A Demographic Approach to Evaluating Tree Population Sustainability
Abstract
:1. Introduction
2. Methods
2.1. Study Areas
2.2. Field Methods
2.3. Model Description
2.4. The Demographic Sustainability Index
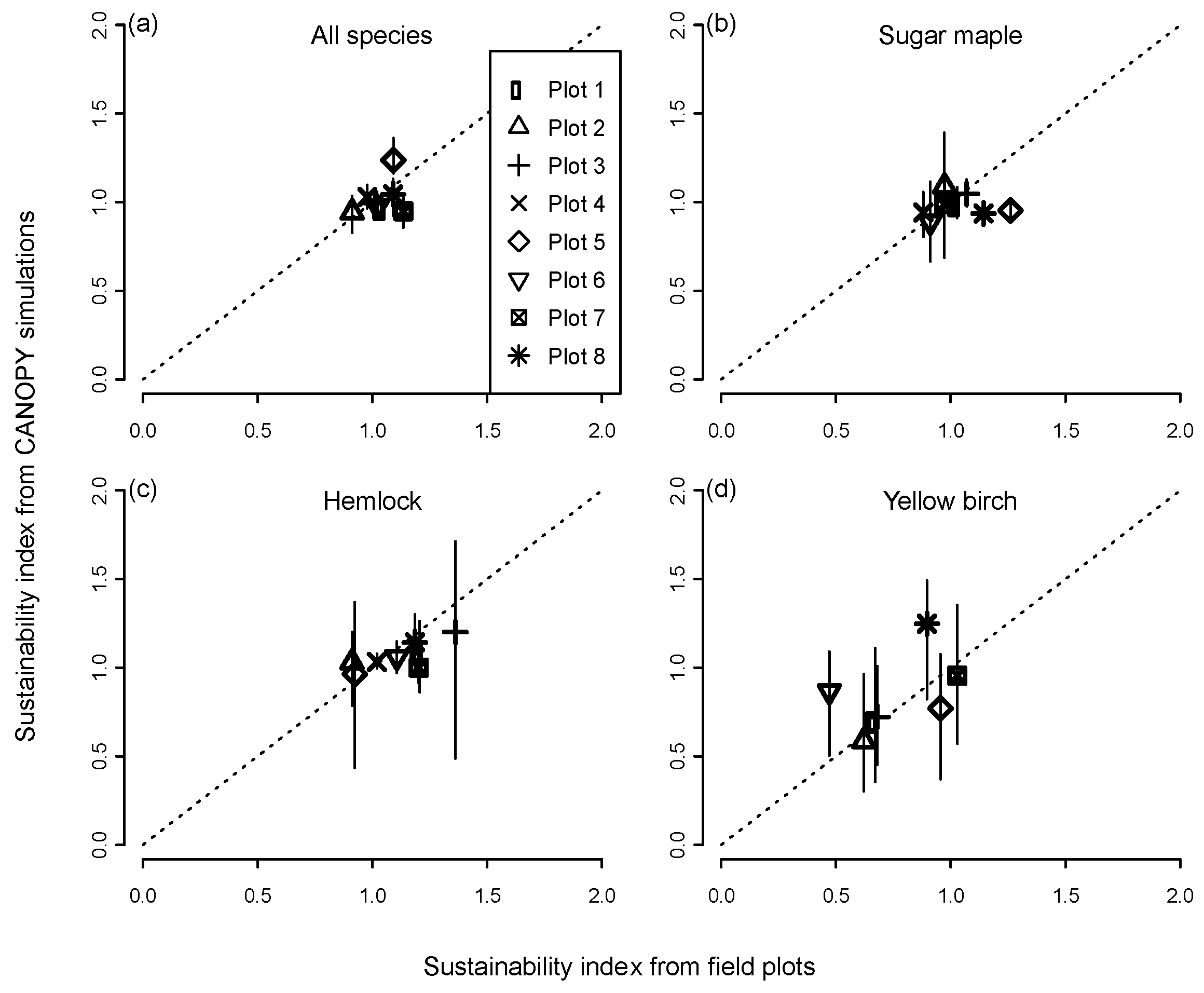
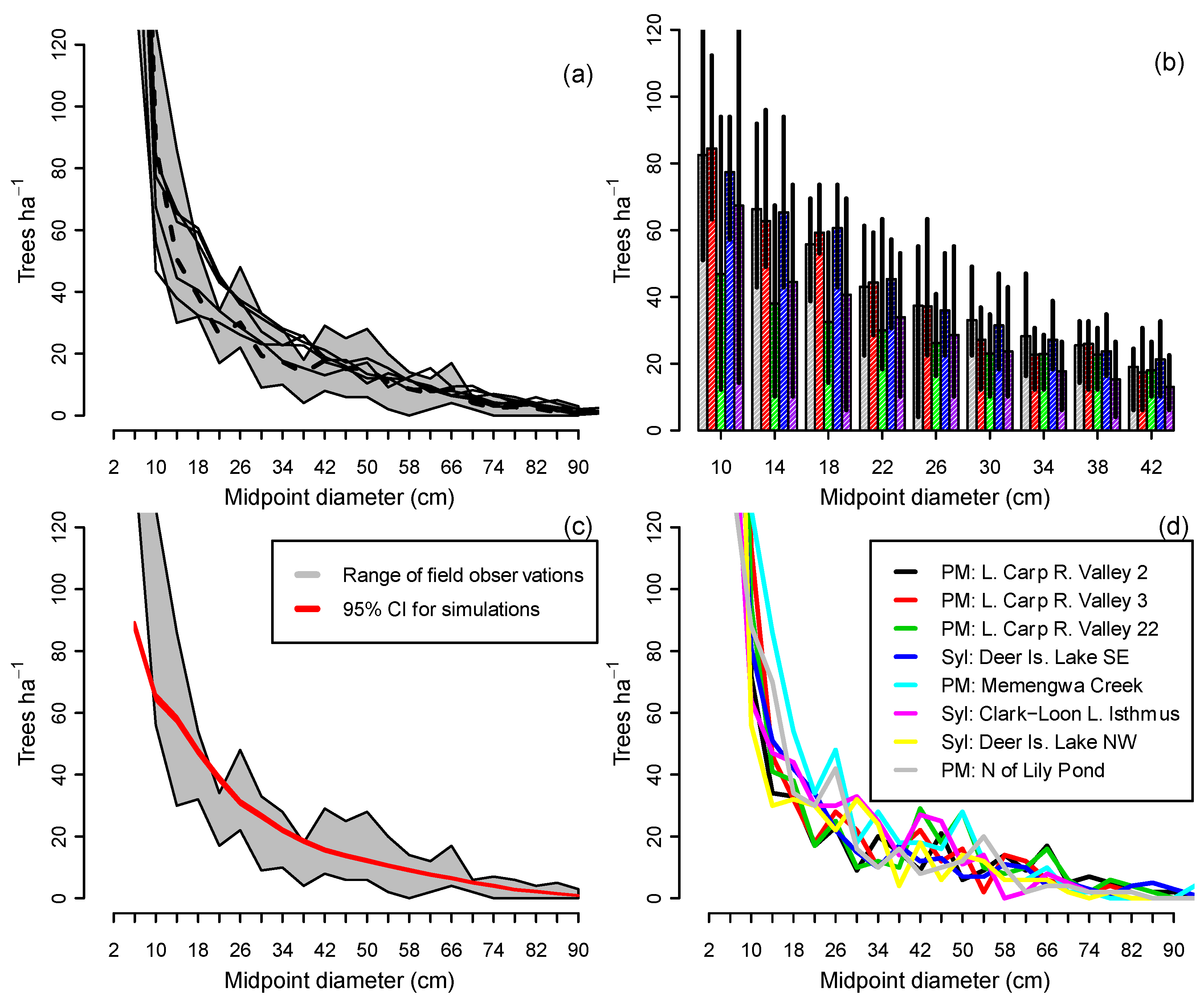
2.5. Evaluation of Model Predictions
2.6. Analytical Techniques
2.7. Simulation Designs
3. Results
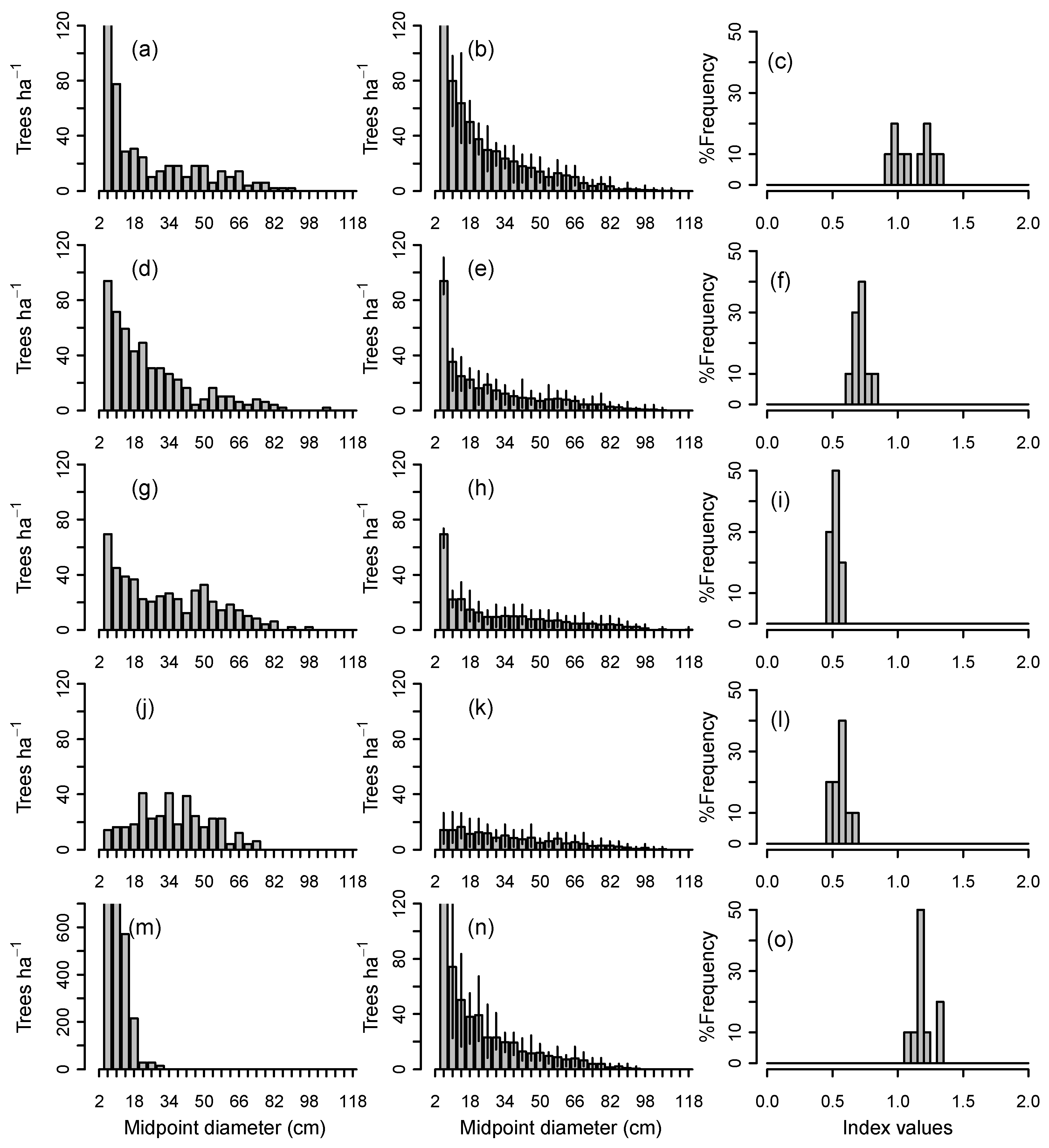
3.1. Range of Demographic Sustainability Index Values for Individual Stands
3.2. Assessing Sustainability at the Landscape Scale
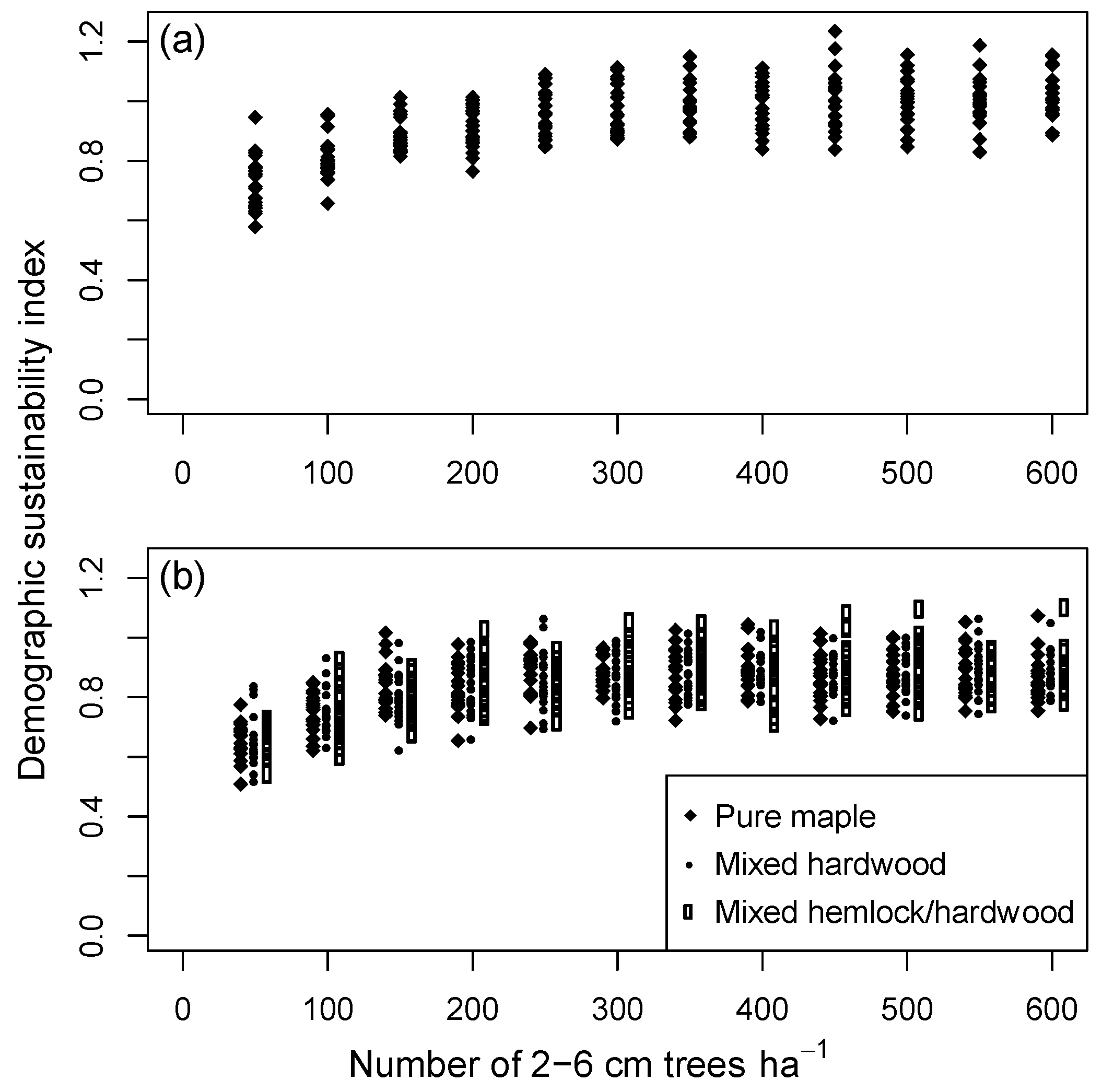
3.3. Predictors of Demographic Sustainability
3.4. Minimum Number of Understory Trees for Sustainability
4. Discussion
4.1. Characteristics and Dynamics of Sustainable Size Distributions
4.2. Sustainability of Shade-Tolerant vs. Gap-Phase Species
4.3. Potential Applications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reed, J.M.; Mills, L.S.; Dunning, J.B.; Menges, E.S.; McKelvey, K.S.; Frye, R.; Beissinger, S.R.; Anstett, M.-C.; Miller, P. Emerging issues in population viability analysis. Conserv. Biol. 2002, 16, 7–19. [Google Scholar] [CrossRef]
- Menges, E.S. The application of minimum viable population theory to plants. In Genetics and Conservation of Rare Plants; Falk, D.A., Holsinger, K.E., Eds.; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- McGraw, J.B.; Furedi, M.A. Deer browsing and population viability of a forest understory plant. Science 2005, 307, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Obioh, G.I.B.; Isichei, A.O. A population viability analysis of serendipity berry (Dioscoreophyllum cumminsii) in a semi-deciduous forest in Nigeria. Ecol. Model. 2007, 201, 558–562. [Google Scholar] [CrossRef]
- Platt, W.J.; Evans, G.W.; Rathbun, S.L. The population dynamics of a long-lived conifer (Pinus palustris). Am. Nat. 1988, 131, 491–525. [Google Scholar] [CrossRef]
- Fraver, S.; Jonsson, B.G.; Jonsson, M.; Esseen, P. Demographics and disturbance history of a boreal old-growth Picea abies forest. J. Veg. Sci. 2008, 19, 789–798. [Google Scholar] [CrossRef]
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M. The Practice of Silviculture: Applied Forest Ecology, 9th ed.; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Nyland, R.D. Silviculture: Concepts and Applications, 2nd ed.; Waveland Press: Long Grove, IL, USA, 2007. [Google Scholar]
