Disturbances in the Northeastern US: Implications for Early Successional Forest Habitats and Regional Age Distributions. For. Ecol. Manag. 2003, 185, 41–64. [Google Scholar] [CrossRef]
- Singer, M.T.; Lorimer, C.G. Crown Release as a Potential Old-Growth Restoration Approach in Northern Hardwoods. Can. J. For. Res. 1997, 27, 1222–1232. [Google Scholar] [CrossRef]
- Keeton, W.S. Managing for Late-Successional/Old-Growth Characteristics in Northern Hardwood-Conifer Forests. For. Ecol. Manag. 2006, 235, 129–142. [Google Scholar] [CrossRef]
- Hanson, J.J.; Lorimer, C.G.; Halpin, C.R.; Palik, B.J. Ecological Forestry in an Uneven-Aged, Late-Successional Forest: Simulated Effects of Contrasting Treatments on Structure and Yield. For. Ecol. Manag. 2012, 270, 94–107. [Google Scholar] [CrossRef]
- Fassnacht, K.S.; Bronson, D.R.; Palik, B.J.; D’Amato, A.W.; Lorimer, C.G.; Martin, K.J. Accelerating the Development of Old-Growth Characteristics in Second-Growth Northern Hardwoods; General Technical Report; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2015.
- McGee, G.G.; Leopold, D.J.; Nyland, R.D. Structural Characteristics of Old-growth, Maturing, and Partially Cut Northern Hardwood Forests. Ecol. Appl. 1999, 9, 1316–1329. [Google Scholar] [CrossRef]
- Keeton, W.S.; Kraft, C.E.; Warren, D.R. Mature and Old-Growth Riparian Forests: Structure, Dynamics, and Effects on Adirondack Stream Habitats. Ecol. Appl. 2007, 17, 852–868. [Google Scholar] [CrossRef] [PubMed]
- Wirth, C.; Messier, C.; Bergeron, Y.; Frank, D.; Fankhänel, A. Old-Growth Forest Definitions: A Pragmatic View. In Old-Growth Forests; Springer: Berlin/Heidelberg, Germany, 2009; pp. 11–33. [Google Scholar]
- Keeton, W.S.; Whitman, A.A.; McGee, G.C.; Goodale, C.L. Late-Successional Biomass Development in Northern Hardwood-Conifer Forests of the Northeastern United States. For. Sci. 2011, 57, 489–505. [Google Scholar]
- McGarvey, J.C.; Thompson, J.R.; Epstein, H.E.; Shugart, H.H. Carbon Storage in Old-growth Forests of the Mid-Atlantic: Toward Better Understanding the Eastern Forest Carbon Sink. Ecology 2015, 96, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.E.; Keeton, W.S. Enhanced Carbon Storage through Management for Old-Growth Characteristics in Northern Hardwood-Conifer Forests. Ecosphere 2016, in press. [Google Scholar]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for Old-Growth Attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, A.W.; Bradford, J.B.; Fraver, S.; Palik, B.J. Forest Management for Mitigation and Adaptation to Climate Change: Insights from Long-Term Silviculture Experiments. For. Ecol. Manag. 2011, 262, 803–816. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F.; Lõhmus, A.; Baker, S.C.; Bauhus, J.; Beese, W.; Brodie, A.; Kiehl, B.; Kouki, J.; Pastur, G.M.; et al. A Major Shift to the Retention Approach for Forestry Can Help Resolve Some Global Forest Sustainability Issues: Retention Forestry for Sustainable Forests. Conserv. Lett. 2012, 5, 421–431. [Google Scholar] [CrossRef]
- Leak, W.B.; Yamasaki, M.; Holleran, R. Silvicultural Guide for Northern Hardwoods in the Northeast; USDA: Washington, DC, USA, 2014.
- Ziegler, S.S. Disturbance Regimes of Hemlock-Dominated Old-Growth Forests in Northern New York, USA. Can. J. For. Res. 2002, 32, 2106–2115. [Google Scholar] [CrossRef]
- McKenny, H.C.; Keeton, W.S.; Donovan, T.M. Effects of Structural Complexity Enhancement on Eastern Red-Backed Salamander (Plethodon cinereus) Populations in Northern Hardwood Forests. For. Ecol. Manag. 2006, 230, 186–196. [Google Scholar] [CrossRef]
- Smith, K.J.; Keeton, W.S.; Twery, M.J.; Tobi, D.R. Understory Plant Responses to Uneven-Aged Forestry Alternatives in Northern Hardwood-Conifer Forests. Can. J. For. Res. 2008, 38, 1303–1318. [Google Scholar] [CrossRef]
- Dove, N.C.; Keeton, W.S. Structural Complexity Enhancement Increases Fungal Species Richness in Northern Hardwood Forests. Fungal Ecol. 2015, 13, 181–192. [Google Scholar] [CrossRef]
- Price, D.T.; Zimmermann, N.E.; van der Meer, P.J.; Lexer, M.J.; Leadley, P.; Jorritsma, I.T.M.; Schaber, J.; Clark, D.F.; Lasch, P.; McNulty, S.; et al. Regeneration in Gap Models: Priority Issues for Studying Forest Responses to Climate Change. Clim. Chang. 2011, 51, 475–508. [Google Scholar] [CrossRef]
- Dodson, E.K.; Burton, J.I.; Puettmann, K.J. Multiscale Controls on Natural Regeneration Dynamics after Partial Overstory Removal in Douglas-Fir Forests in Western Oregon, USA. For. Sci. 2014, 60, 953–961. [Google Scholar] [CrossRef]
- Kneeshaw, D.; Bergeron, Y. Applying Knowledge of Natural Disturbance Regimes to Develop an Ecosystem Management Approach in Forestry. In Ecological Forest Management Handbook; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Augustine, D.J.; McNaughton, S.J. Ungulate Effects on the Functional Species Composition of Plant Communities: Herbivore Selectivity and Plant Tolerance. J. Wildl. Manag. 1998, 62, 1165–1183. [Google Scholar] [CrossRef]
