Laurel Wilt in Natural and Agricultural Ecosystems: Understanding the Drivers and Scales of Complex Pathosystems
Abstract
:1. Introduction
2. Origins
3. Coevolution
- A limited, often specific host range for the pathogen;
- An original geographic distribution of the pathogen that overlaps with that of the host;
- The occurrence of significant disease resistance in the host’s primary center of origin;
- Regional overlap of resistance and pathogenicity factors and phenotypes in the respective host and pathogen populations (i.e., geographic evidence for reciprocal selection);
- Gene-for-gene relationships (although quantitative, non-gene-for-gene interactions can also co-evolve); and
- Tandem speciation (also known as parallel cladogenesis).
4. Ambrosial Symbioses
5. Vectors of Raffaelea lauricola
6. Vector Chemical Ecology and Host Location
7. Pathogen Attributes
8. Hosts of Laurel Wilt
9. Host Responses to Infection by Raffaelea lauricola
10. Ecology and Epidemiology
11. Outlook
12. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.; de Beer, Z.W. Ambrosia and bark beetle-associated tree diseases: Black Swan events in tree pathology? Plant Dis. 2013, 95, 856–872. [Google Scholar] [CrossRef]
- Pautasso, M.; Schlegel, M.; Holdelrieder, O. Forest health in a changing world. Microb. Ecol. 2015, 69, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted forest health: The need for a global strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.J.; Garnas, J.R.; Hajek, A.; Hurley, B.P.; de Beer, Z.W.; Taerum, S.J. Novel and co-evolved associations between insects and microorganisms as drivers of forest pestilence. Biol. Invasions 2016, 18, 1045–1056. [Google Scholar] [CrossRef]
- Anderson, P.K.; Cuningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wingfield, M.J.; Gillette, N.; Sun, J.-H. Do novel genotypes drive the success of an invasive bark beetle–fungus complex? Implications for potential reinvasion. Ecology 2011, 92, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakis, S.L. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 1987, 79, 22–37. [Google Scholar] [CrossRef]
- Ploetz, R.C. Diseases of tropical perennial crops: Challenging problems in diverse environments. Plant Dis. 2007, 91, 644–663. [Google Scholar] [CrossRef]
- Rackham, O. Ancient woodlands: Modern threats. New Phytol. 2008, 180, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E., III; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern USA. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef]
- Haack, R.A. Exotic bark- and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Rabaglia, R.J.; Dole, S.A.; Cognato, A.I. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) Occurring North of Mexico, with an Illustrated Key. Ann. Entomol. Soc. Am. 2006, 99, 1034–1056. [Google Scholar] [CrossRef]
- Barton, C.; Bates, C.; Cutrer, B.; Eickwort, J.; Harrington, S.; Jenkins, D.; Stones, D.M.; Reid, L.; Riggins, J.J.; Trickel, R. Distribution of Counties with Laurel wilt Disease by Year of Initial Detection. Available online: http://www.fs.usda.gov/Internet/FSE_DOCUMENTS/fseprd513913.pdf (accessed on 16 Feb 2017).
- Hughes, M.A.; Riggins, J.J.; Koch, F.H.; Cognato, A.I.; Anderson, C.; Formby, J.P.; Dreaden, T.J.; Ploetz, R.C.; Smith, J.A. No rest for the laurels: Symbioclone invader causes unprecedented damage to southern USA forests. Biol. Invasions 2017, in press. [Google Scholar]
- Goldberg, N.; Heine, J. A comparison of arborescent vegetation pre- (1983) and post- (2008) outbreak of the invasive species the Asian ambrosia beetle Xyleborus glabratus in a Florida maritime hammock. Plant Ecol. Divers. 2009, 2, 77–83. [Google Scholar] [CrossRef]
- Gramling, J.M. Potential effects of laurel wilt on the flora of North America. Southeast Nat. 2010, 9, 827–836. [Google Scholar] [CrossRef]
- Shields, J.; Jose, S.; Freeman, J.; Bunyan, M.; Celis, G.; Hagan, D.; Morgan, M.; Pieterson, E.C.; Zak, J. Short-term impacts of laurel wilt on redbay (Persea borbonia L. Spreng.) in a mixed evergreen-deciduous forest in northern Florida. J. For. 2011, 109, 82–88. [Google Scholar]
- Evans, J.P.; Scheffers, B.R.; Hess, M. Effect of laurel wilt invasion on redbay populations in a maritime forest community. Biol. Invasions 2013, 16, 1581–1588. [Google Scholar] [CrossRef]
- Spiegel, K.S.; Leege, L.M. Impacts of laurel wilt disease on redbay (Persea borbonia (L.) Spreng.) population structure and forest communities in the coastal plain of Georgia, USA. Biol. Invasions 2013, 15, 2467–2487. [Google Scholar] [CrossRef]
- Chupp, A.D.; Battaglia, L.L. Potential for host shifting in Papilio palamedes following invasion of laurel wilt disease. Biol. Invasions 2014, 16, 2639–2651. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Bates, C.; Johnson, J.; Reid, L.; Leininger, T.; Hawkins, T. Susceptibility to laurel wilt and disease incidence in two rare plant species, pondberry and pondspice. Plant. Dis. 2011, 95, 1056–1062. [Google Scholar] [CrossRef]
- Rodgers, L.; Derksen, A.; Pernas, T. Expansion and impact of laurel wilt in the Florida Everglades. Fla. Entomol. 2014, 97, 1247–1250. [Google Scholar] [CrossRef]
- Hughes, M.A.; Smith, J.A.; Ploetz, R.C.; Kendra, P.E.; Mayfield, A.E., III; Hanula, J.L.; Hulcr, J.; Stelinski, L.L.; Cameron, S.; Riggins, J.J.; et al. Recovery plan for laurel wilt on redbay and other forest species caused by Raffaelea lauricola and disseminated by Xyleborus glabratus. Plant Health Progr. 2015, 16, 173–210. [Google Scholar]
