Climate Impacts on Soil Carbon Processes along an Elevation Gradient in the Tropical Luquillo Experimental Forest
Abstract
:1. Introduction
2. Materials and Methods
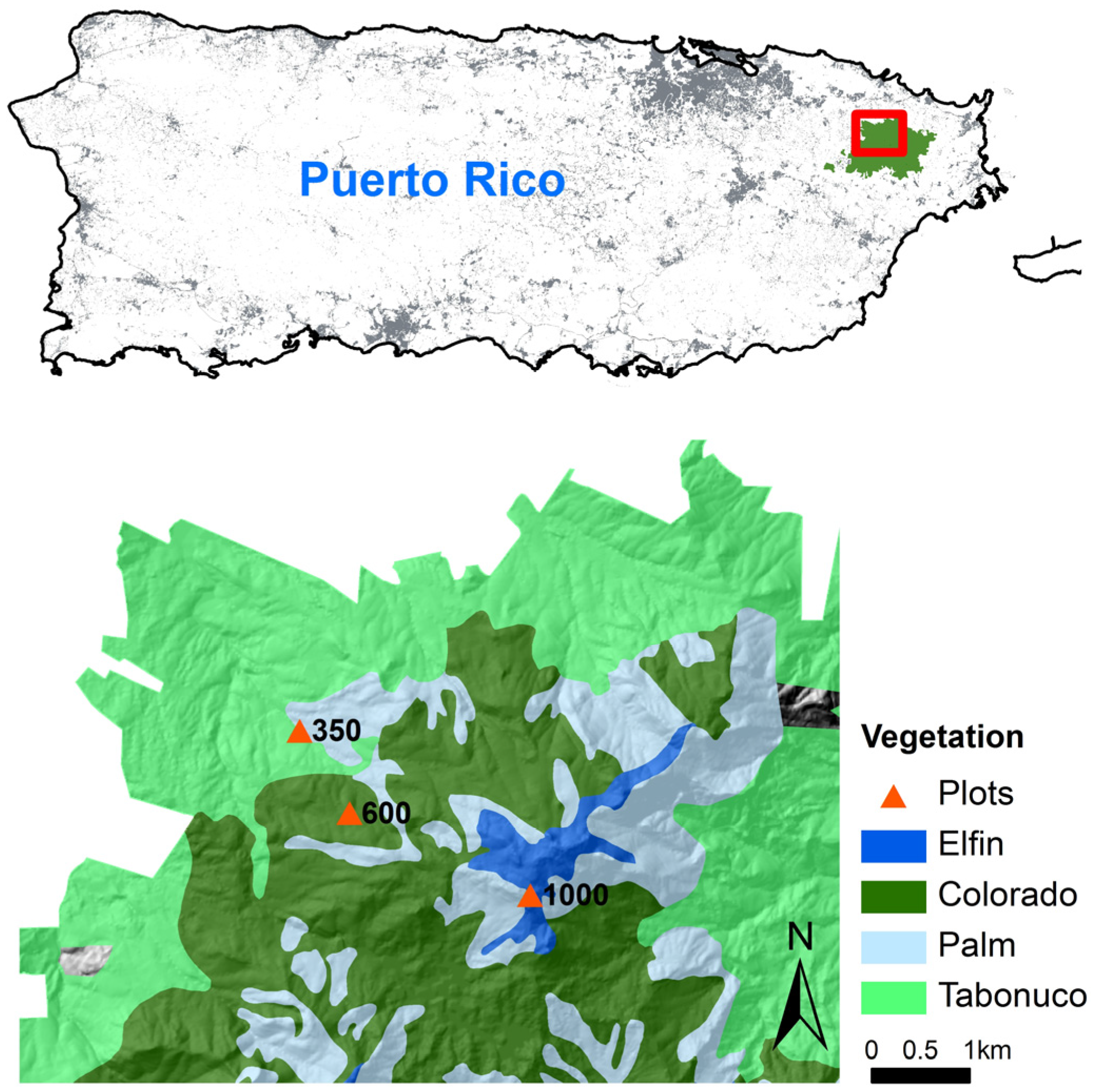
2.1. Study Area
2.2. Soil Translocation Experiment
2.3. Statistical Analyses
3. Results
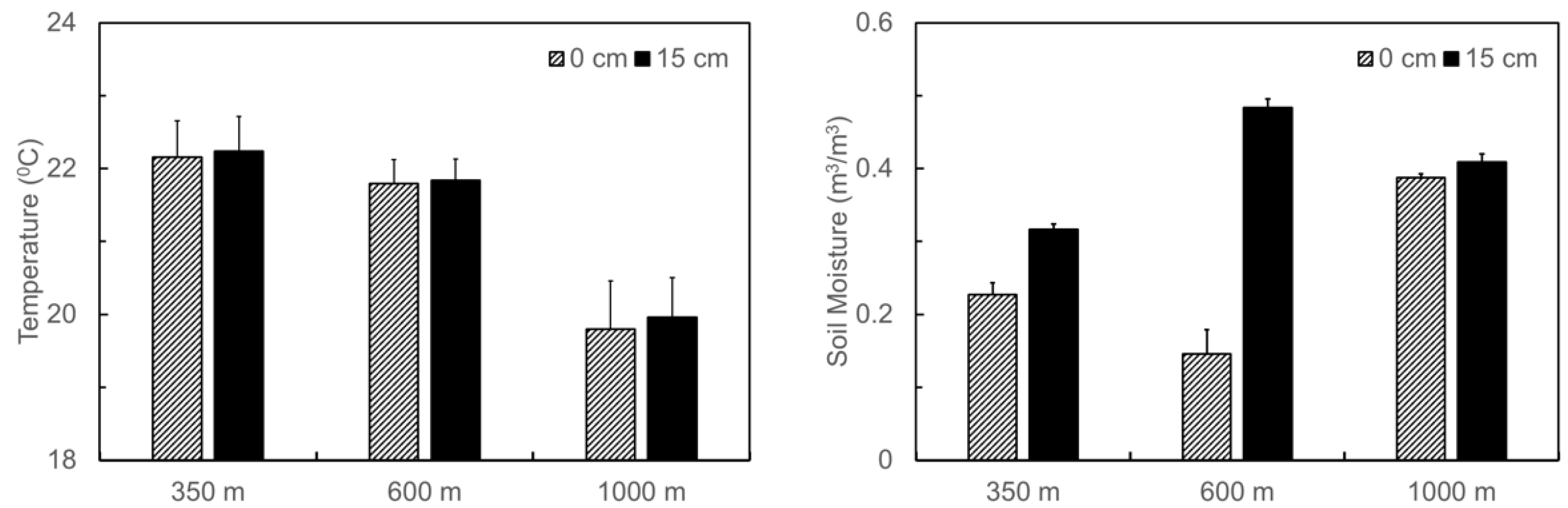
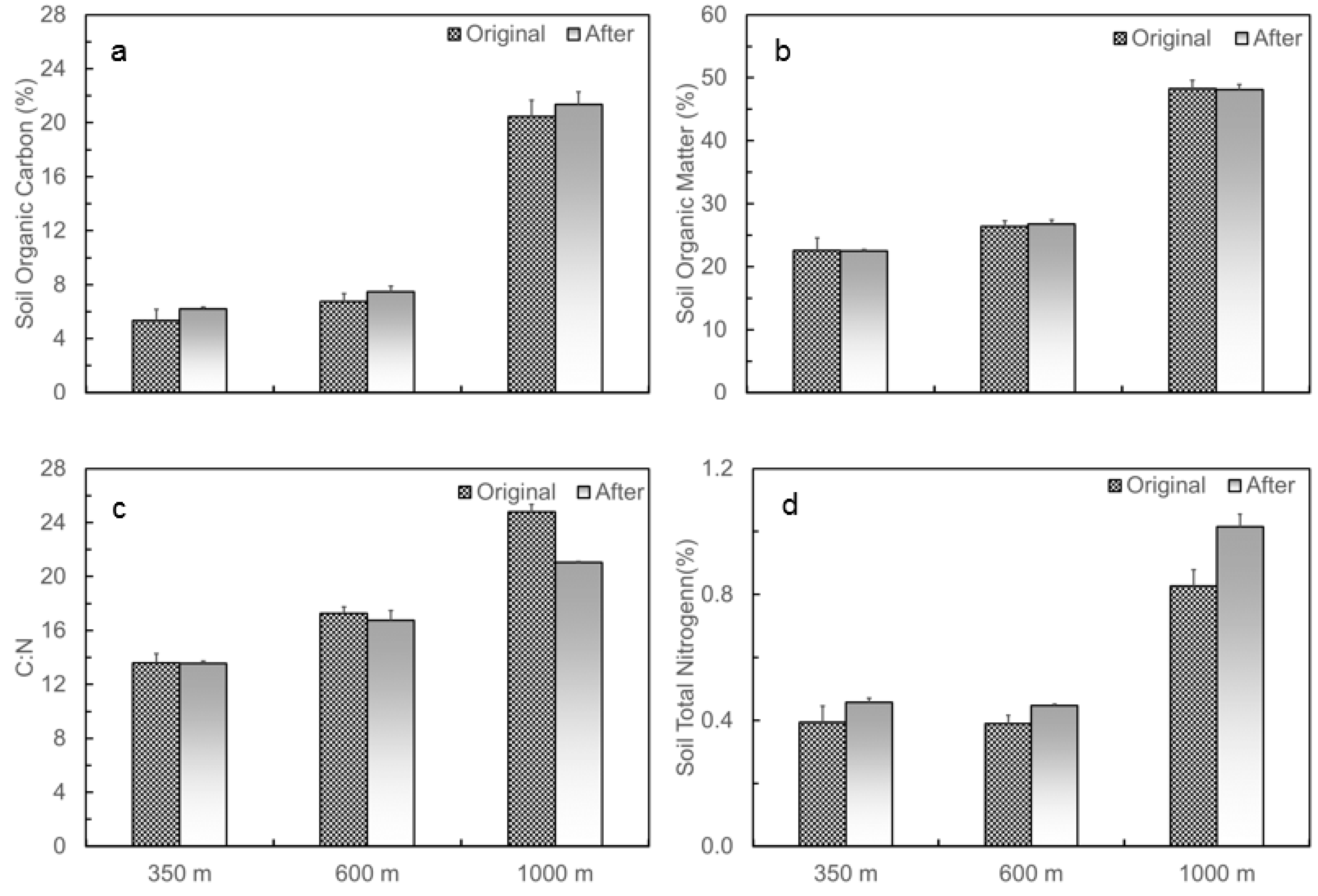
3.1. Naturally Occurred Gradient in Soil Temperature and Moisture and Changes in Soil C and N Contents before and after the Translocation Experiment along the Elevation Gradient
3.2. Impacts of Soil Source Quality on Soil Respiration
3.3. Impacts of Soil Translocated Site and Associated Microclimate on Soil Respiration
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Townsend, A.R.; Vitousek, P.M.; Trumbore, S.E. Soil organic-matter dynamics along gradients in temperature and land-use on the island of Hawaii. Ecology 1995, 76, 721–733. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Silver, W.L. The potential effects of elevated CO2 and climate change on tropical forest soils and biogeochemical cycling. Clim. Chang. 1998, 39, 337–361. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world's forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Meir, P.; Wood, T.E.; Galbraith, D.R.; Brando, P.M.; da Costa, A.C.L.; Rowland, L.; Ferreira, L.V. Threshold responses to soil moisture deficit by trees and soil in tropical rain forests: Insights from field experiments. Bioscience 2015, 65, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.; da Costa, A.C.L.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.R.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.V.; Gloor, M.; Miller, J.B.; Doughty, C.E.; Malhi, Y.; Domingues, L.G.; Basso, L.S.; Martinewski, A.; Correia, C.S.C.; Borges, V.F.; et al. Drought sensitivity of amazonian carbon balance revealed by atmospheric measurements. Nature 2014, 506, 76–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderegg, W.R.L.; Ballantyne, A.P.; Smith, W.K.; Majkut, J.; Rabin, S.; Beaulieu, C.; Birdsey, R.; Dunne, J.P.; Houghton, R.A.; Myneni, R.B.; et al. Tropical nighttime warming as a dominant driver of variability in the terrestrial carbon sink. Proc. Natl. Acad. Sci. USA 2015, 112, 15591–15596. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Norby, R.J.; Ledford, J.; Weltzin, J.F. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Chang. Biol. 2007, 13, 2411–2424. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Yu, M.; Gao, Q.; Gao, C.; Wang, C. Extent of night warming and spatially heterogeneous cloudiness differentiate temporal trend of greenness in mountainous tropics in the new century. Sci. Rep. 2017, 7, 41256. [Google Scholar] [CrossRef] [PubMed]
- Baggs, E.M. Partitioning the components of soil respiration: A research challenge. Plant Soil 2006, 284, 1–5. [Google Scholar] [CrossRef]
- Cramer, W.; Bondeau, A.; Woodward, F.I.; Prentice, I.C.; Betts, R.A.; Brovkin, V.; Cox, P.M.; Fisher, V.; Foley, J.A.; Friend, A.D.; et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: Results from six dynamic global vegetation models. Glob. Chang. Biol. 2001, 7, 357–373. [Google Scholar] [CrossRef]
- Kane, E.S.; Pregitzer, K.S.; Burton, A.J. Soil respiration along environmental gradients in olympic national park. Ecosystems 2003, 6, 326–335. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zou, X.; Xia, Y. Soil CO2 efflux and fungal and bacterial biomass in a plantation and a secondary forest in wet tropics in Puerto Rico. Plant Soil 2005, 268, 151–160. [Google Scholar] [CrossRef]
- Conant, R.T.; Dalla-Betta, P.; Klopatek, C.C.; Klopatek, J.M. Controls on soil respiration in semiarid soils. Soil Biol. Biochem. 2004, 36, 945–951. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meir, P.; Bird, M.I.; Malhi, Y.; Ccahuana, A.J.Q. Climate dependence of heterotrophic soil respiration from a soil-translocation experiment along a 3000 m tropical forest altitudinal gradient. Eur. J. Soil Sci. 2009, 60, 895–906. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Whitaker, J.; Turner, B.L.; Salinas, N.; Zimmermann, M.; Malhi, Y.; Meir, P. Climate warming and soil carbon in tropical forests: Insights from an elevation gradient in the Peruvian Andes. Bioscience 2015, 65, 906–921. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Taylor, J.A. On the temperature-dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Raich, J.W.; Tufekciogul, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Heneghan, L.; Coleman, D.C.; Zou, X.; Crossley, D.A.; Haines, B.L. Soil microarthropod contributions to decomposition dynamics: Tropical-temperate comparisons of a single substrate. Ecology 1999, 80, 1873–1882. [Google Scholar]