- Kohyama, T.S.; Potts, M.D.; Kohyama, T.I.; Kassim, A.R.; Ashton, P.S. Demographic properties shape tree size distribution in a Malaysian rain forest. Am. Nat. 2015, 185, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Goff, F.G.; West, D. Canopy-understory interaction effects on forest population structure. For. Sci. 1975, 21, 98–108. [Google Scholar]
- Bin, Y.; Wanhui, Y.; Muller-Landau, H.C.; Wu, L.; Lian, J.; Cao, H. Unimodal tree size distributions possibly result from relatively strong conservatism in intermediate size classes. PLoS ONE 2012, 7, e52596. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, C.G.; Porter, D.J.; Madej, M.A.; Stuart, J.D.; Viers, S.D.; Norman, S.P.; O’Hara, K.L.; Libby, W.J. Presettlement and modern disturbance regimes in coast redwood forests: Implications for the conservation of old-growth stands. For. Ecol. Manag. 2009, 258, 1038–1054. [Google Scholar] [CrossRef]
- Tyrrell, L.E.; Crow, T.R. Structural characteristics of old-growth hemlock-hardwood forests in relation to age. Ecology 1994, 75, 370–386. [Google Scholar] [CrossRef]
- Busing, R.T. Composition, structure and diversity of cove forest stands in the Great Smoky Mountains: A patch dynamics perspective. J. Veg. Sci. 1998, 9, 881–890. [Google Scholar] [CrossRef]
- Woods, K.D. Dynamics in late-successional hemlock-hardwood forests over three decades. Ecology 2000, 81, 110–126. [Google Scholar]
- Cale, J.A.; Teale, S.A.; West, J.L.; Zhang, L.I.; Castello, D.R.; Devlin, P.; Castello, J.D. A quantitative index of forest structural sustainability. Forests 2014, 5, 1618–1634. [Google Scholar] [CrossRef]
- Marks, C.O.; Canham, C.D. A quantitative framework for demographic trends in size-structured populations: Analysis of threats to floodplain forests. Ecosphere 2015, 6, 232. [Google Scholar] [CrossRef]
- Hanson, J.J.; Lorimer, C.G.; Halpin, C.R. Predicting long-term sapling dynamics and canopy recruitment in northern hardwood forests. Can. J. For. Res. 2011, 41, 903–919. [Google Scholar] [CrossRef]
- Halpin, C.R. Long-term Stand Development and Demographic Sustainability of Tree Populations in Northern Hardwood Forests. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2014. [Google Scholar]
- Halpin, C.R.; Lorimer, C.G. Trajectories and resilience of stand structure in response to variable disturbance severities in northern hardwoods. For. Ecol. Manag. 2016, 365, 69–82. [Google Scholar] [CrossRef]
- Halpin, C.R.; Lorimer, C.G. Long-term trends in biomass and tree demography in northern hardwoods: An integrated field and simulation study. Ecol. Monogr. 2016, 86, 78–93. [Google Scholar] [CrossRef]
