Phenotypic Plasticity Explains Response Patterns of European Beech (Fagus sylvatica L.) Saplings to Nitrogen Fertilization and Drought Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Sapling Cultivation And Treatments
2.3. Measurement of Response Variables
2.4. Statistical Analyses
3. Results
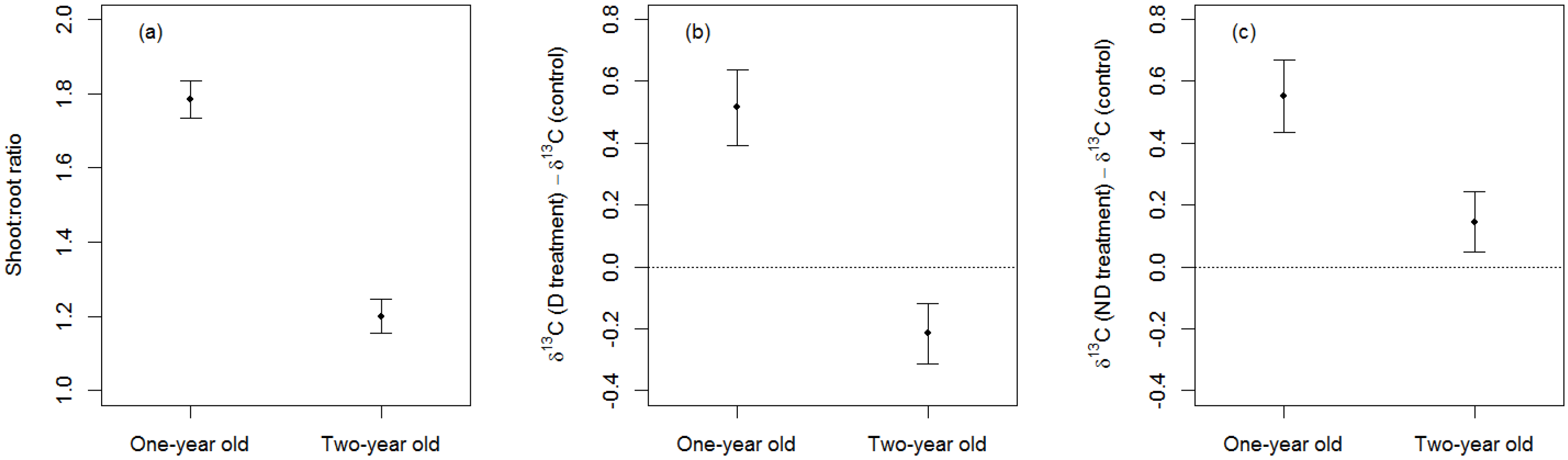
3.1. Effects of N, D, and ND Treatments on Two-Year-Old Saplings
3.2. Effects of Sapling-Age
4. Discussion and Conclusions
4.1. Treatment (N, D, ND) Effects on Sapling Growth
4.2. Effects of Sapling Age
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McLauchlan, K.K.; Craine, J.M. Species-specific trajectories of nitrogen isotopes in Indiana hardwood forests, USA. Biogeoscience 2012, 9, 867–874. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M. The functional role of biodiversity in the context of global change. In Forests and Global Change; Coomes, D.A., Burslem, D.F.R.P., Simonson, W.D., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 195–237. [Google Scholar]
- Jucker, T.; Avăcăriței, D.; Bărnoaiea, I.; Duduman, G.; Bouriaud, O.; Coomes, D.A. Climate modulates the effects of tree diversity on forest productivity. J. Ecol. 2016, 104, 388–398. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochem 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Phil. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobbink, R.; Hornung, M.; Roelofs, J.G.M. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J. Ecol. 1998, 86, 717–738. [Google Scholar] [CrossRef]
- Stevens, C.J.; Dise, N.B.; Gowing, D.J.G.; Mountford, J.O. Loss of forb diversity in relation to nitrogen deposition in the UK: Regional trends and potential controls. Glob. Chang. Biol. 2006, 12, 1823–1833. [Google Scholar] [CrossRef]
- Greaver, T.L.; Clark, C.M.; Compton, J.E.; Vallano, D.; Talhelm, A.F.; Weaver, C.P.; Band, L.E.; Baron, J.S.; Davidson, E.A.; Tague, C.L.; et al. Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 2016, 6, 836–843. [Google Scholar] [CrossRef]
- Zavaleta, E.S.; Shaw, M.R.; Chiariello, N.R.; Mooney, H.A.; Field, C.B. Additive effects of simulated climate changes, elevated CO2, and nitrogen deposition on grassland diversity. Proc. Natl. Acad. Sci. USA 2003, 100, 7650–7654. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Turner, M.M.; Henry, H.A.L. Interactive effects of warming and increased nitrogen deposition on 15N tracer retention in a temperate old field: Seasonal trends. Glob. Chang. Biol. 2009, 15, 2885–2893. [Google Scholar] [CrossRef]
- Friedrich, U.; von Oheimb, G.; Kriebitzsch, W.U.; Schlesselmann, K.; Weber, M.S.; Härdtle, W. Nitrogen deposition increases susceptibility to drought-Experimental evidence with the perennial grass Molinia caerulea L. Moench. Plant Soil 2012, 353, 59–71. [Google Scholar] [CrossRef]