- Mosquera, M.; Evans, E.A.; Ploetz, R. Assessing the profitability of avocado production in south Florida in the presence of laurel wilt. Theor. Econ. Lett. 2015, 5, 343–356. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Hughes, M.A.; Kendra, P.E.; Fraedrich, S.W.; Carrillo, D.; Stelinski, L.L.; Hulcr, J.; Mayfield, A.E., III; Dreaden, T.L.; Crane, J.H.; et al. Recovery Plan for Laurel Wilt of Avocado, caused by Raffaelea lauricola. Plant Health Progr. 2017, 18. in press. [Google Scholar]
- Hughes, M.A.; Shin, K.; Eickwort, J.; Smith, J.A. First report of laurel wilt disease caused by Raffaelea lauricola on silk bay in Florida. Plant Dis. 2012, 96, 910. [Google Scholar] [CrossRef]
- Hughes, M.A.; Brar, G.; Ploetz, R.C.; Smith, J.A. Field and growth chamber inoculations demonstrate Persea indica as a newly recognized host for the laurel wilt pathogen, Raffaelea laurciola. Plant Health Progr. 2013. [Google Scholar] [CrossRef]
- Hughes, M.A.; Black, A.; Smith, J.A. First report of laurel wilt, caused by Raffaelea lauricola, on bay laurel (Laurus nobilis) in the United States. Plant Dis. 2014, 98, 1159. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Konkol, J. First report of gulf licaria, Licaria trianda, as a suscept of laurel wilt. Plant Dis. 2013, 97, 1248. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Best, G.S. Xyleborus glabratus attacks and systemic colonization by Raffaelea lauricola associated with dieback of Cinnamomum camphora in the southeastern United States. For. Pathol. 2015, 45, 60–70. [Google Scholar]
- Hulcr, J.; Lou, Q.-Z. The redbay ambrosia beetle (Coleoptera: Curculionidae) prefers Lauraceae in its native range: Records from the Chinese national insect collection. Fla. Entomol. 2013, 96, 1595–1596. [Google Scholar] [CrossRef]
- Harrington, T.C.; Yun, H.Y.; Lu, S.S.; Goto, H.; Aghayeva, D.N.; Fraedrich, S.W. Isolations from the redbay ambrosia beetle, Xyleborus glabratus, confirm that the laurel wilt pathogen, Raffaelea lauricola, originated in Asia. Mycologia 2011, 103, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.A. The Evaluation of Natural Resistance to Laurel wilt Disease in Redbay (Persea borbonia). Ph.D. Thesis, University of Florida, Gainesville FL, USA, 2013. [Google Scholar]
- Wuest, C.E.; Harrington, T.C.; Fraedrich, S.W.; Yun, H.-Y.; Lu, S.-S. Genetic variation in native populations of the laurel wilt pathogen, Raffaelea lauricola, in Taiwan and Japan and the introduced population in the USA. Plant Dis. 2017, 101. in press. [Google Scholar]
- Ploetz, R.C.; Thant, Y.Y.; Hughes, M.A.; Dreaden, T.J.; Konkol, J.L.; Kyaw, A.T.; Smith, J.A.; Harmon, C.L. Laurel wilt, caused by Raffaelea lauricola, is detected for the first time outside the southeastern USA. Plant Dis. 2016, 100, 2166. [Google Scholar] [CrossRef]
- Erlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Darwin, C.R. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Flor, H.H. Host-parasite interaction in flax rust—Its genetics and other implications. Phytopathology 1955, 45, 680–685. [Google Scholar]
- Crute, I.R. The elucidation and exploitation of gene-for-gene recognition. Plant Pathol. 1998, 47, 107–113. [Google Scholar] [CrossRef]
- Bergelson, J.; Dwyer, G.; Emerson, J.J. Models and data on plant-enemy coevolution. Annu. Rev. Genet. 2001, 35, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Kareiva, P. Coevolutionary arms races: Is victory possible? Proc. Natl. Acad. Sci. USA 1999, 96, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.J.; Thrall, P.H. Spatial and temporal patterns in coevolving plant and pathogen associations. Am. Nat. 1999, 153, S15–S33. [Google Scholar] [CrossRef]
- Thompson, J.N. Specific hypotheses on the geographic mosaic of coevolution. Am. Nat. 1999, 153, S1–S14. [Google Scholar] [CrossRef]
- Burdon, J.J.; Thrall, P.H.; Ericson, L. The current and future dynamics of disease in plant communities. Annu. Rev. Phytopathol. 2006, 44, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. Disease and evolution. La Ric. Sci. (Suppl.) 1949, 19, 68–76. [Google Scholar]
- Harlan, J.R. Diseases as a factor in plant evolution. Annu. Rev. Phytopathol. 1976, 14, 31–51. [Google Scholar] [CrossRef]
- Clay, K.; Kover, P.X. The red queen hypothesis and plant/pathogen interactions. Annu. Rev. Phytopathol. 1996, 34, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Chung, K.-R. Coevolution by common descent of fungal symbionts (Epichloë spp.) and grass hosts. Mol. Biol. Evol. 1997, 14, 133–143. [Google Scholar] [CrossRef]
- Holst-Jensen, A.; Kohn, L.M.; Jakobsen, K.S.; Schumacher, T. Molecular phylogeny and evolution of Monilinia (Sclerotiniaceae) based on coding and noncoding rDNA sequences. Amer. J. Bot. 1997, 84, 686–701. [Google Scholar] [CrossRef]
- Evans, H.C. Invasive neotropical pathogens of tree crops. In Tropical Mycology: Volume 2, Micromycetes; CABI Publishing: Wallingford, UK, 2002; pp. 83–112. [Google Scholar]
- O’Donnell, K.; Sink, S.; Libeskind-Hadas, R.; Ploetz, R.C.; Konkol, J.L.; Ploetz, J.N.; Carrillo, D.; Campbell, A.; Duncan, R.E.; Kasson, M.T.; et al. Cophylogenetic analysis of the Fusarium–Euwallacea (Coleoptera: Scolytinae) mutualism suggests their discordant phylogenies are due to repeated host shifts. Fungal Genet. Biol. 2015, 82, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, L.R. Ecology and evolution of biased sex ratios in bark and ambrosia beetles. In Evolution and Diversity of Sex Ratio: Insects and Mites; Wrensch, D.L., Ebbert, M.A., Eds.; Chapman and Hall: New York, NY, USA, 1993; pp. 235–345. [Google Scholar]