- Halpin, C.R. Simulated Long-term Effects of Group Selection on Production, Efficiency, Composition, and Structure in Northern Hardwood and Hemlock-hardwood Forests. Master’s Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2009. [Google Scholar]
- Hanson, J.J.; Lorimer, C.G.; Halpin, C.R.; Palik, B.J. Ecological forestry in an uneven-aged, late-successional forest: Simulated effects of contrasting treatments on structure and yield. For. Ecol. Manag. 2012, 270, 94–107. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Halpin, C.R. Classification and dynamics of developmental stages in late-successional temperate forests. For. Ecol. Manag. 2014, 334, 344–357. [Google Scholar] [CrossRef]
- Kotar, J.; Burger, T.L.; Kovach, J.A. A Guide to Forest Communities and Habitat Types of Northern Wisconsin; Department of Forest Ecology and Management, University of Wisconsin-Madison: Madison, WI, USA, 2002. [Google Scholar]
- Coffman, M.S. Field Guide: Habitat Classification System for Upper Peninsula of Michigan and Northeast Wisconsin; School of Forestry and Wood Products, Michigan Technological University: Houghton, MI, USA, 1980. [Google Scholar]
- Frelich, L.E.; Lorimer, C.G. Natural disturbance regimes in hemlock-hardwood forests of the upper Great Lakes region. Ecol. Monogr. 1991, 61, 145–164. [Google Scholar] [CrossRef]
- Lugo, A.E.; Scatena, F.N. Background and catastrophic mortality in tropical moist, wet, and rain forests. Biotropica 1996, 28, 585–599. [Google Scholar] [CrossRef]
- Vanclay, J.K. A growth model for north Queensland rain forests. For. Ecol. Manag. 1989, 27, 245–271. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.D.; Swan, J.M.A. Reconstructing forest history from live and dead plant material—An approach to the study of forest succession in southwest New Hampshire. Ecology 1974, 55, 772–783. [Google Scholar] [CrossRef]
- Peterson, C.J. Damage and recovery of tree species after two different tornadoes in the same old growth forest: A comparison of infrequent wind disturbances. For. Ecol. Manag. 2000, 135, 237–252. [Google Scholar] [CrossRef]
- Lang, K.D.; Schulte, L.A.; Guntenspergen, G.R. Windthrow and salvage logging in an old-growth hemlock-northern hardwoods forest. For. Ecol. Manag. 2009, 259, 56–64. [Google Scholar] [CrossRef]
- Zhang, Q.; Pregitzer, K.S.; Reed, D.D. Catastrophic disturbance in the presettlement forests of the upper peninsula of Michigan. Can. J. For. Res. 1999, 29, 106–114. [Google Scholar] [CrossRef]