- Meyer-Grünefeldt, M.; Friedrich, U.; Klotz, M.; von Oheimb, G.; Härdtle, W. Nitrogen deposition and drought events have non-additive effects on plant growth-evidence from greenhouse experiments. Plant Biosyst. 2015, 149, 424–432. [Google Scholar] [CrossRef]
- Dziedek, C.; Härdtle, W.; von Oheimb, G.; Fichtner, A. Nitrogen addition enhances drought sensitivity of young deciduous tree species. Front. Plant Sci. 2016, 7, 1100. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Thomas, S.C. The nature of tree growth and the “age-related decline in forest productivity”. Oikos 2001, 94, 374–376. [Google Scholar] [CrossRef]
- Thomas, S.C. Age-related changes in tree growth and functional biology: The role of reproduction. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Berlin, Germany, 2011; pp. 33–64. [Google Scholar]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen; Ulmer: Stuttgart, Germany, 2010. [Google Scholar]
- Rose, L.; Leuschner, C.; Köckemann, B.; Buschmann, H. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes? Eur. J. For. Res. 2009, 128, 335–343. [Google Scholar] [CrossRef]
- Magri, D.; Vendramin, G.G.; Comps, B.; Dupanloup, I.; Geburek, T.; Gomory, D.; Latalowa, M.; Litt, T.; Paule, L.; Roure, J.M.; et al. A new scenario for the Quaternary history of European beech populations: Palaeobotanical evidence and genetic consequences. New Phytol. 2006, 171, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Widmer, A.; Lexer, C. Glacial refugia: Sanctuaries for allelic richness, but not for gene diversity. Trends Ecol. Evol. 2001, 16, 267–269. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jump, A.S.; Hunt, J.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Hampe, A.; Jump, A.S. Climate relicts: Past, present, future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Dziedek, C.; von Oheimb, G.; Calvo, L.; Fichtner, A.; Kriebitzsch, W.U.; Marcos, E.; Pitz, W.T.; Härdtle, W. Does excess nitrogen supply increase the drought sensitivity of European beech (Fagus sylvatica L.) seedlings? Plant Ecol. 2016, 217, 393–405. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book; Wiley: Chichester, UK, 2007. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model selection and Multimodel Inference: A Practical Information-Theoretical Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. 2014. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.1-6. Available online: http://CRAN.R-project.org/package=lme4 (accessed on 18 November 2016).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest: Tests for Random and Fixed Effects for Linear Mixed Effect Models Lmer Objects of lme4 Package. R Package Version 2.0-6. Available online: http://CRAN.R-project.org/package=lmerTest (accessed on 18 November 2016).