- Jordal, B.H.; Normark, B.B.; Farrell, B.D. Evolutionary radiation of a haplodiploid beetle lineage (Curculionidae, Scolytinae). Biol. J. Linn. Soc. 2000, 71, 483–499. [Google Scholar] [CrossRef]
- Jordal, B.H.; Cognato, A.I. Molecular phylogeny of bark and ambrosia beetles reveals multiple origins of fungus farming during periods of global warming. BMC Evol. Biol. 2012, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Maner, M.L.; Hanula, J.L.; Braman, S.K. Gallery productivity, emergence, and flight activity of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2013, 42, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.D.; Sequeira, A.; O’Meara, B.; Normark, B.B.; Chung, J.; Jordal, B. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L. Bark beetle-fungus symbioses. In Insect Symbiosis; Bourtzis, K., Miller, T., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 97–114. [Google Scholar]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: From evolutionary ecology to practical management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Francke-Grosmann, H. Ectosymbiosis in wood-inhabiting insects. In Symbiosis (Volume 2—Associations of Invertebrates, Birds, Ruminants and Other Biota; Henry, S.M., Ed.; Academic Press: New York, NY, USA, 1967; pp. 141–206. [Google Scholar]
- Hulcr, J.; Cognato, A.I. Repeated evolution of crop theft in fungus-farming ambrosia beetles. Evolution 2010, 64, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Ngoan, N.D.; Wilkinson, R.C.; Short, D.E.; Moses, C.S.; Mangold, J.R. Biology of an introduced ambrosia beetle, Xylosandrus compactus, in Florida. Ann. Entomol. Soc. Am. 1976, 69, 872–876. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Persad, A.B.; Herms, D.A. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agric. For. Entomol. 2010, 12, 177–185. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Pruett, G.E.; Mayfield, A.E., III; MacKenzie, M.; Deyrup, M.A.; Bauchan, G.R.; Ploetz, R.C.; Epsky, N.D. North American Lauraceae: Terpenoid emissions, relative attraction and boring preferences of redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2014, 9, e102086. [Google Scholar] [CrossRef] [PubMed]
- Kuhnholz, S.; Borden, J.H.; Uzunovic, A. Secondary ambrosia beetles in apparently healthy trees: Adaptions, potential causes and suggested research. Integr. Pest Manag. Rev. 2002, 6, 209–219. [Google Scholar] [CrossRef]
- Hulcr, J.; Dunn, R.R. The sudden emergence of pathogenicity in insect–fungus symbioses threatens naive forest ecosystems. Proc. Roy. Soc. B Sci. 2011, 278, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, D.; Duncan, R.E.; Ploetz, J.N.; Campbell, A.; Ploetz, R.C.; Peña, J.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Narvaez, T.; Duncan, R.E.; Saucedo, R.J.; Campbell, A.; Mantilla, J.; Carrillo, D.; Kendra, P.E. Presence and prevalence of Raffaelea lauricola, cause of laurel wilt, in different species of ambrosia beetle in Florida USA. J. Econ. Entomol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Batra, L.R. Ambrosia fungi: Extent of specificity to ambrosia beetles. Science 1966, 173, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.C. Ecology and evolution of mycophagous bark beetles and their fungal partners. In Insect-Fungal Associations. Ecology and Evolution; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 257–291. [Google Scholar]
- Gebhardt, H.; Begerow, D.; Oberwinkler, F. Identification of the ambrosia fungus of Xyleborus monographus and X. dryographus (Coleoptera: Curculionidae, Scolytinae). Mycol. Prog. 2004, 3, 95–102. [Google Scholar] [CrossRef]
- Kostovcik, M.; Bateman, C.C.; Kolarik, M.; Stelinski, L.L.; Jordal, B.H.; Hulcr, J. The ambrosia symbiosis is specific in some species and promiscuous in others: Evidence from community pyrosequencing. ISME J. 2015, 9, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.C.; Aghayeva, D.N.; Fraedrich, S.W. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiela, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 2010, 111, 337–361. [Google Scholar] [CrossRef]
- Harrington, T.C.; Fraedrich, S.W. Quantification of propagules of the laurel wilt fungus and other mycangial fungi from the redbay ambrosia beetle, Xyleborus glabratus. Phytopathology 2010, 100, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Hulcr, J.; Mann, R.; Stelinski, L.L. The scent of a partner: Ambrosia beetles are attracted to volatiles from their fungal symbionts. J. Chem. Ecol. 2011, 37, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Hanula, J.L.; Mayfield, A.E., III; Fraedrich, S.W.; Rabaglia, R.J. Biology and host associations of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae), exotic vector of laurel wilt killing redbay trees in the southeastern United States. J. Econ. Entomol. 2008, 101, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Rabaglia, R.J. Ethanol and (−)-α-pinene: Attractant kairomones for bark and ambrosia beetles in the southeastern U.S. J. Chem. Ecol. 2009, 35, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Hanula, J.L.; Sullivan, B. Manuka oil and phoebe oil are attractive baits for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), the vector of laurel wilt. Environ. Entomol. 2008, 37, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Peña, J.E.; Capinera, J.L.; Brar, G.; Epsky, N.D.; Heath, R.R. Attraction of the redbay ambrosia beetle, Xyleborus glabratus, to avocado, lychee, and essential oil lures. J. Chem. Ecol. 2011, 37, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Niogret, J.; Kendra, P.E.; Epsky, N.D.; Heath, R.R. Comparative analysis of terpenoid emissions from Florida host trees of the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2011, 94, 1010–1017. [Google Scholar] [CrossRef]