- Schulte, L.A.; Mladenoff, D.J. Severe wind and fire regimes in northern forests: Historical variability at the regional scale. Ecology 2005, 86, 431–445. [Google Scholar] [CrossRef]
- Hanson, J.J. Emulating Natural Disturbance Dynamics in Northern Hardwood Forests: Long-term Effects on Species Composition, Forest Structure, and Yield. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2009. [Google Scholar]
- Lorimer, C.G.; Dahir, S.E.; Nordheim, E.V. Tree mortality rates and longevity in mature and old-growth hemlock-hardwood forests. J. Ecol. 2001, 89, 960–971. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Holdsworth, A.R.; Frelich, L.E.; Reich, P.B. Effects of earthworm invasion on plant species richness in northern hardwood forests. Conserv. Biol. 2007, 21, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.J.; Orwig, D.A. An invasive urban forest pest invades natural environments—Asian longhorned beetle in northeastern US hardwood forests. Can. J. For. Res. 2011, 41, 1729–1742. [Google Scholar] [CrossRef]
- Mayer, D.G.; Butler, D.G. Statistical validation. Ecol. Model. 1993, 68, 21–32. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Lima, R.A.F.; Muller-Landau, H.C.; Prado, P.I.; Condit, R. How do size distributions relate to currently measured demographic rates? Evidence from over 150 tree species in Panama. J. Trop. Ecol. 2016, 32, 179–192. [Google Scholar] [CrossRef]
- Rubin, B.D.; Manion, P.D.; Faber-Langendoen, D. Diameter distributions and structural sustainability in forests. For. Ecol. Manag. 2006, 222, 427–438. [Google Scholar] [CrossRef]
- O’Hara, K.L. Dynamics and stocking-level relationships of multi-aged Ponderosa pine stands. For. Sci. Monogr. 1996, 42, 1–34. [Google Scholar]
- Goodburn, J.M.; Lorimer, C.G. Population structure in old-growth and managed northern hardwoods: An examination of the balanced diameter distribution concept. For. Ecol. Manag. 1999, 118, 11–29. [Google Scholar] [CrossRef]
- Marquis, D.A. Application of uneven-aged silviculture and management on public and private lands. In Uneven-Aged Silviculture and Management in the United States; USDA Forest Service, Timber Management Research: Washington, DC, USA, 1978; pp. 25–61. [Google Scholar]
- Crow, T.R.; Jacobs, R.D.; Oberg, R.R.; Tubbs, C.H. Stocking and Structure for Maximum Growth in Sugar Maple Selection Stands; Research Paper NC-199; USDA Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1981.