- González, G.; Seastedt, T.R. Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 2001, 82, 955–964. [Google Scholar] [CrossRef]
- Dechaine, J.; Ruan, H.; Sánchez-de León, Y.; Zou, X. Correlation between earthworms and plant litter decomposition in a tropical wet forest of Puerto Rico. Pedobiologia 2005, 49, 601–607. [Google Scholar] [CrossRef]
- Sjögersten, S.; Wookey, P.A. Climatic and resource quality controls on soil respiration across a forest-tundra ecotone in Swedish Lapland. Soil Biol. Biochem. 2002, 34, 1633–1646. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Neff, J.C.; Schimel, J.P. Does adding microbial mechanisms of decomposition improve soil organic matter models? A comparison of four models using data from a pulsed rewetting experiment. Soil Biol. Biochem. 2009, 41, 1923–1934. [Google Scholar] [CrossRef]
- Mikan, C.J.; Schimel, J.P.; Doyle, A.P. Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol. Biochem. 2002, 34, 1785–1795. [Google Scholar] [CrossRef]
- Xu, L.; Baldocchi, D.D.; Tang, J. How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, M.; Wu, J. Temperature sensitivity of soil respiration and its effects on ecosystem carbon budget: Nonlinearity begets surprises. Ecol. Model. 2002, 153, 131–142. [Google Scholar] [CrossRef]
- Rastetter, E.B.; Ryan, M.G.; Shaver, G.R.; Melillo, J.M.; Nadelhoffer, K.J.; Hobbie, J.E.; Aber, J.D. A general biogeochemical model describing the responses of the C-cycle and N-cycle in terrestrial ecosystems to changes in CO2, climate, and N-deposition. Tree Physiol. 1991, 9, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R. The global carbon cycle: A viewpoint on the missing sink. Funct. Plant Biol. 1994, 21, 1–15. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zou, X. Heterotrophic soil respiration in relation to environmental factors and microbial biomass in two wet tropical forests. Plant Soil 2006, 281, 193–201. [Google Scholar] [CrossRef]