- Hanula, J.L.; Sullivan, B.T.; Wakarchuk, D. Variation in manuka oil lure efficacy for capturing Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), and cubeb oil as an alternative attractant. Environ. Entomol. 2013, 42, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Epsky, N.D. An uncertain future for American Lauraceae: A lethal threat from redbay ambrosia beetle and laurel wilt disease. Amer. J. Plant Sci. 2013, 4, 727–738. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Schnell, E.Q.; Deyrup, M.A.; Epsky, N.D. Evaluation of seven essential oils identifies cubeb oil as most effective attractant for detection of Xyleborus glabratus. J. Pest Sci. 2014, 87, 681–689. [Google Scholar] [CrossRef]
- Kendra, P.E.; Niogret, J.; Montgomery, W.S.; Deyrup, M.A.; Epsky, N.D. Cubeb oil lures: Terpenoid emissions, trapping efficacy, and longevity for attraction of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2015, 108, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Deyrup, M.A.; Wakarchuk, D. Improved lure for redbay ambrosia beetle developed by enrichment of α-copaene content. J. Pest Sci. 2016, 89, 427–438. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Epsky, N.D. Efficacy of α-copaene, cubeb, and eucalyptol lures for detection of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2016, 109, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Tang, M. Laboratory evaluation of flight activity of Dendroctonus armando (Coleoptera: Curculionidae: Scolytinae). Canad. Entomol. 2010, 142, 378–387. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Deyrup, M.A.; Guillén, L.; Epsky, N.D. Xyleborus glabratus, X. affinis, and X. ferrugineus (Coleoptera: Curculionidae: Scolytinae): Electroantennogram responses to host-based attractants and temporal patterns in host-seeking flight. Environ. Entomol. 2012, 41, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, E.H.; Martini, X.; Tribuiani, Y.; Coy, M.; Gibbard, C.; Peña, J.; Hulcr, J.; Stelinski, L.L. Eucalyptol is an attractant of the redbay ambrosia beetle, Xyleborus glabratus. J. Chem. Ecol. 2014, 40, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Martini, X.; Hughes, M.A.; Smith, J.A.; Stelinski, L.L. Attraction of redbay ambrosia beetle, Xyleborus glabratus, to leaf volatiles of its host plants in North America. J. Chem. Ecol. 2015, 41, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A.E., III; Brownie, C. The redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) uses stem silhouette diameter as a visual host-finding cue. Environ. Entomol. 2013, 42, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Niogret, J.; Epsky, N.D.; Schnell, R.J.; Boza, E.J.; Kendra, P.E.; Heath, R.R. Terpenoid variations within and among half-sibling avocado trees, Persea americana Mill. (Lauraceae). PLoS ONE 2013, 8, e73601. [Google Scholar] [CrossRef] [PubMed]
- Maner, M.L.; Hanula, J.L.; Horn, S. Population trends of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae): Does utilization of small diameter redbay trees allow populations to persist? Fla. Entomol. 2014, 97, 208–216. [Google Scholar] [CrossRef]
- Kuhns, E.H.; Tribuiani, Y.; Martini, X.; Meyer, W.L.; Peña, J.; Hulcr, J.; Stelinski, L.L. Volatiles from the symbiotic fungus Raffaelea lauricola are synergistic with manuka lures for increased capture of the redbay ambrosia beetle Xyleborus glabratus. Agric. For. Entomol. 2014, 16, 87–94. [Google Scholar] [CrossRef]
- Kim, K.-H.; Choi, Y.J.; Seo, S.-T.; Shin, H.-D. Raffaelea quercus-mongolicae sp. nov. associated with Platypus koryoensis on oak in Korea. Mycotaxon 2009, 110, 189–197. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kimura, K.; Ito, S.-I. Genetic characterization of Raffaelea quercivora isolates collected from areas of oak wilt in Japan. Mycoscience 2010, 51, 310–316. [Google Scholar] [CrossRef]
- Six, D.L.; Wingfield, M.J. The role of phytopathogenicity in bark beetle-fungus symbioses: A challenge to the classic paradigm. Annu. Rev. Entomol. 2011, 56, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Rollins, J.A.; Ploetz, R.C.; Zhang, Y. Genomic insights into the mechanisms of pathogenesis in Raffaelea lauricola, causal agent of laurel wilt disease. University of Florida: Gainesville, FL, USA, Unpublished data. 2017. [Google Scholar]
- Dreaden, T.J.; Davis, J.M.; de Beer, W.Z.; Ploetz, R.C.; Soltis, P.S.; Wingfield, M.J.; Smith, J.A. Phylogeny of ambrosia beetle symbionts in the genus Raffaelea. Fungal Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.R.; de Beer, Z.W.; Huang, Y.-T.; Bateman, C.C.; Campbell, A.; Dreaden, T.J.; Li, Y.; Ploetz, R.C.; Li, H.-F.; Chen, C.-Y.; et al. New Raffaelea species (Ophiostomataceae) from the United States and Taiwan associated with ambrosia beetles and plant hosts. IMA Fungus 2016, 7, 265–273. [Google Scholar] [PubMed]