- Collins, B. Ground Layer Competition and Herbivory Effects on Cherrybark Oak (Quercus pagoda Raf.) Regeneration in Experimental Canopy Gaps. J. Torrey Bot. Soc. 2003, 130, 147–157. [Google Scholar] [CrossRef]
- Andreozzi, H.A.; Pekins, P.J.; Langlais, M.L. Impact of Moose Browsing on Forest Regeneration in Northeast Vermont. Alces J. Devoted Biol. Manag. Moose 2014, 50, 67–79. [Google Scholar]
- Houston, D.R. Beech Bark Disease—The Aftermath Forests Are Structured for a New Outbreak. J. For. 1975, 73, 660–663. [Google Scholar]
- Giencke, L.M.; Dovčiak, M.; Mountrakis, G.; Cale, J.A.; Mitchell, M.J. Beech Bark Disease: Spatial Patterns of Thicket Formation and Disease Spread in an Aftermath Forest in the Northeastern United States. Can. J. For. Res. 2014, 44, 1042–1050. [Google Scholar] [CrossRef]
- Gauthier, M.-M.; Lambert, M.-C.; Bédard, S. Effects of Harvest Gap Size, Soil Scarification, and Vegetation Control on Regeneration Dynamics in Sugar Maple-Yellow Birch Stands. For. Sci. 2016, 62, 237–246. [Google Scholar] [CrossRef]
- Lambert, J.-B.; Ameztegui, A.; Delagrange, S.; Messier, C. Birch and Conifer Deadwood Favour Early Establishment and Shade Tolerance in Yellow Birch Juveniles Growing in Sugar Maple Dominated Stands. Can. J. For. Res. 2016, 46, 114–121. [Google Scholar] [CrossRef]
- Huggett, B.A.; Schaberg, P.G.; Hawley, G.J.; Eagar, C. Long-Term Calcium Addition Increases Growth Release, Wound Closure, and Health of Sugar Maple (Acer saccharum) Trees at the Hubbard Brook Experimental Forest. Can. J. For. Res. 2007, 37, 1692–1700. [Google Scholar] [CrossRef]
- Nyland, R.D. Silviculture: Concepts and Applications; McGraw-Hill Series in Forest Resources; Waveland Press: Long Grove, IL, USA, 2007. [Google Scholar]
- Blankenhorn, P.R.; Labosky, P., Jr.; Janowiak, J.J.; Manbeck, H. The Development of Preservation Treatment Recommendations for Red Oak and Red Maple Glued-Laminated Timber Bridge Members. For. Prod. J. 1999, 49, 87. [Google Scholar]
- Wang, X.; Ross, R.J.; Green, D.W.; Brashaw, B.; Englund, K.; Wolcott, M. Stress Wave Sorting of Red Maple Logs for Structural Quality. Wood Sci. Technol. 2004, 37, 531–537. [Google Scholar] [CrossRef]
- Hannah, P.R. Species Composition and Dynamics in Two Hardwood Stands in Vermont: A Disturbance History. For. Ecol. Manag. 1999, 120, 105–116. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Catanzaro, P.F.; Fletcher, L.S. Early Regeneration and Structural Responses to Patch Selection and Structural Retention in Second-Growth Northern Hardwoods. For. Sci. 2015, 61, 183–189. [Google Scholar] [CrossRef]
- Seymour, R.S.; White, A.S.; deMaynadier, P.G. Natural Disturbance Regimes in Northeastern North America—Evaluating Silvicultural Systems Using Natural Scales and Frequencies. For. Ecol. Manag. 2002, 155, 357–367. [Google Scholar] [CrossRef]
- Goff, F.G.; West, D. Canopy-Understory Interaction Effects on Forest Population Structure. For. Sci. 1975, 21, 98–108. [Google Scholar]
- Goodburn, J.M.; Lorimer, C.G. Population Structure in Old-Growth and Managed Northern Hardwoods: An Examination of the Balanced Diameter Distribution Concept. For. Ecol. Manag. 1999, 118, 11–29. [Google Scholar] [CrossRef]
- Peet, R.K.; Wentworth, T.R.; White, P.S. A Flexible, Multipurpose Method for Recording Vegetation Composition and Structure. Castanea 1998, 63, 262–274. [Google Scholar]
- Faison, E.K.; Motzkin, G.; Foster, D.R.; McDonald, J.E. Moose Foraging in the Temperate Forests of Southern New England. Northeast. Nat. 2010, 17, 1–18. [Google Scholar] [CrossRef]
- Create a monthly or seasonal time series of climate variables. Available online: https://www.esrl.noaa.gov/psd/data/timeseries/ (accessed on 1 May 2016).
- Zwolinski, J.; Donald, D.; Van Laar, A.; Groenewald, W. Regeneration Procedures of Pinus radiata in the Southern Cape Province: Part V: Post Planting Mortality and Growth of Trees in Response to the Experimental Treatments and Planting Site Environment. S. Afr. For. J. 1994, 168, 7–21. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized Linear Mixed Models: A Practical Guide for Ecology and Evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data. Statistics for Biology and Health; Springer: New York, NY, USA; London, UK, 2007. [Google Scholar]
- Littlefield, C.E.; Keeton, W.S. Bioenergy Harvesting Impacts on Ecologically Important Stand Structure and Habitat Characteristics. Ecol. Appl. 2012, 22, 1892–1909. [Google Scholar] [CrossRef] [PubMed]
- Nyland, R.D.; Bashant, A.L.; Bohn, K.K.; Verostek, J.M. Interference to Hardwood Regeneration in Northeastern North America: Controlling Effects of American Beech, Striped Maple, and Hobblebush. North. J. Appl. For. 2006, 23, 122–132. [Google Scholar]
- Ward, J.; Worthley, T.E.; Smallidge, P.J.; Bennet, K.P. Northeastern Forest Regeneration Handbook; USDA: Newtown Square, PA, USA, 2013.