- Wang, W.J.; Dalal, R.C.; Moody, P.W.; Smith, C.J. Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol. Biochem. 2003, 35, 273–284. [Google Scholar] [CrossRef]
- Vance, E.D.; Chapin Iii, F.S. Substrate limitations to microbial activity in taiga forest floors. Soil Biol. Biochem. 2001, 33, 173–188. [Google Scholar] [CrossRef]
- Blagodatsky, S.; Blagodatskaya, E.; Yuyukina, T.; Kuzyakov, Y. Model of apparent and real priming effects: Linking microbial activity with soil organic matter decomposition. Soil Biol. Biochem. 2010, 42, 1275–1283. [Google Scholar] [CrossRef]
- Smith, D.L.; Johnson, L. Vegetation-mediated changes in microclimate reduce soil respiration as woodlands expand into grasslands. Ecology 2004, 85, 3348–3361. [Google Scholar] [CrossRef]
- Kleb, H.R.; Wilson, S.D. Vegetation effects on soil resource heterogeneity in prairie and forest. Am. Nat. 1997, 150, 283–298. [Google Scholar] [PubMed]
- Bruijnzeel, L.A.; Veneklaas, E.J. Climatic conditions and tropical montane forest productivity: The fog has not lifted yet. Ecology 1998, 79, 3–9. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Givnish, T.J. Altitudinal gradients in tropical forest composition, structure, and diversity in the sierra de manantlán. J. Ecol. 1998, 86, 999–1020. [Google Scholar]
- Gould, W.A.; González, G.; Carrero, R.G. Structure and composition of vegetation along an elevational gradient in Puerto Rico. J. Veg. Sci. 2006, 17, 653–664. [Google Scholar] [CrossRef]
- Barone, J.A.; Thomlinson, J.; Cordero, P.A.; Zimmerman, J.K. Metacommunity structure of tropical forest along an elevation gradient in Puerto Rico. J. Trop. Ecol. 2008, 24, 525–534. [Google Scholar] [CrossRef]
- González, G.; García, E.; Cruz, V.; Borges, S.; Zalamea, M.; Rivera, M.M. Earthworm communities along an elevation gradient in northeastern Puerto Rico. Eur. J. Soil Biol. 2007, 43, S24–S32. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meir, P.; Bird, M.I.; Malhi, Y.; Ccahuana, A.J.Q. Temporal variation and climate dependence of soil respiration and its components along a 3000 m altitudinal tropical forest gradient. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Martinó, A.G.; Warner, G.S.; Scatena, F.N.; Civco, D.L. Rainfall, runoff and elevation relationships in the Luquillo mountains of Puerto Rico. Caribb. J. Sci. 1996, 32, 413–424. [Google Scholar]