- Leak, W.B.; Solomon, D.S.; DeBald, P.S. Silvicultural Guide for Northern Hardwood Types in the Northeast (Revised); Research Paper NE-603; USDA Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1987.
- Janowiak, M.K.; Webster, C.R. Promoting ecological sustainability in woody biomass harvesting. J. For. 2010, 108, 16–23. [Google Scholar]
- Aguilar, F.X.; Daniel, M.J.; Narine, L.L. Opportunities and challenges to the supply of woody biomass for energy from Missouri nonindustrial privately owned forestlands. J. For. 2013, 111, 249–260. [Google Scholar] [CrossRef]
- Keeton, W.S. Managing for late-successional/old-growth characteristics in northern hardwood-conifer forests. For. Ecol. Manag. 2006, 235, 129–142. [Google Scholar] [CrossRef]
- Busing, R.T.; Wu, X. Size-specific mortality, growth, and structure of a Great Smoky Mountains red spruce population. Can. J. For. Res. 1990, 20, 206–210. [Google Scholar] [CrossRef]
- Westphal, C.; Tremer, N.; von Oheimb, G.; Hansen, J.; von Gadow, K.; Hardtle, W. Is the reverse J-shaped diameter distribution universally applicable in European virgin beech forests? For. Ecol. Manag. 2006, 223, 75–83. [Google Scholar] [CrossRef]
- Alessandrini, A.; Biondi, F.; Di Filippo, A.; Ziaco, E.; Piovesan, G. Tree size distribution at increasing spatial scales converges to the rotated sigmoid form in two old-growth beech stands of the Italian Apennines. For. Ecol. Manag. 2011, 262, 1950–1962. [Google Scholar] [CrossRef]
- Trotsiuk, V.; Hobi, M.L.; Commarmot, B. Age structure and disturbance dynamics of the relic virgin beech forest Uholka (Ukrainian Carpathians). For. Ecol. Manag. 2012, 265, 181–190. [Google Scholar] [CrossRef]
- Hobi, M.L.; Commarmot, B.; Bugmann, H. Pattern and process in the largest primeval beech forest of Europe (Ukrainian Carpathians). J. Veg. Sci. 2015, 26, 323–336. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Frelich, L.E. A simulation of equilibrium diameter distributions of sugar maple (Acer saccharum). Bull. Torrey Bot. Club 1984, 111, 193–199. [Google Scholar] [CrossRef]
- Janowiak, M.K.; Nagel, L.M.; Webster, C.R. Spatial scale and stand structure in northern hardwood forests: Implications for quantifying diameter distributions. For. Sci. 2008, 54, 497–506. [Google Scholar]
- Runkle, J.R. Canopy tree turnover in old-growth mesic forests of eastern North America. Ecology 2000, 81, 554–567. [Google Scholar] [CrossRef]
- Busing, R.T. Tree mortality, canopy turnover, and woody detritus in old cove forests of the southern Appalachians. Ecology 2005, 86, 73–84. [Google Scholar] [CrossRef]
- Lines, E.R.; Coomes, D.A.; Purves, D.W. Influences of forest structure, climate and species composition on tree mortality across the eastern US. PLoS ONE 2010, 5, e13212. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.R.; Lorimer, C.G. Single-tree versus group selection in hemlock-hardwood forests: Are smaller openings less productive? Can. J. For. Res. 2002, 32, 591–604. [Google Scholar] [CrossRef]
- Woods, K.D. Long-term change and spatial pattern in a late-successional hemlock-northern hardwood forest. J. Ecol. 2000, 88, 267–282. [Google Scholar] [CrossRef]
- Fahey, R.T.; Lorimer, C.G.; Mladenoff, D.J. Habitat heterogeneity and life-history traits influence presettlement distribution of early-successional tree species in a late-successional, hemlock-hardwood landscape. Landsc. Ecol. 2012, 27, 999–1013. [Google Scholar] [CrossRef]
- Graham, S.A. Causes of Hemlock Mortality in Northern Michigan; Bulletin 10, School of Forestry & Conservation, University of Michigan: Ann Arbor, MI, USA, 1943. [Google Scholar]
- Hough, A.F. Silvical Characteristics of Eastern Hemlock (Tsuga canadensis); Station Paper SP-NE-132; USDA Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1960.