- Kern, C.; Reich, P.B.; Montgomery, R.A.; Strong, T.F. Do Deer and Shrubs Override Canopy Gap Size Effects on Growth and Survival of Yellow Birch, Northern Red Oak, Eastern White Pine, and Eastern Hemlock Seedlings? For. Ecol. Manag. 2012, 267, 134–143. [Google Scholar] [CrossRef]
- Curtis, R.O. Notes: A Simple Index of Stand Density for Douglas-Fir. For. Sci. 1982, 28, 92–94. [Google Scholar]
- Durbin, J.; Watson, G. Testing for Serial Correlation in Least Squares Regression, III. Biometrika 1971, 58, 1–19. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.A.; van Pelt, R.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and Structural Development of Natural Forest Ecosystems with Silvicultural Implications, Using Douglas-Fir Forests as an Example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Mader, S.F.; Nyland, R.D. Six-Year Response of Northern Hardwoods to the Selection System. North. J. Appl. For. 1984, 1, 87–91. [Google Scholar]
- Jones, R.H.; Nyland, R.D.; Raynal, D.J. Response of American Beech Regeneration to Selection Cutting of Northern Hardwoods in New York. North. J. Appl. For. 1989, 6, 34–36. [Google Scholar]
- Donoso, P.J.; Nyland, R.D.; Zhang, L. Growth of Saplings after Selection Cutting in Northern Hardwoods. North. J. Appl. For. 2000, 17, 149–152. [Google Scholar]
- Poznanovic, S.K.; Webster, C.R.; Bump, J.K. Maintaining Mid-Tolerant Tree Species with Uneven-Aged Forest Management: 9-Year Results from a Novel Group-Selection Experiment. Forestry 2013, 86, 555–567. [Google Scholar] [CrossRef]
- Woods, K.D. Reciprocal Replacement and the Maintenance of Codominance in a Beech-Maple Forest. Oikos 1979, 33, 31–39. [Google Scholar] [CrossRef]
- Whittaker, R.; Levin, S. The Role of Mosaic Phenomena in Natural Communities. Theor. Popul. Biol. 1977, 12, 117–139. [Google Scholar] [CrossRef]
- McClure, J.W.; Lee, T.D.; Leak, W.B. Gap Capture in Northern Hardwoods: Patterns of Establishment and Height Growth in Four Species. For. Ecol. Manag. 2000, 127, 181–189. [Google Scholar] [CrossRef]
- Ricard, J.-P.; Messier, C.; Delagrange, S.; Beaudet, M. Do Understory Sapling Respond to Both Light and below-Ground Competition? A Field Experiment in a North-Eastern American Hardwood Forest and a Literature Review. Ann. For. Sci. 2003, 60, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Nolet, P. Coexistence et Sylviculture de L’érable À Sucre et Du Hêtre À Grandes Feuilles Dans Un Contexte de Changements Globaux; Université du Québec à Montréal: Montréal, QC, Canada, 2016. [Google Scholar]
- Beaudet, M.; Messier, C. Growth and Morphological Responses of Yellow Birch, Sugar Maple, and Beech Seedlings Growing under a Natural Light Gradient. Can. J. For. Res. 1998, 28, 1007–1015. [Google Scholar] [CrossRef]
- Beaudet, M.; Messier, C.; Paré, D.; Brisson, J.; Bergeron, Y. Possible Mechanisms of Sugar Maple Regeneration Failure and Replacement by Beech in the Boisé-Des-Muir Old-Growth Forest, Québec. Écoscience 1999, 6, 264–271. [Google Scholar] [CrossRef]
- Gasser, D.; Messier, C.; Beaudet, M.; Lechowicz, M.J. Sugar Maple and Yellow Birch Regeneration in Response to Canopy Opening, Liming and Vegetation Control in a Temperate Deciduous Forest of Quebec. For. Ecol. Manag. 2010, 259, 2006–2014. [Google Scholar] [CrossRef] [Green Version]
- Hane, E.N.; Hamburg, S.P.; Barber, A.L.; Plaut, J.A. Phytotoxicity of American Beech Leaf Leachate to Sugar Maple Seedlings in a Greenhouse Experiment. Can. J. For. Res. 2003, 33, 814–821. [Google Scholar] [CrossRef]
- Sage, R.W.; Porter, W.F.; Underwood, H.B. Windows of Opportunity: White-Tailed Deer and the Dynamics of Northern Hardwood Forests of the Northeastern US. J. Nat. Conserv. 2003, 10, 213–220. [Google Scholar] [CrossRef]
- Horsley, S.B.; Stout, S.L.; DeCalesta, D.S. White-Tailed Deer Impact on the Vegetation Dynamics of a Northern Hardwood Forest. Ecol. Appl. 2003, 13, 98–118. [Google Scholar] [CrossRef]
- Healy, W.M. Influence of Deer on the Structure and Composition of Oak Forests in Central Massachusetts. In The Science of Overabundance: Deer Ecology and Population Management; Smithsonian Institution Press: Washington, DC, USA, 1997; pp. 249–266. [Google Scholar]
- Paine, C.E.T.; Beck, H. Seed Predation by Neotropical Rain Forest Mammals Increases Diversity in Seedling Recruitment. Ecology 2007, 88, 3076–3087. [Google Scholar] [CrossRef] [PubMed]
- Marx, L.M.; Walters, M.B. Effects of Nitrogen Supply and Wood Species on Tsuga canadensis and Betula alleghaniensis Seedling Growth on Decaying Wood. Can. J. For. Res. 2006, 36, 2873–2884. [Google Scholar] [CrossRef]
- Decker, K.L.M.; Wang, D.; Waite, C.; Scherbatskoy, T. Snow Removal and Ambient Air Temperature Effects on Forest Soil Temperatures in Northern Vermont. Soil Sci. Soc. Am. J. 2003, 67, 1234. [Google Scholar] [CrossRef]
- Christenson, L.M.; Mitchell, M.J.; Groffman, P.M.; Lovett, G.M. Cascading Effects of Climate Change on Forest Ecosystems: Biogeochemical Links Between Trees and Moose in the Northeast USA. Ecosystems 2013, 17, 442–457. [Google Scholar] [CrossRef]
- Drescher, M.; Thomas, S.C. Snow Cover Manipulations Alter Survival of Early Life Stages of Cold-Temperate Tree Species. Oikos 2013, 122, 541–554. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate Change and Forests of the Future: Managing in the Face of Uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Rustad, L.; Campbell, J.; Dukes, J.S.; Huntington, T.; Lambert, K.F.; Mohan, J.; Rodenhouse, N. Changing Climate, Changing Forests: The Impacts of Climate Change on Forests of the Northeastern United States and Eastern Canada; USDA: Washington, DC, USA, 2012.