- Dreaden, T.J.; Campbell, A.S.; Gonzalez-Benecke, C.A.; Ploetz, R.C.; Smith, J.A. Response of swamp bay, Persea palustris, and redbay, P. borbonia, to Raffaelea spp. isolated from Xyleborus glabratus. For. Pathol. 2016. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; van der Werff, H.; Renner, S.S. Phylogeny and historical biogeography of Lauraceae: Evidence from the chloroplast and nuclear genomes. Ann. Missouri Bot. Garden 2001, 88, 104–134. [Google Scholar] [CrossRef] [Green Version]
- Drinnan, A.; Crane, P.; Friis, E.; Pedersen, K. Lauraceous flowers form the Potomac Group (Mid-Cretaceous) of Eastern North America. Bot. Gaz. 1990, 151, 370–384. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Rohwer, J.G.; van der Werff, H.; Wang, Z.-H.; Li, H.-W. Molecular phylogenetic analysis of the Persea group (Lauraceae) and its biogeographic implications on the evolution of tropical and subtropical amphi-Pacific disjunctions. Am. J. Bot. 2011, 98, 1520–1536. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Li, L.; van der Werff, H.; Li, H.-W.; Rohwer, J.G.; Crayn, D.M.; Meng, H.H.; van der Merwe, M.; Conran, J.G.; Li, J. Origins and evolution of cinnamon and camphor: A phylogenetic and historical biogeographical analysis of the Cinnamomum group (Lauraceae). Molec. Phylogenet. Evol. 2016, 96, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Langeland, K.A.; Cherry, H.M.; McCormick, C.M.; Burks, K.A.C. Identification and Biology of Nonnative Plants in Florida’s Natural Areas; IFAS Communication Services, University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Schaffer, B.; Gil, P.M.; Mickelbart, M.V.; Whiley, A.W. Ecophysiology. In The Avocado: Botany, Production and Uses, 2nd ed.; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CAB International Publishing: Wallingford, UK, 2013; pp. 168–199. [Google Scholar]
- Gentry, A. Neotropical floristic diversity: Phytogeographical connections between Central and South America, Pleistocene climate fluctuations, or an accident of the Andean orogeny? Ann. Mo. Bot. Gard. 1982, 69, 557–593. [Google Scholar] [CrossRef]
- Bosque, C.; Ramírez, R.; Rodríguez, D. The diet of the oilbird in Venezuela. Ornitol. Neotropical 1995, 6, 67–80. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 21 December 2016).
- Evans, E.A.; Current losses of avocado to laurel wilt. University of Florida, Homestead, FL, USA. Personal communication, 2017.
- Podger, F.D. Phytophthora cinnamomi, a cause of lethal disease in indigenous plant communities in Western Australia. Phytopathology 1972, 62, 972–981. [Google Scholar] [CrossRef]
- Lahav, E.; Lavi, U. Genetics and breeding. In The Avocado: Botany, Production and Uses, 2nd ed.; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CAB International Publishing: Wallingford, UK, 2013; pp. 51–85. [Google Scholar]
- Whiley, A.W.; Schaffer, B. Avocado. In Environmental Physiology of Fruit Crops, Vol. 2, Subtropical and Tropical Crops; Schaffer, B., Andersen, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 165–197. [Google Scholar]
- Ploetz, R.C.; Pérez-Martínez, J.M.; Smith, J.A.; Hughes, M.; Dreaden, T.J.; Inch, S.A.; Fu, Y. Responses of avocado to laurel wilt, caused by Raffaelea lauricola. Plant Pathol. 2012, 61, 801–808. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Schaffer, B.; Vargas, A.I.; Konkol, J.L.; Salvatierra, J.; Wideman, R. Impact of laurel wilt, caused by Raffaelea lauricola, on leaf gas exchange and xylem sap flow in avocado, Persea americana. Phytopathology 2015, 105, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C.; Hughes, M.A. Susceptibility of gulf licaria and lancewood to laurel wilt. University of Florida: Homestead, FL, USA; University of Hawaii: Hilo, HI, USA, Unpublished data. 2017. [Google Scholar]
- Hughes, M.A.; Smith, J.A. Vegetative propagation of putatively laurel wilt-resistant redbay (Persea borbonia). Native Plants 2014, 115, 42–50. [Google Scholar] [CrossRef]
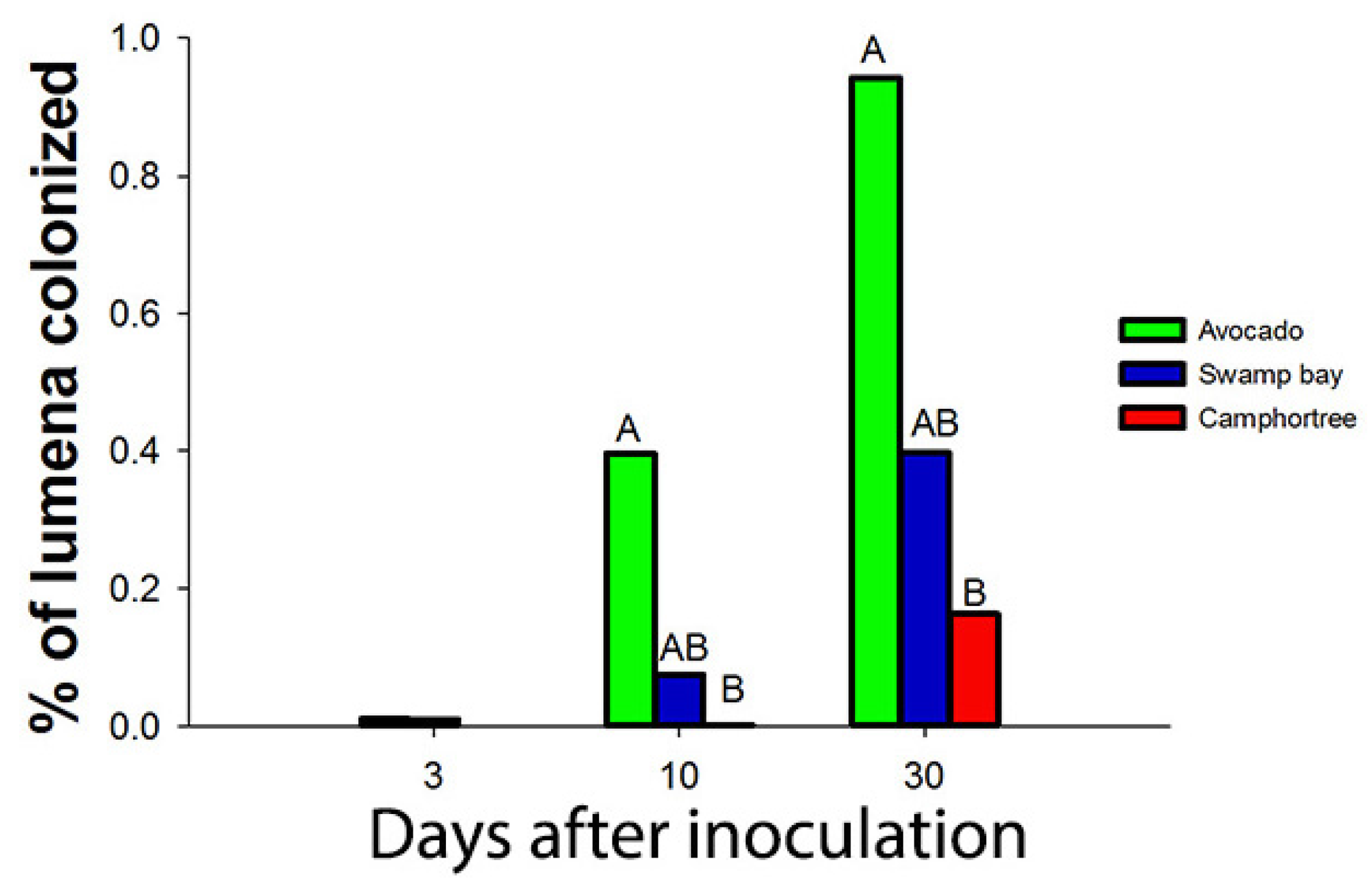
- Campbell, A.S.; Ploetz, R.C.; Rollins, J.A. Comparing avocado, swamp bay, and camphortree as hosts of Raffaelea lauricola using a green fluorescent protein (GFP)-labeled strain of the pathogen. Phytopathology 2016, 107, 70–74. [Google Scholar] [CrossRef] [PubMed]
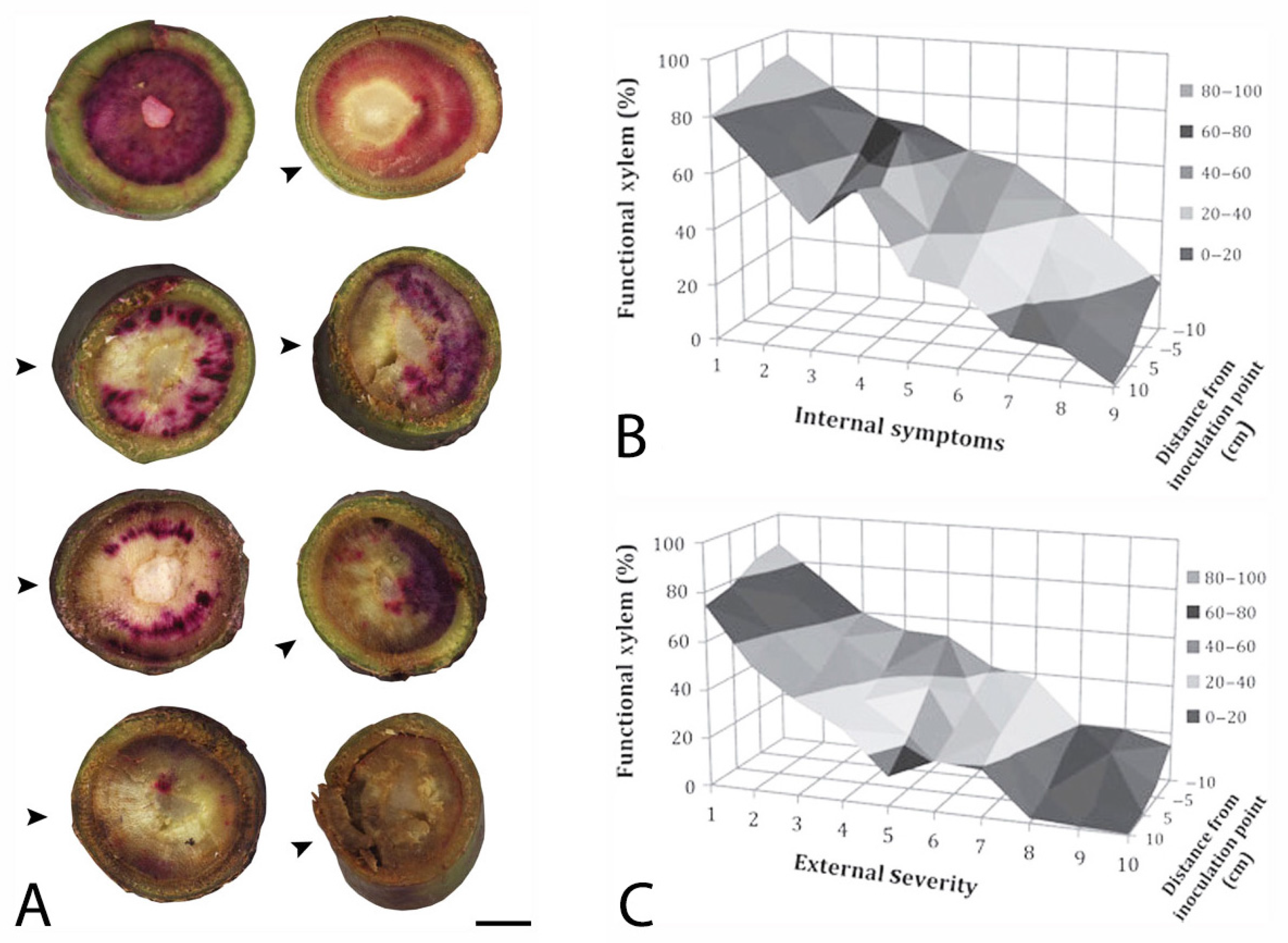
- Inch, S.A.; Ploetz, R.C. Impact of laurel wilt, caused by Raffaelea lauricola, on xylem function in avocado. Forest Pathol. 2012, 42, 239–245. [Google Scholar] [CrossRef]
- Inch, S.A.; Ploetz, R.C.; Held, B.; Blanchette, R. Histological and anatomical responses in avocado, Persea americana, induced by the vascular wilt pathogen, Raffaelea lauricola. Botany 2012, 90, 627–635. [Google Scholar] [CrossRef]
- Beckman, C.H. Host response to vascular infection. Annu. Rev. Phytopathol. 1964, 2, 231–252. [Google Scholar] [CrossRef]
- Bonsen, K.J.M.; Kucera, L.J. Vessel occlusions in plants: morphological functional and evolutionary aspects. IAWA J. 1990, 11, 393–399. [Google Scholar] [CrossRef]
- Bishop, C.D.; Cooper, R.M. Ultrastructure of vascular colonization by fungal wilt pathogens. II. Invasion of resistant cultivars. Physiol. Plant Pathol. 1984, 24, 277–289. [Google Scholar] [CrossRef]
- Struckmeyer, B.E.; Beckman, C.H.; Kuntz, J.E.; Ricker, A.J. Plugging of vessels by tyloses and gums in wilting oaks. Phytopathology 1954, 44, 148–153. [Google Scholar]
- Jacobi, W.R.; MacDonald, W.L. Colonization of resistant and susceptible oaks by Ceratocystis fagacearum. Phytopathology 1980, 70, 618–623. [Google Scholar] [CrossRef]
- Rioux, D.; Ouellette, G.B. Light microscope observations of histological changes induced by Ophiostoma ulmi in various nonhost trees and shrubs. Can. J. Bot. 1989, 67, 2335–2351. [Google Scholar] [CrossRef]