- Weaver, P.L. Environmental gradients affect forest structure in Puerto Rico's Luquillo mountains. Interciencia 2000, 25, 254–259. [Google Scholar]
- Weaver, P.L.; Gould, W.A. Forest vegetation along environmental gradients in northeastern Puerto Rico. Ecol. Bull. 2013, 54, 43–65. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Bond-Lamberty, B.; Bolton, H.; Fansler, S.; Heredia-Langner, A.; Liu, C.; McCue, L.A.; Smith, J.; Bailey, V. Soil respiration and bacterial structure and function after 17 years of a reciprocal soil transplant experiment. PLoS ONE 2016, 11, e0150599. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, M.U.F. The temperature dependence of organic-matter decomposition—Still a topic of debate. Soil Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar] [CrossRef]
- Sayer, E.J.; Heard, M.S.; Grant, H.K.; Marthews, T.R.; Tanner, E.V.J. Soil carbon release enhanced by increased tropical forest litterfall. Nat. Clim. Chang. 2011, 1, 304–307. [Google Scholar] [CrossRef]
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Ruan, H.H.; Zou, X.M.; Scatena, E.; Zimmerman, J.K. Asynchronous fluctuation of soil microbial biomass and plant litterfall in a tropical wet forest. Plant Soil 2004, 260, 147–154. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, H.; Wang, B. Effects of soil microarthropods on plant litter decomposition across an elevation gradient in the Wuyi mountains. Soil Biol. Biochem. 2009, 41, 891–897. [Google Scholar] [CrossRef]
- Malhi, Y.; Grace, J. Tropical forests and atmospheric carbon dioxide. Trends Ecol. Evol. 2000, 15, 332–337. [Google Scholar] [CrossRef]
- Clark, D.A. Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Plante, A.F.; Conant, R.T.; Carlson, J.; Greenwood, R.; Shulman, J.M.; Haddix, M.L.; Paul, E.A. Decomposition temperature sensitivity of isolated soil organic matter fractions. Soil Biol. Biochem. 2010, 42, 1991–1996. [Google Scholar] [CrossRef]