- Canham, C.D.; Murphy, L. The Demography of Tree Species Response to Climate: Seedling Recruitment and Survival. Ecosphere 2016, 7, e01424. [Google Scholar] [CrossRef]
- Iverson, L.; Prasad, A.; Matthews, S. Modeling Potential Climate Change Impacts on the Trees of the Northeastern United States. Mitig. Adapt. Strateg. Glob. Chang. 2008, 13, 487–516. [Google Scholar] [CrossRef]
- Fisichelli, N.A.; Frelich, L.E.; Reich, P.B. Climate and Interrelated Tree Regeneration Drivers in Mixed Temperate–Boreal Forests. Landsc. Ecol. 2013, 28, 149–159. [Google Scholar] [CrossRef]
- Fisichelli, N.; Wright, A.; Rice, K.; Mau, A.; Buschena, C.; Reich, P.B. First-Year Seedlings and Climate Change: Species-Specific Responses of 15 North American Tree Species. Oikos 2014, 123, 1331–1340. [Google Scholar] [CrossRef]
- Mallett, A.L. Management of Understory American Beech by Manual and Chemical Control Methods; State University of New York, College of Environmental Science and Forestry: Syracuse, NY, USA, 2002. [Google Scholar]
- Kochenderfer, J.D.; Zedaker, S.M.; Johnson, J.E.; Smith, D.W.; Miller, G.W. Herbicide Hardwood Crop Tree Release in Central West Virginia. North. J. Appl. For. 2001, 18, 46–54. [Google Scholar]
- Nelson, A.S.; Wagner, R.G. Improving the Composition of Beech-Dominated Northern Hardwood Understories in Northern Maine. North. J. Appl. For. 2011, 28, 186–193. [Google Scholar]
- Forrester, J.A.; Lorimer, C.G.; Dyer, J.H.; Gower, S.T.; Mladenoff, D.J. Response of Tree Regeneration to Experimental Gap Creation and Deer Herbivory in North Temperate Forests. For. Ecol. Manag. 2014, 329, 137–147. [Google Scholar] [CrossRef]
- Arseneault, J.E.; Saunders, M.R.; Seymour, R.S.; Wagner, R.G. First Decadal Response to Treatment in a Disturbance-Based Silviculture Experiment in Maine. For. Ecol. Manag. 2011, 262, 404–412. [Google Scholar] [CrossRef]