- Fry, S.M.; Milholland, R.D. Response of resistant, tolerant, and susceptible grapevine tissues to invasion by pierce’s disease bacterium, Xylella fastidiosa. Phytopathology 1990, 80, 66–69. [Google Scholar] [CrossRef]
- Rioux, D.; Nicole, M.; Simard, M.; Ouellette, G.B. Immunocytochemical evidence that secretion of pectin occurs during gel (gum) and tylosis formation in trees. Phytopathology 1998, 88, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.R.; Parke, J.L.; Lachenbruch, B.; Hansen, E.M. The effects of Phytophthora ramorum infection on hydralic conductivity and tylosis formation in tanoak sapwood. Can. J. For. Res. 2009, 39, 1766–1776. [Google Scholar] [CrossRef]
- VanderMolen, G.E.; Beckman, C.H.; Rodehorst, E. Vascular gelation: A general response phenomenon following infection. Physiol. Plant Pathol. 1977, 11, 85–100. [Google Scholar] [CrossRef]
- Aist, J.R. Structural responses as resistance mechanisms. In The dynamics of host defense; Bailey, J.A., Deverall, B.J., Eds.; Academic Press: Sydney, Australia, 1983; pp. 33–70. [Google Scholar]
- Gagnon, C. Histochemical studies on the alteration of lignin and pectic substances in white elm infected by Ceratocystis ulmi. Can. J. Bot. 1967, 45, 1619–1623. [Google Scholar] [CrossRef]
- Hughes, M.A.; Inch, S.A.; Ploetz, R.C.; Er, H.L.; van Bruggen, A.H.C.; Smith, J.A. Responses of swamp bay, Persea palustris, and avocado, Persea americana, to the laurel wilt pathogen, Raffaelea lauricola. For. Pathol. 2015, 45, 111–119. [Google Scholar] [CrossRef]
- Urban, J.; Dvořák, M. Occlusion of sap flow in elm after artificial inoculation with Ophiostoma novo-ulmi. Acta Hort. 2013, 991, 301–306. [Google Scholar] [CrossRef]
- Park, J.-H.; Juzwik, J.; Cavender-Bares, J. Multiple Ceratocystis smalleyi infections associated with reduced stem water transport in bitternut hickory. Phytopathology 2013, 103, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Yamada, T.; Ito, S. Changes in water status in seedlings of six species in the Fagaceae after inoculation with Raffaelea quercivora Kubono et Shin-Ito. J. For. Res. 2005, 10, 251–255. [Google Scholar] [CrossRef]
- Parke, J.L.; Oh, E.; Voelker, S.; Hansen, E.M.; Buckles, G.; Lachenbruch, B. Phytophthora ramorum colonizes tanoak xylem and is associated with reduced stem water transport. Phytopathology 2007, 97, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.X.; Lin, J.G.; Liu, J.; Jiang, M.S.; Chu, L.X. Chemical Composition and Antifungal Activity of Extracts from the Xylem of Cinnamomum camphora. BioResources 2014, 9, 2560–2571. [Google Scholar] [CrossRef]
- Solla, A.; Gil, L. Xylem vessel diameter as a factor in resistance of Ulmus minor to Ophiostoma novo-ulmi. For. Pathol. 2002, 32, 123–134. [Google Scholar] [CrossRef]
- Solla, A.; Martín, J.A.; Corral, P.; Gil, L. Seasonal changes in wood formation of Ulmus pumila and U. minor and its relation with Dutch elm disease. New Phytol. 2005, 166, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Venturas, M.; Lopez, R.; Martın, R.A.; Gasco, A.; Gil, L. Heritability of Ulmus minor resistance to Dutch elm disease and its relationship to vessel size, but not to xylem vulnerability to drought. Plant Pathol. 2014, 63, 500–509. [Google Scholar] [CrossRef]
- Cameron, R.S.; Hanula, J.; Fraedrich, S.W.; Bates, C. Progression of laurel wilt disease within redbay and sassafras populations in southeast Georgia. Southeast Nat. 2015, 14, 650–674. [Google Scholar] [CrossRef]
- Smith, C.K.; Landreaux, E.; Steinmann, H.; McGrath, D.; Hayes, C.; Hayes, R. Redbay survival eleven years after infection with an exotic disease on St. Catherines Island, Georgia, USA. Environ. Nat. Resour. Res. 2015, 6, 27–34. [Google Scholar] [CrossRef]
- Cameron, R.S.; Bates, C.; Johnson, J. Progression of laurel wilt disease in Georgia: 2009–2011 (Project SC-EM-08-02). In Forest Health Monitoring: National Status, Trends and Analysis; Potter, K.M., Conlking, B.L., Eds.; Southern Research Station, USDA-ARS: Asheville, NC, USA, 2012; pp. 145–151. [Google Scholar]
- Best, S.; Fraedrich, S. The impact of laurel wilt caused by Raffaelea lauricola on clonal populations of pondberry (Lindera melissifolia). Phytopathology 2016, 106, S4.124. [Google Scholar]
- Beckman, F.C. Laurel Wilt: Assessing the Risk of Pruning Tool Transmission of Raffaelea lauricola. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2012. [Google Scholar]
- Ploetz, R.C.; Inch, S.A.; Pérez-Martínez, J.M.; White, T.L., Jr. Systemic infection of avocado, Persea americana, by Raffaelea lauricola, does not progress into fruit pulp or seed. J. Phytopathol. 2012, 160, 491–495. [Google Scholar] [CrossRef]
- Ploetz, R.C.; White, T.; Konkol, J. Raffaelea lauricola is not transmitted through scions of avocado that are used for grafting. University of Florida: Homestead, FL, USA, Unpublished data. 2017. [Google Scholar]
- Evans, E.A.; Ballen, F.H. An econonmetric demand model for Florida green-skin avocados. HortTechnology 2015, 25, 405–411. [Google Scholar]
- Ploetz, R.C.; Peña, J.E.; Smith, J.A.; Dreaden, T.L.; Crane, J.H.; Schubert, T.; Dixon, W. Laurel wilt is confirmed in Miami-Dade County, center of Florida’s commercial avocado production. Plant Dis. 2011, 95, 1599. [Google Scholar] [CrossRef]