- Goerlich, D.L.; Nyland, R.D. Natural regeneration of eastern hemlock: A review. In Proceedings: Symposium on Sustainable Management of Hemlock Ecosystems in Eastern North America; McManus, K.A., Shields, K.S., Souto, D.R., Eds.; General Technical Report NE-267; USDA Forest Service, Northeastern Forest Experiment Station: Newtown Square, PA, USA, 2000; pp. 14–22. [Google Scholar]
- Marx, L.M.; Walters, M.B. Survival of tree seedlings on different species of decaying wood maintains tree distribution in Michigan hemlock-hardwood forests. J. Ecol. 2008, 96, 505–513. [Google Scholar] [CrossRef]
- Shields, J.M.; Webster, C.R.; Nagel, L.M. Factors influencing tree species diversity and Betula alleghaniensis establishment in silvicultural openings. Forestry 2007, 80, 293–307. [Google Scholar] [CrossRef]
- Bolton, N.W.; D’Amato, A.W. Regeneration responses to gap size and coarse woody debris within natural disturbance-based silvicultural systems in northeastern Minnesota, USA. For. Ecol. Manag. 2011, 262, 1215–1222. [Google Scholar] [CrossRef]
- Lambert, J.-B.; Ameztegui, A.; Delagrange, S.; Messier, C. Birch and conifer deadwood favour early establishment and shade tolerance in yellow birch juveniles growing in sugar maple dominated stands. Can. J. For. Res. 2016, 46, 114–121. [Google Scholar] [CrossRef]
- Schreiber, S.G.; Bearlin, A.R.; Nicol, S.J.; Todd, C.R. Adaptive management: A synthesis of current understanding and effective application. Ecol. Manag. Restor. 2004, 5, 177–182. [Google Scholar] [CrossRef]
- Williams, B.K. Passive and active adaptive management: Approaches and an example. For. Ecol. Manag. 2011, 92, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.R.; Leopold, D.J.; Eichenberger, J.K. Tree dynamics in an old-growth, deciduous forest. For. Ecol. Manag. 1985, 11, 31–57. [Google Scholar] [CrossRef]
- Redmond, M.D.; Wilbur, R.B.; Wilbur, H.M. Recruitment and dominance of Quercus rubra and Quercus alba in a previous oak-chestnut forest from the 1980s to 2008. Am. Midl. Nat. 2012, 168, 427–442. [Google Scholar] [CrossRef]
- Frelich, L.E.; Lorimer, C.G. Current and predicted long-term effects of deer browsing in hemlock forests in Michigan, USA. Biol. Conserv. 1985, 34, 99–120. [Google Scholar] [CrossRef]
- Kaya, I.; Buongiorno, J. Economic harvesting of uneven-aged northern hardwood stands under risk: A Markovian decision model. For. Sci. 1987, 33, 889–907. [Google Scholar]
- Caspersen, J.P.; Vanderwel, M.C.; Cole, W.G.; Purves, D.W. How stand productivity results from size- and competition-dependent growth and mortality. PLoS ONE 2011, 6, e28660. [Google Scholar] [CrossRef] [PubMed]
- Eschtruth, A.K.; Cleavitt, N.L.; Battles, J.J.; Evans, R.A.; Fahey, T.J. Vegetation dynamics in declining eastern hemlock stands: 9 years of forest response to hemlock woolly adelgid infestation. Can. J. For. Res. 2006, 36, 1435–1450. [Google Scholar] [CrossRef]
- Orwig, D.A.; Foster, D.R.; Mausel, D.L. Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. J. Biogeogr. 2002, 29, 1475–1487. [Google Scholar] [CrossRef]



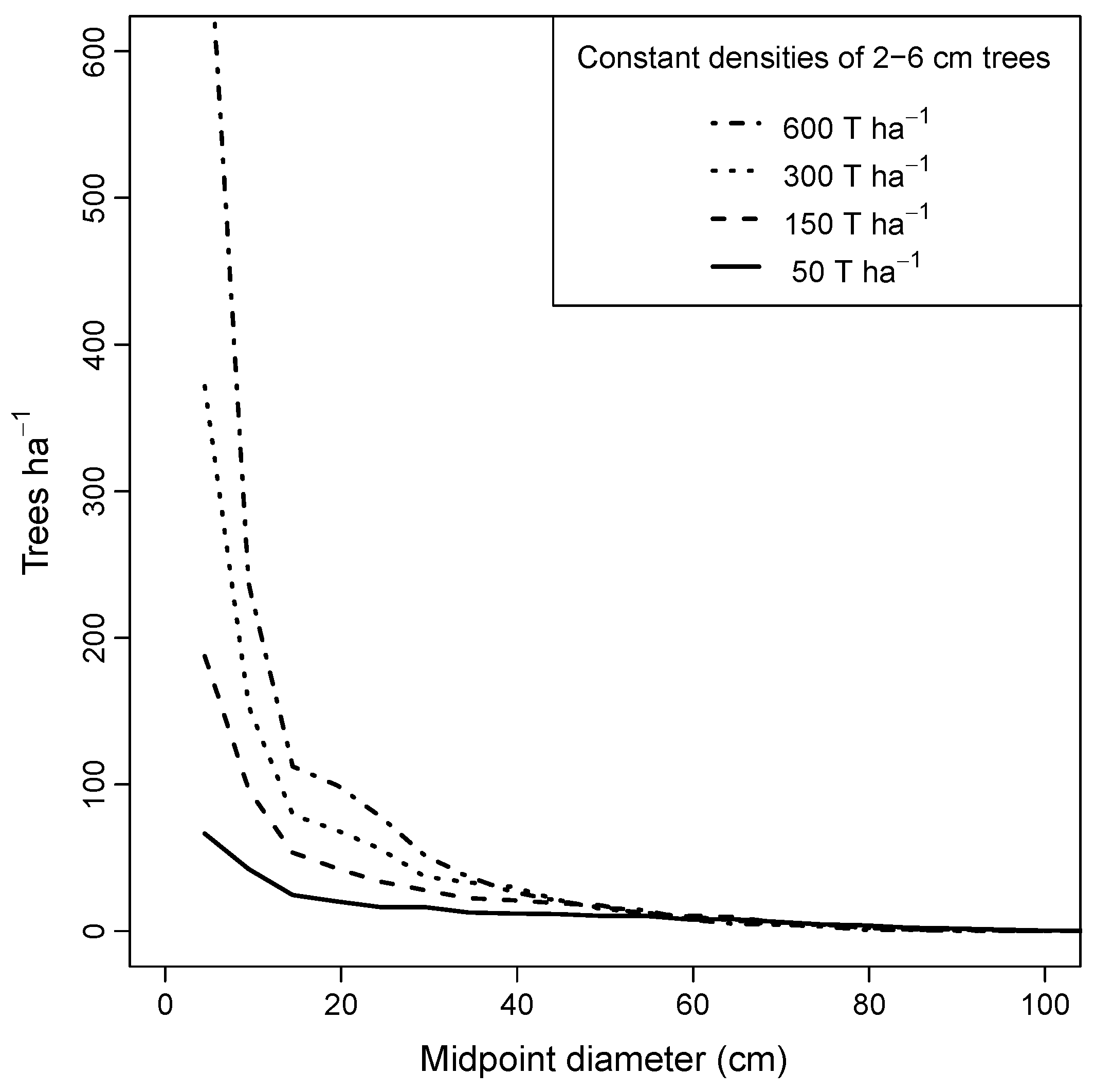

| Simulation number | Recruitment mode | Mortality | Species of recruits | Scale | Data set | Duration (years) |
|---|---|---|---|---|---|---|
| Group 1a | Fixed at initial | Background | Density dep. | Dual 1 | 70 indiv. plots | 500 |
| Group 1b | Density dep. | Background | Density dep. | Dual | 70 indiv. plots | 500 |
| Group 1c | Density dep. | Nat. Disturb. | Density dep. | Dual | 70 indiv. plots | 500 |