- Bolton, N.W.; D’Amato, A.W. Regeneration Responses to Gap Size and Coarse Woody Debris within Natural Disturbance-Based Silvicultural Systems in Northeastern Minnesota, USA. For. Ecol. Manag. 2011, 262, 1215–1222. [Google Scholar] [CrossRef]
- Kern, C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Harvest-Created Canopy Gaps Increase Species and Functional Trait Diversity of the Forest Ground-Layer Community. For. Sci. 2014, 60, 335–344. [Google Scholar] [CrossRef]
- Harmon, M.E.; Ferrell, W.K.; Franklin, J.F. Effects on Carbon Storage of Conversion of Old-Growth Forests to Young Forests. Science 1990, 247, 699–703. [Google Scholar] [CrossRef] [PubMed]
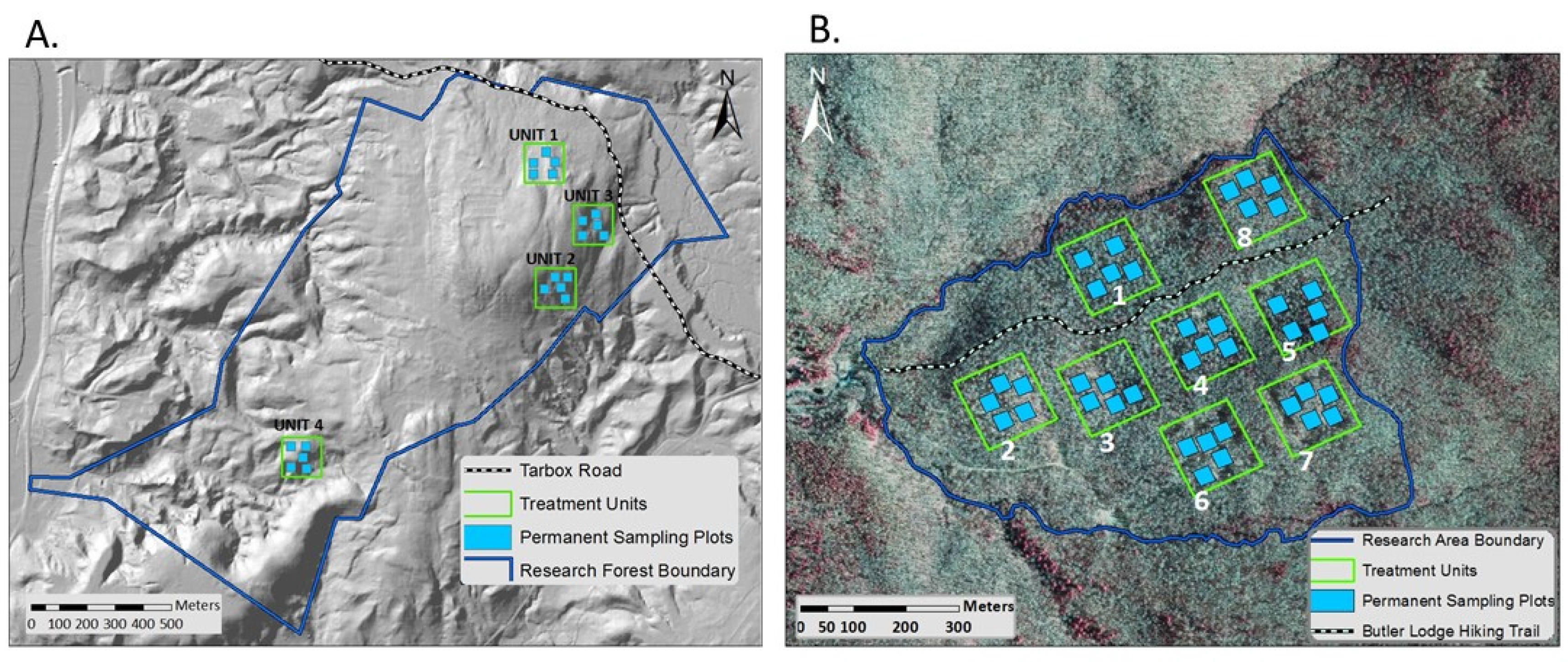
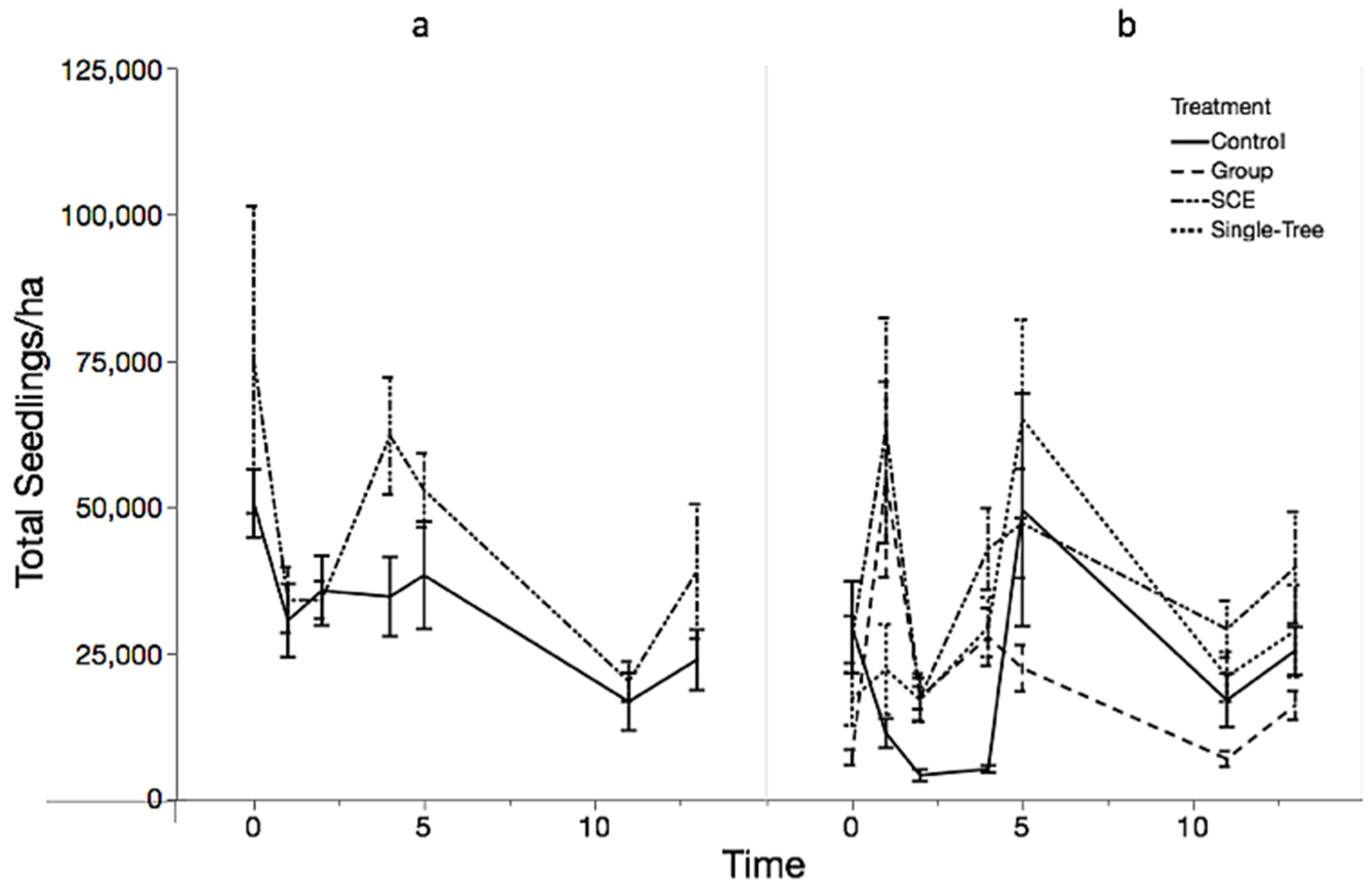
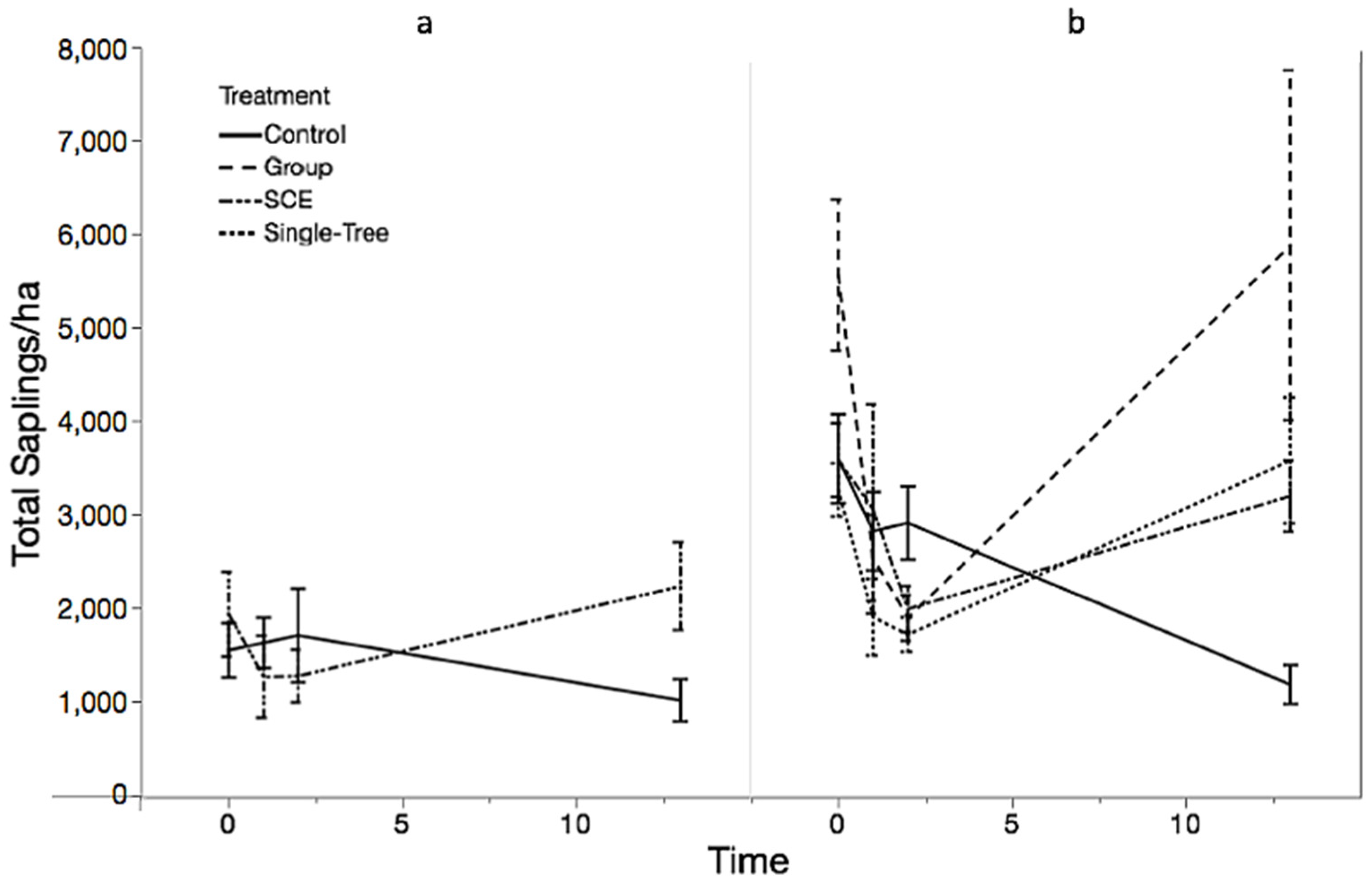
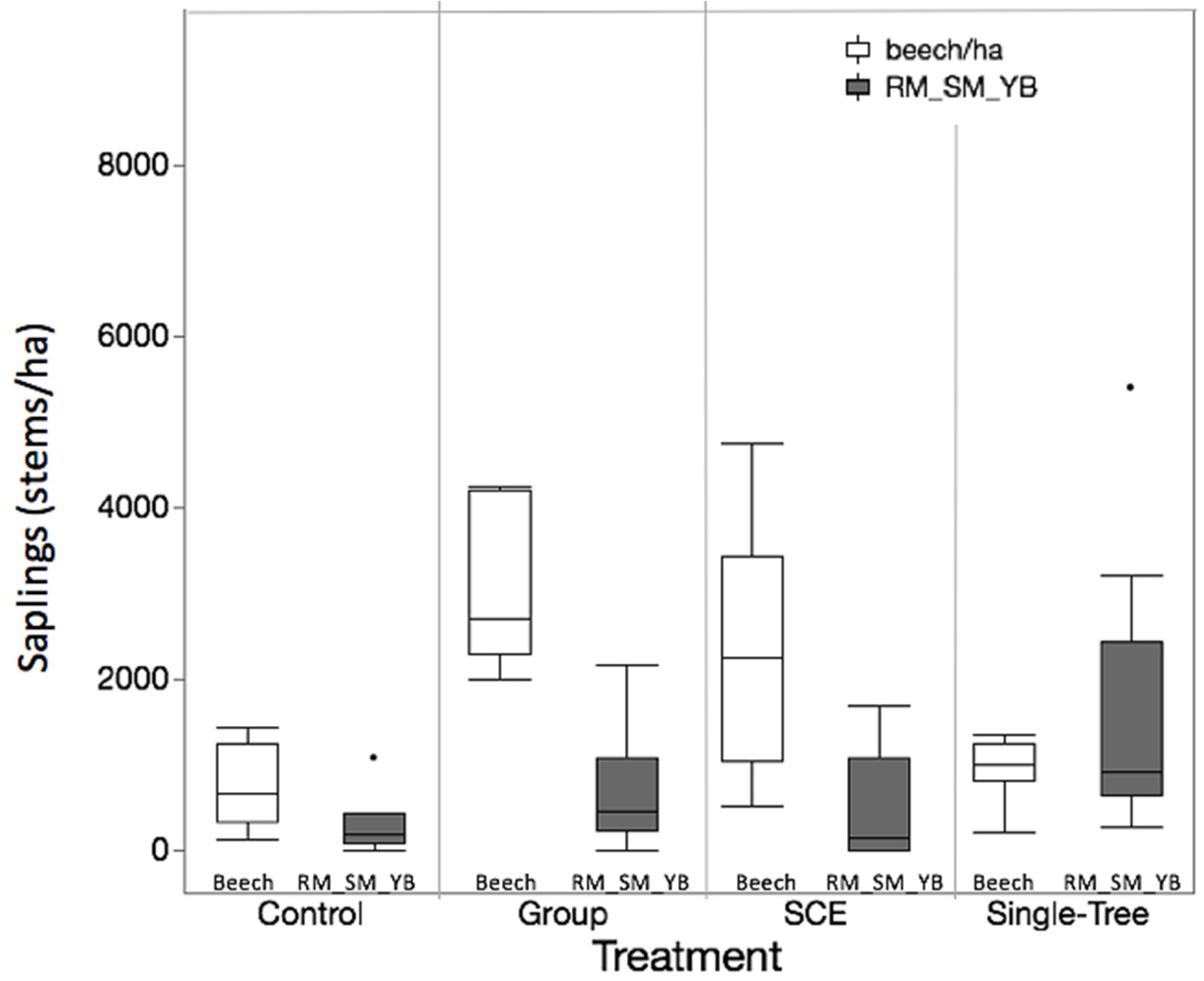
| Site | Unit | Treatment | Site Index | Slope (%) | Aspect (deg) | % Hardwood | Total BA (m2/ha) | Total Stem Density (trees/ha) | Live QMD 1 (cm) |
|---|---|---|---|---|---|---|---|---|---|
| Mansfield | 1 | Control | 70 | 28.8 | 276 | 99.7 | 33.5 | 728 | 24.2 |
| Mansfield | 2 | SCE | 55 | 22.2 | 290 | 99.7 | 36.4 | 1044 | 21.1 |
| Mansfield | 3 | SCE | 55 | 13.0 | 260 | 99.7 | 28.5 | 1056 | 18.5 |
| Mansfield | 4 | Single-Tree | 60 | 29.6 | 272 | 95.9 | 33.9 | 750 | 24 |
| Mansfield | 5 | Single-Tree | 60 | 37.0 | 273 | 97.5 | 31.9 | 750 | 23.3 |
| Mansfield | 6 | Group | 60 | 19.4 | 249 | 98.7 | 30.1 | 1140 | 18.3 |
| Mansfield | 7 | Group | 60 | 26.4 | 250 | 99.4 | 30.8 | 1144 | 18.5 |
| Mansfield | 8 | Control | 55 | 22.3 | 320 | 98.2 | 27.6 | 1066 | 18.2 |
| Jericho | 1 | Control | 60 | 27.1 | 188 | 53.1 | 35.4 | 1186 | 19.5 |
| Jericho | 2 | SCE | 60 | 27.8 | 146 | 83.0 | 33.5 | 1040 | 20.2 |
| Jericho | 3 | SCE | 60 | 42.6 | 147 | 54.8 | 44 | 1034 | 23.3 |
| Jericho | 4 | Control | 60 | 34.2 | 99 | 74.2 | 30.2 | 940 | 20.2 |
| Treatment | Target Residual Basal Area (m2·ha−1) | Max Diameter (cm) | q-Factor | Structural Objective | Silvicultural Prescription |
|---|---|---|---|---|---|