- Ågren, G.I.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127. [Google Scholar] [CrossRef]
- Potocic, N.; Seletkovic, I.; Cater, M.; Cosic, T.; Sango, M.; Vedris, M. Ecophysiological response of sun-exposed Common Beech (Fagus sylvatica L.) seedlings under different fertilization levels. Sumarski List 2009, 133, 289–300. [Google Scholar]
- Högberg, P.; Johannisson, C.; Hallgren, J.E. Studies of 13C in the foliage reveal interactions between nutrients and water in forest fertilization experiments. Plant Soil 1993, 152, 207–214. [Google Scholar] [CrossRef]
- Gordon, C.; Woodin, S.J.; Alexander, I.J.; Mullins, C.E. Effects of increased temperature, drought and nitrogen supply on two upland perennials of contrasting functional type: Calluna vulgaris and Pteridium aquilinum. New Phytol. 1999, 142, 243–258. [Google Scholar] [CrossRef]
- Jokela, A.; Back, J.; Huttunen, S.; Jalkanen, R. Excess nitrogen-fertilization and the structure of Scots-Pine needles. Eur. J. For. Pathol. 1995, 25, 109–124. [Google Scholar] [CrossRef]
- Hamp, R.; Shi, L.; Guttenberger, M.; Nehls, U. Mykorrhizierung und Stresstoleranz von Ökotypen der Buche (Fagus sylvatica L.): “Conventwaldprojekt”. Forschungsbericht 1999. FZKA-BWPLUS. [Google Scholar]
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: Fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Huet, S.; Forgeard, F.; Nys, C. Above- and belowground distribution of dry matter and carbon biomass of Atlantic beech (Fagus sylvatica L.) in a time sequence. Ann. For. Sci. 2004, 61, 683–694. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.Y.H. Observations from old forests underestimate climate change effects on tree mortality. Nat. Commun. 2013, 4, 1655. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.K.; HilleRisLambers, J. Climate isn’t everything: Competitive interactions and variation by life stage will also affect range shifts in a warming world. Am. J. Bot. 2013, 100, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Marcos, E.; Calvo, L.; Marcos, J.A.; Taboada, A.; Tarrega, R. Tree effects on the chemical topsoil features of oak, beech and pine forests. Eur. J. For. Res. 2010, 129, 25–30. [Google Scholar] [CrossRef]
- Rivas-Martinez, S. Estudio de la vegetacion y flora de las Sierras de Guadarrama y Gredos. An. del Inst. Bot. A.J. Cavanilles 1963, 21, 5–330. [Google Scholar]
| Morphological Variables | Stem Increment | Height Increment | Leaf Biomass Increment | Aboveground Biomass | ||||||||
| Estimate | t-value | p-value | Estimate | t-value | p-value | Estimate | t-value | p-value | Estimate | t-value | p-value | |
| Intercept | 1.867 | 16.828 | <0.001 | 3.837 | 3.617 | 0.004 | 2.546 | 9.986 | <0.001 | 18.121 | 19.998 | <0.001 |
| D | 0.026 | 0.260 | 0.793 | 0.048 | 0.054 | 0.957 | 0.158 | 1.158 | 0.248 | 0.609 | 0.709 | 0.479 |
| N | 0.566 | 5.649 | <0.001 | 2.021 | 2.292 | 0.023 | 0.397 | 2.932 | 0.004 | 2.712 | 3.170 | 0.002 |
| ND | 0.567 | 5.675 | <0.001 | 2.599 | 2.956 | 0.003 | 0.461 | 3.413 | <0.001 | 3.593 | 4.211 | <0.001 |