- Gottwald, T.R.; Hughes, G.; Graham, J.H.; Sun, X.; Riley, T. The citrus canker epidemic in Florida: The scientific basis of regulatory eradication policy for an invasive species. Phytopathology 2001, 91, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, T.R.; Wierenga, E.; Luo, W.; Parnell, S. Epidemiology of Plum pox ‘D’strain in Canada and the USA. Can. J. Plant Pathol. 2013, 35, 442–457. [Google Scholar] [CrossRef]
- Drew, J.; Anderson, N.; Andow, D. Conundrums of a complex vector for invasive species control: A detailed examination of the horticultural industry. Biol. Invas. 2010, 12, 2837–2851. [Google Scholar] [CrossRef]
- Fry, W.E.; McGrath, M.T.; Seaman, A.; Zitter, T.A.; McLeod, A.; Danies, G.; Gugino, B.K. The 2009 late blight pandemic in the eastern United States-Causes and results. Plant Dis. 2013, 97, 296–306. [Google Scholar] [CrossRef]
- Brar, G.S.; Capinera, J.L.; Kendra, P.E.; McLean, S.; Peña, J.E. Life cycle, development, and culture of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2013, 96, 1158–1167. [Google Scholar] [CrossRef]
- Plantegenest, M.; Le May, C.; Fabre, F. Landscape epidemiology of plant diseases. J. R. Soc. Interface 2007, 4, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, D.; Choudrey, R.; Garrett, K. Spatial distribution of laurel wilt outbreaks in commercial avocado production areas in south Florida suggest no connection with outbreaks of the disease in neighboring natural areas. University of Florida: Homestead, FL, USA, Unpublished data. 2017. [Google Scholar]
- La Rue, C.D. Root grafting in trees. Am. J. Bot. 1934, 21, 121–126. [Google Scholar] [CrossRef]
- La Rue, C.D. Root-grafting in tropical trees. Science 1952, 115, 296. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.H. Root graft transmission of tree pathogens. Annu. Rev. Phytopathol. 1978, 16, 181–192. [Google Scholar] [CrossRef]
- Himelick, E.B.; Neely, D. Root grafting of city-planted American elms. Plant Dis. Rep. 1962, 46, 86–87. [Google Scholar]
- Sinclair, W.A.; Lyon, J. Diseases of Trees and Shrubs, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 2005; pp. 1–680. [Google Scholar]
- Horne, W.T.; Parker, E.R. The avocado disease called sunblotch. Phytopathology 1931, 21, 235–238. [Google Scholar]
- Wallace, J.M.; Drake, R.J. A high rate of seed transmission of avocado sun blotch virus from symptomless trees and the origin of such trees. Phytopathology 1962, 52, 237–241. [Google Scholar]
- Dann, E.; Ploetz, R.C.; Pegg, K.G.; Coates, L. Diseases of avocado. In The Avocado, 2nd ed.; Schaffer, B., Ed.; CABI: Wallingford, UK, 2012; pp. 380–422. [Google Scholar]
- Ploetz, R.C.; Konkol, J. Herbicide treatment to establish barriers around laurel wilt affected avocado trees. University of Florida: Homestead, FL, USA, Unpublished data. 2017. [Google Scholar]
- Koch, F.H.; Smith, W.D. Spatio-temporal analysis of Xyleborus glabratus (Coleoptera: Circulionidae: Scolytinae) invasion in eastern US forests. Environ. Entomol. 2008, 37, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.S.; Bates, C.; Johnson, J. Distribution and Spread of Laurel Wilt Disease in Georgia: 2006–2008 Survey and Field Observations; Georgia Forestry Commission Report, 2006–2008; Georgia Forestry Commission: Tifton, GA, USA, 2008. [Google Scholar]
- Riggins, J.J.; Hughes, M.; Smith, J.A.; Mayfield, A.E., III; Layton, B.; Balbalian, C.; Campbell, R. First occurrence of laurel wilt disease caused by Raffaelea lauricola on redbay trees in Mississippi. Plant Dis. 2010, 94, 634. [Google Scholar] [CrossRef]
- Bates, C.A.; Fraedrich, S.W.; Harrington, T.C.; Cameron, R.S.; Menard, R.D.; Best, G.S. First report of laurel wilt, caused by Raffaelea lauricola, on sassafras (Sassafras albidum) in Alabama. Southeast Nat. 2015, 14, 650–674. [Google Scholar]
- Fraedrich, S.W.; Johnson, C.W.; Menard, R.D.; Harrington, T.C.; Olatinwo, R.; Best, G.S. First report of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae) and laurel wilt in Louisiana, USA: The disease continues westward on sassafras. Fla. Entomol. 2015, 98, 1266–1268. [Google Scholar] [CrossRef]
- Olatinwo, R.; Barton, C.; Fraedrich, S.W.; Johnson, W.; Hwang, J. First report of laurel wilt, caused by Raffaelea lauricola, on sassafras (Sassafras albidum) in Arkansas. Plant Dis. 2016, 100, 2331. [Google Scholar] [CrossRef]
- Shearman, T.M.; Wang, G.G.; Bridges, W.C. Population dynamics of redbay (Persea borbonia) after laurel wilt disease: An assessment based on forest inventory and analysis data. Biol. Invasions 2015, 17, 1371–1382. [Google Scholar] [CrossRef]
- Moslonka-Lefebvre, M.; Finley, A.; Dorigatti, I.; Dehnen-Schmutz, K.; Harwood, T.; Jeger, M.J.; Xu, X.M.; Holdenrieder, O.; Pautasso, M. Networks in plant epidemiology: From genes to landscapes, countries, and continents. Phytopathology 2011, 101, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.W.; Pautasso, M. Networks and plant disease management: Concepts and applications. Annu. Rev. Phytopathol. 2014, 52, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Harwood, T.D.; Xu, X.; Pautasso, M.; Jeger, M.J.; Shaw, M.W. Epidemiological risk assessment using linked network and grid based modelling: Phytophthora ramorum and Phytophthora kernoviae in the UK. Ecol. Model. 2009, 220, 3353–3361. [Google Scholar] [CrossRef]