| Single-Tree Selection | 18.4 | 60 | 1.3 | Increased post-harvest target structural retention | Elevated target residual basal area Slash/un-merchentable bole retention |
| Group Selection | 18.4 | 60 | 1.3 | Increased post-harvest target structural retention Variable horizontal density Vertically differentiated canopy Increased horizontal complexity | Elevated target residual basal area Slash/un-merchantable bole retention Variable density marking Release advanced regeneration Spatially aggregated harvest (patches ~ 0.05 ha) |
| SCE | 34 | 90 | 2.0/1.1/1.3 | Re-allocation of the basal area to larger size class Vertically differentiated canopy Recruitment of greater densities of large trees Elevated coarse woody material inputs for added structure | Rotated sigmoid diameter dist. High max. D and the target basal area Retention of trees >60 cm dbh Single-tree sel. with target diameter distribution Release advanced regeneration Full (3- or 4-sided) and partial (2-sided) crown release Tree girdling/felling and leaving trees |
| A. Type III Tests of Fixed Effects MMSF Seedlings | ||||
| Effect | Number df | Density df | F Value | Pr > F |
| Pre-treatment | 1 | 208 | 31.94 | <0.0001 |
| Treatment | 3 | 208 | 24.73 | <0.0001 |
| Time | 5 | 208 | 22.43 | <0.0001 |
| Treatment × Time | 15 | 208 | 5.72 | <0.0001 |
| B. Type III Tests of Fixed Effects MMSF Saplings | ||||
| Effect | Number df | Density df | F Value | Pr > F |
| Pre-treatment | 1 | 36.85 | 0.17 | 0.6861 |
| Treatment | 3 | 3.244 | 0.65 | 0.6315 |
| Time | 2 | 61.74 | 2.91 | 0.0618 |
| Treatment × Time | 6 | 64.33 | 6.66 | <0.0001 |
| Seedlings | ||||||||
| Site | Treatment | N | Total Stems/ha Mean | Min | Max | Beech | SM_RM_YB | H-Index |
| JRF | Control | 10 | 23,687 ± 5128 | 4269 | 56,293 | 3004 ± 1382 | 17,204 ± 4201 | 1.1 ± 0.14 |
| SCE | 10 | 38,726 ± 11,438 | 12,018 | 122,233 | 1850 ± 464 | 27,973 ± 10,809 | 1.42 ± 0.11 | |