| Morphological Variables | Belowground Biomass | Shoot:Root Ratio | No. of Necrotic Leaves | No. of Dead Branches | ||||||||
| Estimate | t-value | p-value | Estimate | t-value | p-value | Estimate | χ2 | p-value | Estimate | χ2 | p-value | |
| Intercept | 2.669 | 41.668 | <0.001 | 0.073 | 3.690 | 0.002 | 0.550 | 2.363 | 0.018 | 0.072 | −0.270 | 0.787 |
| D | 0.047 | 0.599 | 0.550 | 0.008 | −0.375 | 0.709 | 0.464 | 1.675 | 0.094 | 0.079 | −0.386 | 0.700 |
| N | 0.068 | 0.866 | 0.389 | 0.059 | 2.857 | 0.005 | −0.620 | −2.130 | 0.033 | 0.216 | 1.101 | 0.270 |
| ND | 0.109 | 0.079 | 0.169 | 0.043 | 2.061 | 0.041 | −0.330 | −1.163 | 0.245 | 0.512 | 2.724 | 0.006 |
| Physiological Variables | C Concentration Leaves | N Concentration Leaves | C:N Ratio | Leaf δ13C Signature | ||||||||
| Estimate | t-value | p-value | Estimate | t-value | p-value | Estimate | t-value | p-value | Estimate | t-value | p-value | |
| Intercept | 463.205 | 1.987 | <0.001 | 15.413 | 27.799 | <0.001 | 30.777 | 29.853 | <0.001 | 29.049 | −167.820 | <0.001 |
| D | −3.738 | 2.931 | 0.004 | −1.354 | −4.296 | <0.001 | 2.420 | 4.634 | <0.001 | −0.197 | −2.175 | 0.030 |
| N | −2.466 | 1.934 | 0.054 | 3.138 | 9.954 | <0.001 | −5.380 | 10.302 | <0.001 | −0.094 | −0.134 | 0.302 |
| ND | −2.482 | 1.945 | 0.053 | 2.557 | 8.107 | <0.001 | −4.718 | −9.031 | <0.001 | 0.083 | 0.920 | 0.358 |
| Fixed Effects | Estimate | t-Value | p-Value |
|---|---|---|---|
| (a) Shoot:root ratio | |||
| Intercept | 1.747 | 22.185 | <0.001 |
| D | 0.023 | 4.649 | <0.001 |
| N | 0.089 | 2.078 | 0.038 |
| Age (1 year vs. 2 years) | −0.456 | −4.392 | <0.001 |
| D × Age | −0.258 | −2.621 | 0.009 |
| (b) Leaf δ13C signature | |||
| Intercept | −29.688 | −159.014 | <0.001 |
| D | 0.442 | 5.431 | <0.001 |
| N | −0.034 | −0.516 | 0.606 |
| Age (1 year vs. 2 years) | 1.058 | 8.188 | <0.001 |
| D × N | 0.191 | 2.035 | 0.042 |
| D × Age | −0.546 | −5.804 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedek, C.; Fichtner, A.; Calvo, L.; Marcos, E.; Jansen, K.; Kunz, M.; Walmsley, D.; Von Oheimb, G.; Härdtle, W. Phenotypic Plasticity Explains Response Patterns of European Beech (Fagus sylvatica L.) Saplings to Nitrogen Fertilization and Drought Events. Forests 2017, 8, 91. https://doi.org/10.3390/f8030091
Dziedek C, Fichtner A, Calvo L, Marcos E, Jansen K, Kunz M, Walmsley D, Von Oheimb G, Härdtle W. Phenotypic Plasticity Explains Response Patterns of European Beech (Fagus sylvatica L.) Saplings to Nitrogen Fertilization and Drought Events. Forests. 2017; 8(3):91. https://doi.org/10.3390/f8030091
Chicago/Turabian StyleDziedek, Christoph, Andreas Fichtner, Leonor Calvo, Elena Marcos, Kirstin Jansen, Matthias Kunz, David Walmsley, Goddert Von Oheimb, and Werner Härdtle. 2017. "Phenotypic Plasticity Explains Response Patterns of European Beech (Fagus sylvatica L.) Saplings to Nitrogen Fertilization and Drought Events" Forests 8, no. 3: 91. https://doi.org/10.3390/f8030091