- Conant, R.T.; Drijber, R.A.; Haddix, M.L.; Parton, W.J.; Paul, E.A.; Plante, A.F.; Six, J.; Steinweg, J.M. Sensitivity of organic matter decomposition to warming varies with its quality. Glob. Chang. Biol. 2008, 14, 868–877. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I. Temperature sensitivity of soil organic matter decomposition—What do we know? Biol. Fertil. Soils 2009, 46, 1–15. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.; et al. Temperature and soil organic matter decomposition rates—Synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Balser, T.C.; Wixon, D.L. Investigating biological control over soil carbon temperature sensitivity. Glob. Chang. Biol. 2009, 15, 2935–2949. [Google Scholar] [CrossRef]

| Fixed Effects Variable | Value | Standard Error | df | t-Value | p-Value |
|---|---|---|---|---|---|
| log(Rs)~Translocation|source | AIC = 105.6 BIC = 116.9 #observation = 74 #group = 3 | ||||
| T_350 | 0.34 | 0.12 | 69 | 2.87 | 0.005 |
| T_600 | −0.08 | 0.12 | 69 | −0.68 | 0.50 |
| T_1000 | 0.26 | 0.12 | 69 | 2.15 | 0.04 |
| log(Rs)~Translocation + Temp. + M. + Temp. × M.|source | AIC = 91.0 BIC = 107.4 #observation = 63 #group = 3 | ||||
| T_350 | −3.33 | 1.91 | 55 | −1.75 | 0.09 |
| T_600 | −3.57 | 1.84 | 55 | −1.95 | 0.06 |
| T_1000 | −3.09 | 1.92 | 55 | −1.61 | 0.11 |
| Temperature | 0.17 | 0.09 | 55 | 1.98 | 0.05 |
| Moisture | 6.95 | 6.40 | 55 | 1.09 | 0.28 |
| Temperature × Moisture | −0.35 | 0.30 | 55 | −1.18 | 0.24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Yu, M.; González, G.; Zou, X.; Gao, Q. Climate Impacts on Soil Carbon Processes along an Elevation Gradient in the Tropical Luquillo Experimental Forest. Forests 2017, 8, 90. https://doi.org/10.3390/f8030090
Chen D, Yu M, González G, Zou X, Gao Q. Climate Impacts on Soil Carbon Processes along an Elevation Gradient in the Tropical Luquillo Experimental Forest. Forests. 2017; 8(3):90. https://doi.org/10.3390/f8030090
Chicago/Turabian StyleChen, Dingfang, Mei Yu, Grizelle González, Xiaoming Zou, and Qiong Gao. 2017. "Climate Impacts on Soil Carbon Processes along an Elevation Gradient in the Tropical Luquillo Experimental Forest" Forests 8, no. 3: 90. https://doi.org/10.3390/f8030090