| MMSF | Control | 10 | 25,206 ± 4089 | 11,701 | 50,127 | 1265 ± 294 | 21,743 ± 4271 | 1 ± 0.13 |
| Group | 10 | 15,939 ± 2454 | 7748 | 34,156 | 3447 ± 553 | 11,196 ± 2554 | 1.12 ± 0.09 | |
| SCE | 10 | 39,374 ± 9506 | 14,231 | 111,006 | 6578 ± 1983 | 29,823 ± 9687 | 1.16 ± 0.08 | |
| Single-Tree | 9 | 28,586 ± 7812 | 4111 | 66,414 | 1125 ± 289 | 26,320 ± 7592 | 0.81 ± 0.1 | |
| Saplings | ||||||||
| JRF | Control | 10 | 1036 ± 226 | 160 | 2160 | 768 ± 238 | 92 ± 70 | 0.56 ± 0.12 |
| SCE | 10 | 2260 ± 471 | 240 | 4480 | 1596 ± 501 | 16 ± 12 | 0.63 ± 0.12 | |
| MMSF | Control | 10 | 1204 ± 208 | 320 | 2400 | 736 ± 144 | 292 ± 100 | 0.93 ± 0.1 |
| Group | 10 | 5912 ± 1874 | 2200 | 22,400 | 4788 ± 1831 | 652 ± 206 | 0.67 ± 0.09 | |
| SCE | 10 | 3228 ± 382 | 1280 | 4760 | 2240 ± 433 | 476 ± 206 | 0.81 ± 0.12 | |
| Single-Tree | 9 | 3609 ± 674 | 1520 | 7520 | 964 ± 117 | 1662 ± 549 | 1.25 ± 0.06 | |
| A. Seedlings | |||||||
| Treatment | Treatment | Estimate | Standard Error | df | t Value | Pr > |t| | |
| Time 1 | Control | Group | −2.0489 | 0.3068 | 208 | −6.68 | <0.0001 |
| Control | SCE | −1.8638 | 0.2964 | 208 | −6.29 | <0.0001 | |
| Control | STS | −0.9610 | 0.3079 | 208 | −3.12 | 0.0021 | |
| Group | SCE | 0.1851 | 0.3039 | 208 | 0.61 | 0.5431 | |
| Group | STS | 1.0878 | 0.3059 | 208 | 3.56 | 0.0005 | |
| SCE | STS | 0.9027 | 0.3060 | 208 | 2.95 | 0.0035 | |
| Time 13 | Control | Group | −0.0363 | 0.3065 | 208 | −0.12 | 0.9060 |
| Control | SCE | −0.4215 | 0.2959 | 208 | −1.42 | 0.1559 | |
| Control | STS | −0.2814 | 0.3072 | 208 | −0.92 | 0.3607 | |
| Group | SCE | −0.3852 | 0.3045 | 208 | −1.27 | 0.2072 | |
| Group | STS | −0.2451 | 0.3063 | 208 | −0.80 | 0.4244 | |
| SCE | STS | 0.1401 | 0.3060 | 208 | 0.46 | ||
| B. Saplings | |||||||
| Simple Effect Level | Treatment | Treatment | Estimate | Standard Error | df | t Value | Pr > |t| |
| Time 1 | Control | Group | 0.1478 | 0.3502 | 10.19 | 0.42 | 0.6818 |
| Control | SCE | −0.079 | 0.3333 | 8.811 | −0.24 | 0.8177 | |