| Group 1d | Fixed at initial | Background | Fixed at initial | Landscape | 70 plots pooled 2 | 500 |
| Group 2a | Fixed, prespecified | Background | Sugar maple | Stand | 20 sim. AA plots 3 | 1000 |
| Group 2b | Capped, prespecified | Background | Sugar maple | Stand | 20 sim. AA plots | 1000 |
| Group 2c | Capped, prespecified | Background | Dens. dep, no Hem. | Stand | 20 sim. AA plots | 1000 |
| Group 2d | Capped, prespecified | Background | Density dep. | Stand | 20 sim. AA plots | 1000 |
| Group 3 | Constant q | Background | Density dep. | Stand | 20 sim. AA plots | 1000 |
| Group 4 | Variable q | Background | Density dep. | Stand | 20 sim. AA plots | 1000 |
| Simulation group | Percentage of plots by sustainability class | Pop. overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <0.2 | <0.4 | <0.6 | <0.8 | <0.9 | 0.9+ | avg. plot 1 | pop. Overall 2 | where present | |
| All spp. pooled | |||||||||
| (1a) Const. 2–6 cm·T·ha−1, BM 3 | 0 | 8 | 23 | 48 | 58 | 42 | 0.82 | 0.83 | NA |
| (1b) Density dependent, BM 4 | 0 | 0 | 0 | 11 | 24 | 76 | 1.02 | 1.08 | NA |
| (1c) Density dependent, HD 5 | 0 | 2 | 16 | 49 | 66 | 34 | 0.82 | 0.85 | NA |
| Sugar maple | |||||||||
| (1a) Const. 2–6 cm·T·ha−1, BM | 1 | 4 | 11 | 24 | 32 | 68 | 1.35 | 1.06 | 1.06 |
| (1b) Density dependent, BM | 4 | 15 | 27 | 39 | 44 | 56 | 1.23 | 0.95 | 0.95 |
| (1c) Density dependent, HD | 1 | 6 | 15 | 27 | 35 | 65 | 1.38 | 1.06 | 1.06 |
| Hemlock | |||||||||
| (1a) Const. 2–6 cm·T·ha−1, BM | 15 | 27 | 40 | 50 | 55 | 45 | 1.31 | 0.72 | 0.71 |
| (1b) Density dependent, BM | 0 | 1 | 4 | 8 | 13 | 87 | 2.28 | 1.65 | 1.64 |
| (1c) Density dependent, HD | 10 | 23 | 38 | 51 | 56 | 44 | 1.16 | 0.82 | 0.81 |
| Yellow birch | |||||||||
| (1a) Const. 2–6 cm·T·ha−1, BM | 57 | 78 | 84 | 88 | 89 | 11 | NA 6 | 0.20 | 0.19 |
| (1b) Density dependent, BM | 48 | 70 | 81 | 86 | 87 | 13 | NA | 0.22 | 0.22 |
| (1c) Density dependent, HD | 59 | 79 | 86 | 89 | 90 | 10 | NA | 0.18 | 0.18 |
| Ash | |||||||||
| (1a) Const. 2–6 cm·T·ha−1, BM | 31 | 41 | 49 | 55 | 59 | 41 | NA | 2.27 | 0.78 |
| (1b) Density dependent, BM | 16 | 31 | 44 | 50 | 53 | 47 | NA | 2.57 | 0.81 |
| (1c) Density dependent, HD | 24 | 37 | 46 | 52 | 57 | 43 | NA | 2.49 | 0.80 |
| Basswood | |||||||||
| (1a) Const. 2–6 cm·T·ha−1, BM | 26 | 37 | 46 | 53 | 55 | 45 | NA | 0.78 | 0.58 |
| (1b) Density dependent, BM | 14 | 27 | 38 | 47 | 51 | 49 | NA | 0.90 | 0.68 |
| (1c) Density dependent, HD | 28 | 45 | 54 | 62 | 64 | 36 | NA | 0.57 | 0.42 |
| Red maple | |||||||||
| (1a) Const. 2–6 cm·T·ha−1, BM | 66 | 78 | 84 | 86 | 88 | 12 | NA | 0.28 | 0.19 |
| (1b) Density dependent, BM | 61 | 73 | 79 | 84 | 85 | 15 | NA | 0.29 | 0.19 |
| (1c) Density dependent, HD | 61 | 74 | 82 | 86 | 87 | 13 | NA | 0.28 | 0.21 |
| Background mortality | Historic dist. regime | |||||
|---|---|---|---|---|---|---|
| Variable | Const. recruitment 1 | Density-dep recruitment 2 | Density-dep recruitment 3 | |||
| Group 1a | Group 1b | Group 1c | ||||
| Estimate | p | Estimate | p | Estimate | p | |
| All species pooled | ||||||
| (Intercept) | 1.0150 | <0.001 | 0.7190 | <0.001 | 0.5753 | <0.001 |
| 2–6 cm·T·ha−1 | 0.0008 | <0.001 | −0.0002 | <0.001 | −0.0002 | 0.039 |
| Initial BA | −0.0266 | <0.001 | −0.0128 | <0.001 | −0.0165 | <0.001 |
| BA-weighted avg. q | 0.0320 | <0.001 | 0.0249 | 0.072 | ||
| Deviation from SS 4 | −0.0112 | <0.001 | −0.0022 | 0.012 | ||
| Modal DBH | −0.0020 | 0.452 | −0.0030 | <0.001 | −0.0020 | 0.023 |
| R2 | 0.77 | 0.63 | 0.47 | |||