- Nopsa, J.F.H.; Daglish, G.J.; Hagstrum, D.W.; Leslie, J.F.; Phillips, T.W.; Scoglio, C.; Thomas-Sharma, S.; Walter, G.H.; Garrett, K.A. Ecological networks in stored grain: Key postharvest nodes for emerging pests, pathogens, and mycotoxins. Bioscience 2015, 65, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Sutrave, S.; Scoglio, C.; Isard, S.A.; Hutchinson, J.M.S.; Garrett, K.A. Identifying highly connected counties compensates for resource limitations when evaluating national spread of an invasive pathogen. PLoS ONE 2012, 7, e37793. [Google Scholar] [CrossRef] [PubMed]
- Garrett, K.A. Impact Network Analysis: A framework for evaluating the effects of information and other technologies through linked socioeconomic and biophysical networks. bioRxiv 2017, in press. [Google Scholar]
- Grimm, V.; Revilla, E.; Berger, U.; Jeltsch, F.; Mooij, W.M.; Railsback, S.F.; Thulke, H.H.; Weiner, J.; Wiegand, T.; DeAngelis, D.L. Pattern-oriented modeling of agent-based complex systems: Lessons from ecology. Science 2005, 310, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Garrett, K.A. Information networks for plant disease: Commonalities in human management networks and within-plant signaling networks. Eur. J. Plant Pathol. 2012, 133, 75–88. [Google Scholar] [CrossRef]
- Mills, P.; Dehnen-Schmutz, K.; Ilbery, B.; Jeger, M.; Jones, G.; Little, R.; MacLeod, A.; Parker, S.; Pautasso, M.; Pietravalle, S.; et al. Integrating natural and social science perspectives on plant disease risk, management and policy formulation. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2035–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebaudo, F.; Dangles, O. Coupled information diffusion-pest dynamics models predict delayed benefits of farmer cooperation in pest management programs. PLoS Comput. Biol. 2011, 7, e1002222. [Google Scholar] [CrossRef] [PubMed]
- McRoberts, N.; Hall, C.; Madden, L.V.; Hughes, G. Perceptions of disease risk: From social construction of subjective judgments to rational decision making. Phytopathology 2011, 101, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Borer, E.; Laine, A.-L.; Seabloom, E. A multiscale approach to plant disease using the metacommunity concept. Annu. Rev. Phytopathol. 2016, 54, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, T.J.; Davis, J.M.; Harmon, C.L.; Ploetz, R.C.; Palmateer, A.J.; Soltis, P.S.; Smith, J.A. Development of multilocus PCR assays for Raffaelea lauricola, causal agent of laurel wilt disease. Plant Dis. 2014, 98, 379–383. [Google Scholar] [CrossRef]
- Sankaran, S.; Ehsani, R.; Inch, S.A.; Ploetz, R.C. Evaluation of visible-near infrared reflectance spectra of avocado leaves as a non-destructive sensing tool for detection of vascular infection by the laurel wilt pathogen, Raffaelea lauricola. Plant Dis. 2012, 96, 1683–1689. [Google Scholar] [CrossRef]
- De Castro, A.I.; Ehsani, R.; Ploetz, R.; Crane, J.H.; Abdulridh, J. Optimum spectral and geometric parameters for early detection of laurel wilt disease in avocado. Rem. Sens. Environ. 2015, 171, 33–44. [Google Scholar] [CrossRef]
- De Castro, A.I.; Ehsani, R.; Ploetz, R.C.; Crane, J.H.; Buchanon, S. Detection of laurel wilt disease in avocado using low altitude aerial imaging. PLoS ONE 2015, 10, e0124642. [Google Scholar] [CrossRef] [PubMed]
- Pero, J. Bug off my guacamole. Mech. Engin.-CIME 2015, 137, 96–97. [Google Scholar]
- Angle, C.; Waggoner, L.P.; Ferrando, A.; Haney, P.; Passler, T. Canine detection of the volatilome: A review of implications for pathogen and disease detection. Front. Vet. Sci. 2016, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Mills, D. The Science of Laurel wilt Canine Detection; Seminar Series; Tropical Research & Education Center, University of Florida: Homestead, FL, USA, 2017. [Google Scholar]
- Adkins, J. Canines Detect Deadly Disease in Historic Avocado Trees. Available online: https://news.fiu.edu/2015/10/canines-detect-deadly-disease-in-historic-avocado-trees/92863 (accessed on 31 January 2017).
- Campbell, A.S.; Ploetz, R.C.; Kendra, P.E.; Montgomery, W.S.; Dreaden, T.J. Geographic variation in mycangial communities of Xyleborus glabratus. Mycologia 2016, 108, 657–667. [Google Scholar] [CrossRef] [PubMed]
- An, L. Modeling human decisions in coupled human and natural systems: review of agent-based models. Ecol. Model. 2012, 229, 25–36. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Pérez-Martínez, J.M.; Fernandez, R. Management of laurel wilt of avocado, caused by Raffaelea lauricola. Eur. J. Plant Pathol. 2017. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Barnard, E.L.; Smith, J.A.; Bernick, S.C.; Eickwort, J.M.; Dreaden, T.J. Effect of propiconazole on laurel wilt disease development in redbay trees and on the pathogen in vitro. Arboric. Urban For. 2008, 34, 317–324. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ploetz, R.C.; Kendra, P.E.; Choudhury, R.A.; Rollins, J.A.; Campbell, A.; Garrett, K.; Hughes, M.; Dreaden, T. Laurel Wilt in Natural and Agricultural Ecosystems: Understanding the Drivers and Scales of Complex Pathosystems. Forests 2017, 8, 48. https://doi.org/10.3390/f8020048
Ploetz RC, Kendra PE, Choudhury RA, Rollins JA, Campbell A, Garrett K, Hughes M, Dreaden T. Laurel Wilt in Natural and Agricultural Ecosystems: Understanding the Drivers and Scales of Complex Pathosystems. Forests. 2017; 8(2):48. https://doi.org/10.3390/f8020048
Chicago/Turabian StylePloetz, Randy C., Paul E. Kendra, Robin Alan Choudhury, Jeffrey A. Rollins, Alina Campbell, Karen Garrett, Marc Hughes, and Tyler Dreaden. 2017. "Laurel Wilt in Natural and Agricultural Ecosystems: Understanding the Drivers and Scales of Complex Pathosystems" Forests 8, no. 2: 48. https://doi.org/10.3390/f8020048