| Control | STS | 0.3746 | 0.3398 | 9.447 | 1.10 | 0.2977 | |
| Group | SCE | −0.2269 | 0.3504 | 10.2 | −0.65 | 0.5315 | |
| Group | STS | 0.2268 | 0.3596 | 11.1 | 0.63 | 0.5411 | |
| SCE | STS | 0.4537 | 0.3398 | 9.445 | 1.34 | 0.2131 | |
| Time 13 | Control | Group | −1.6100 | 0.3426 | 9.484 | −4.70 | 0.0010 |
| Control | SCE | −1.0472 | 0.3333 | 8.815 | −3.14 | 0.0122 | |
| Control | STS | −1.1555 | 0.3398 | 9.449 | −3.40 | 0.0073 | |
| Group | SCE | 0.5627 | 0.3427 | 9.49 | 1.64 | 0.1333 | |
| Group | STS | 0.4544 | 0.3518 | 10.35 | 1.29 | 0.2246 | |
| SCE | STS | −0.1083 | 0.3398 | 9.443 | −0.32 | 0.7569 | |
| Dependent Variable | Effect | Estimate | Standard Error | df | t Value | Pr > |t| |
|---|---|---|---|---|---|---|
| Sapling Diversity (H’) | Percent Browse | −0.00447 | 0.002122 | 48.7 | −2.11 | 0.0403 |
| Percent Fine Litter Substrate | −0.01230 | 0.005158 | 46.3 | −2.38 | 0.0213 | |
| DSF | −0.02103 | 0.7502 | 46.4 | −0.03 | 0.9778 | |
| Curtis RD | −0.00542 | 0.03665 | 47.6 | 0.15 | 0.8830 | |
| Beech sapling | Percent Browse | 0.01130 | 0.005656 | 49 | 2.00 | 0.0512 |
| Percent Fine Litter Substrate | 0.02944 | 0.01371 | 46.55 | 2.15 | 0.0369 | |
| DSF | 0.4978 | 1.9390 | 46.31 | 0.26 | 0.7985 | |
| Curtis RD | −0.06259 | 0.09770 | 48.21 | −0.64 | 0.5248 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottesman, A.J.; Keeton, W.S. Regeneration Responses to Management for Old-Growth Characteristics in Northern Hardwood-Conifer Forests. Forests 2017, 8, 45. https://doi.org/10.3390/f8020045
Gottesman AJ, Keeton WS. Regeneration Responses to Management for Old-Growth Characteristics in Northern Hardwood-Conifer Forests. Forests. 2017; 8(2):45. https://doi.org/10.3390/f8020045
Chicago/Turabian StyleGottesman, Aviva J., and William S. Keeton. 2017. "Regeneration Responses to Management for Old-Growth Characteristics in Northern Hardwood-Conifer Forests" Forests 8, no. 2: 45. https://doi.org/10.3390/f8020045