| Sugar maple | ||||||
| (Intercept) | 1.0941 | <0.001 | −1.5188 | <0.001 | 1.2119 | <0.001 |
| 2–6 cm·T·ha−1 | 0.0012 | <0.001 | 0.0010 | <0.001 | 0.0009 | 0.003 |
| Initial BA | −0.0254 | <0.001 | −0.0057 | 0.053 | −0.0204 | <0.001 |
| Avg. q below mode 5 | 0.1478 | <0.001 | 1.4382 | <0.001 | 0.2942 | 0.008 |
| QMD | 0.0391 | <0.001 | 0.0274 | <0.001 | 0.0226 | <0.001 |
| 2–6 cm·SM·ha−1 | −0.0010 | <0.001 | −0.0013 | <0.001 | ||
| Initial SM BA | −0.0397 | <0.001 | −0.0470 | <0.001 | −0.0379 | <0.001 |
| Avg. q below mode for SM | 0.0031 | 0.104 | ||||
| QMD for SM | −0.0170 | <0.001 | −0.0104 | 0.002 | −0.0205 | <0.001 |
| R2 | 0.68 | 0.45 | 0.60 | |||
| Hemlock | ||||||
| (Intercept) | 2.9959 | <0.001 | 1.6742 | <0.001 | −0.2405 | 0.404 |
| 2–6 cm·T·ha−1 | −0.0012 | 0.002 | 0.0004 | 0.071 | 0.0007 | 0.049 |
| Initial BA | 0.0613 | <0.001 | −0.0041 | 0.159 | 0.0148 | 0.012 |
| Avg. q below mode | −0.2965 | 0.071 | −0.7328 | <0.001 | ||
| QMD | −0.1779 | <0.001 | 0.0128 | 0.065 | 0.0284 | 0.012 |
| 2–6 cm·Hem·ha−1 | 0.0056 | 0.003 | ||||
| Initial Hem BA | −0.0649 | <0.001 | −0.0307 | <0.001 | −0.0262 | <0.001 |
| Avg. q below mode for Hem | 0.0115 | 0.049 | −0.0400 | <0.001 | ||
| QMD for Hem | −0.0359 | <0.001 | ||||
| R2 | 0.57 | 0.29 | 0.29 | |||
| Yellow birch | ||||||
| (Intercept) | −0.0820 | 0.740 | 1.0638 | 0.010 | 2.0188 | 0.004 |
| 2–6 cm·T·ha−1 | −0.0011 | 0.004 | ||||
| Initial BA | 0.0501 | <0.001 | −0.0199 | <0.001 | 0.0409 | <0.001 |
| Avg. q below mode | −0.8325 | <0.001 | −1.9549 | <0.001 | ||
| QMD | −0.0682 | <0.001 | −0.0485 | <0.001 | ||
| 2–6 cm·YB·ha−1 | −0.0033 | 0.079 | 0.0042 | 0.026 | ||
| Initial YB BA | −0.0458 | 0.002 | −0.1621 | <0.001 | −0.0614 | <0.001 |
| Avg. q below mode for YB | −0.0473 | <0.001 | −0.0344 | <0.001 | ||
| QMD for YB | −0.0492 | <0.001 | 0.0130 | <0.001 | −0.0371 | <0.001 |
| R2 | 0.34 | 0.27 | 0.34 | |||
| 1. Simulated SS stand | Steady-state field data | |||
| T·ha−1 | q | T·ha−1 | q | |
| 2–7 cm | 337 | 3.83 | 686 (290–1080) | 4.52 (1.65–7.39) |
| 7–12 cm | 88 | 1.74 | 153 (119–189) | 2.20 (1.84–2.56) |
| 12–17 cm | 51 | 1.05 | 71 (55–86) | 1.60 (1.29–1.91) |
| 17–22 cm | 49 | – | 45 (37–52) | – |
| 2. Number of 12–17 cm trees needed to maintain 49, 17–22 cm trees | ||||
| q | 12–17 cm·T·ha−1 | Predicted 17–22 cm·T·ha−1 | ||
| 1.05 | 51 | 30.1 | (24.9–35.3) | |
| 1.10 | 53 | 35.9 | (30.7–41.2) | |
| 1.15 | 56 | 48.5 | (41.9–55.0) | |
| 3. Number of 7–12 cm trees needed to maintain 56, 12–17 cm trees | ||||
| q | 7–12 cm·T·ha−1 | Predicted 12–17 cm·T·ha−1 | ||
| 1.60 | 90 | 34.5 | (30.3–38.7) | |
| 1.80 | 101 | 45.0 | (39.6–50.4) | |
| 1.90 | 106 | 56.3 | (49.3–62.9) | |
| 4. Number of 2–7 cm trees needed to maintain 106, 7–12 cm trees | ||||
| q | 2–7 cm·T·ha−1 | Predicted 7–12 cm·T·ha−1 | ||
| 3.50 | 370 | 72.3 | (65.5–79.2) | |
| 3.60 | 382 | 88.8 | (79.0–100.6) | |
| 3.70 | 392 | 98.5 | (88.5–108.6) | |
| 3.75 | 398 | 106.7 | (93.7–119.6) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halpin, C.R.; Lorimer, C.G. A Demographic Approach to Evaluating Tree Population Sustainability. Forests 2017, 8, 46. https://doi.org/10.3390/f8020046
Halpin CR, Lorimer CG. A Demographic Approach to Evaluating Tree Population Sustainability. Forests. 2017; 8(2):46. https://doi.org/10.3390/f8020046
Chicago/Turabian StyleHalpin, Corey R., and Craig G. Lorimer. 2017. "A Demographic Approach to Evaluating Tree Population Sustainability" Forests 8, no. 2: 46. https://doi.org/10.3390/f8020046




