Biomass and Carbon Sequestration by Juglans regia Plantations in the Karst Regions of Southwest China
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Sites
2.2. Field Sampling and Measurements
2.3. Data Analysis
3. Results
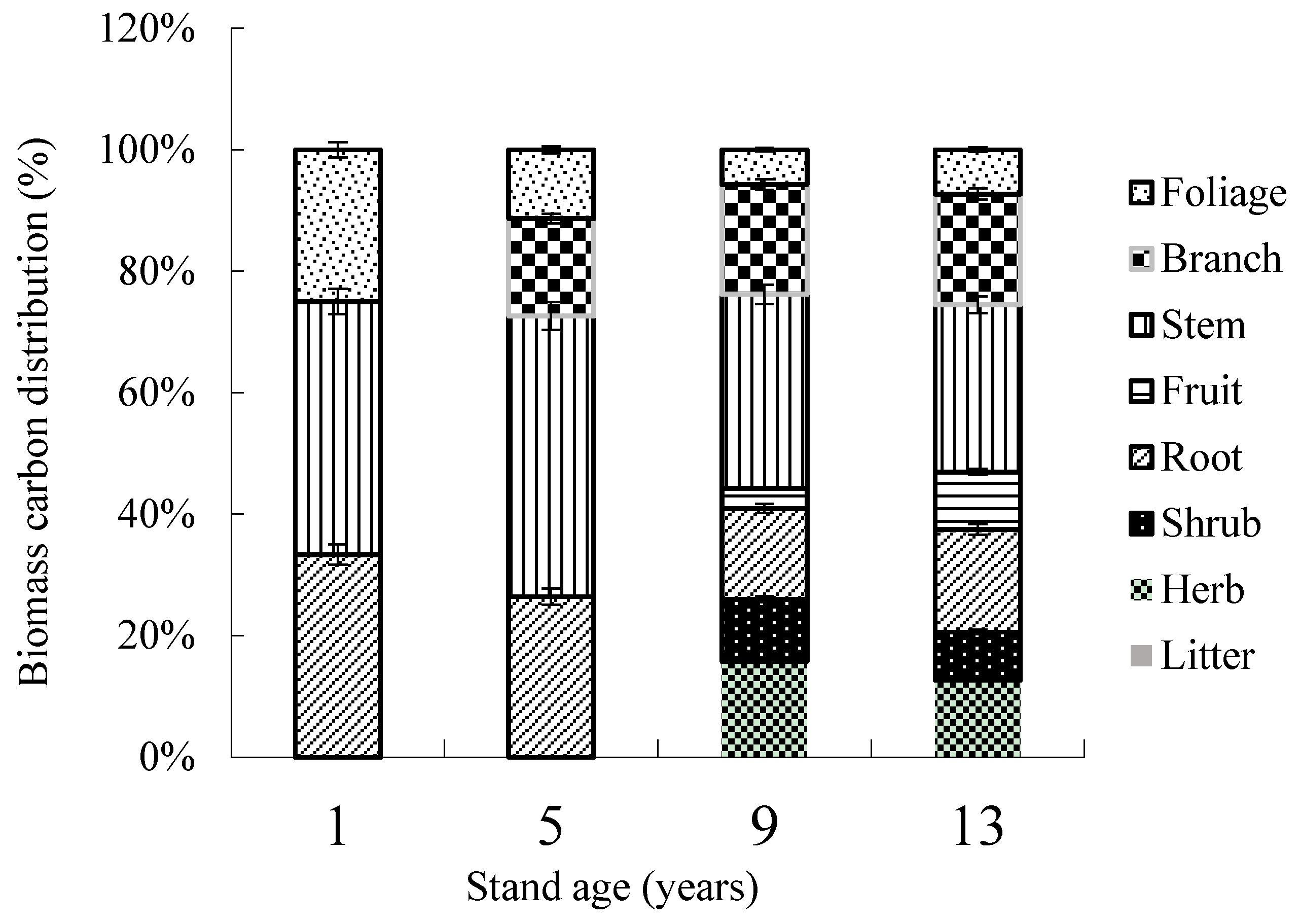
3.1. Biomass Distribution in Ecosystem Components
3.2. Carbon Concentration and Accumulation in Biomass
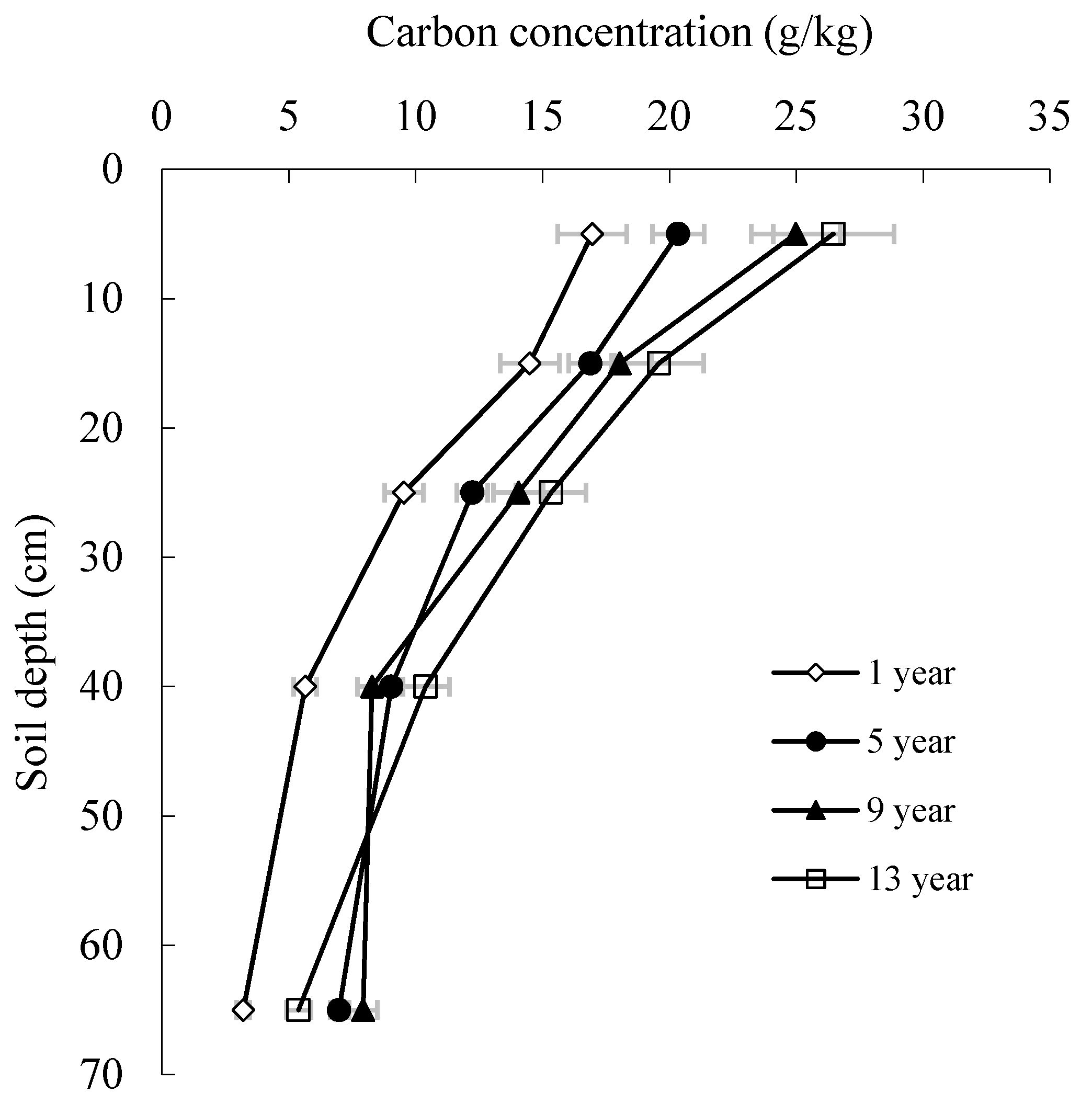
3.3. Carbon Concentration and Storage in Soil
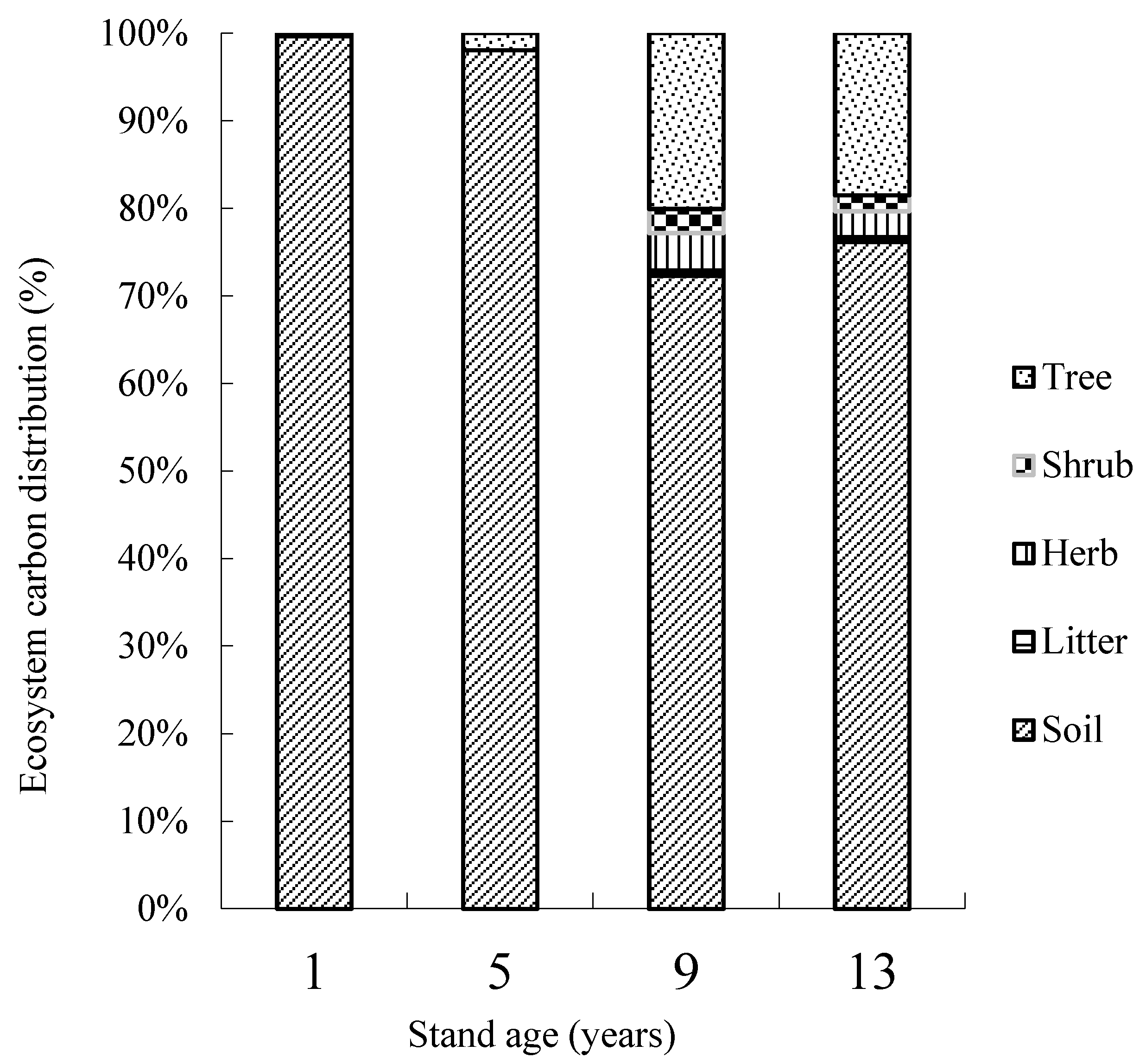
3.4. Carbon Storage in the J. regia Plantation Ecosystem
4. Discussion
4.1. Biomass of J. regia Plantations of Different Ages
4.2. Biomass C of J. regia Plantations of Different Ages
4.3. Soil Organic Carbon (SOC) Storage in J. regia Plantations
4.4. Influence of GGP on C Sequestration in the Karst Region
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, J.Y.; Chen, A.P.; Peng, C.H.; Zhao, S.Q.; Ci, L.J. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration in China through agricultural intensification, and restoration of degraded and desertified ecosystems. Land Degrad. Dev. 2002, 13, 469–478. [Google Scholar] [CrossRef]
- Pacaldo, R.S.; Volk, T.A.; Briggs, R.D. Greenhouse gas (GHG) potentials of shrub willow biomass crop (Salix x dasyclados) based on the above- and belowground biomass production across a 19-year chronosequence. BioEnergy Res. 2013, 6, 252–262. [Google Scholar] [CrossRef]
- Pacaldo, R.S.; Volk, T.A.; Briggs, R.D. No differences in soil organic carbon in short rotation willow (Salix x dasyclados) along a 19-year chronosequence. Biomass Bioenergy 2013. [Google Scholar] [CrossRef]
- Pacaldo, R.S.; Volk, T.A.; Briggs, R.D. Carbon sequestration in fine roots and foliage offsets soil CO2 effluxes along a 19-year chronosequence of Shrub Willow Biomass Crops. BioEnergy Res. 2014, 7, 769–776. [Google Scholar] [CrossRef]
- Chang, R.Y.; Fu, B.J.; Liu, G.H.; Liu, S.G. Soil carbon sequestration potential for ‘Grain for Green’ Project in Loess Plateau, China. Environ. Manag. 2011, 48, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- State Forestry Administration. China Forestry Statistical Yearbook 1999–2011; China Forestry Publishing House: Beijing, China, 2012.
- Chen, X.G.; Zhang, X.Q.; Zhang, Y.P.; Wan, C.B. Carbon sequestration potential of the stands under the grain for green program in Yunnan Province, China. For. Ecol. Manag. 2009, 258, 199–206. [Google Scholar] [CrossRef]
- Zhang, H.; Song, T.Q.; Wang, K.L.; Du, H.; Yue, Y.M.; Wang, G.X.; Zeng, F.P. Biomass and carbon storage in an age-sequence of Cyclobalanopsis glauca plantations in southwest China. Ecol. Eng. 2014, 73, 184–191. [Google Scholar] [CrossRef]
- Zhang, H.; Song, T.Q.; Wang, K.L.; Yang, H.; Yue, Y.M.; Zeng, Z.X.; Peng, W.X.; Zeng, F.P. Influences of stand characteristics and environmental factors on forest biomass and root-shoot allocation in southwest China. Ecol. Eng. 2016, 91, 7–15. [Google Scholar] [CrossRef]
- Yuan, D.X. The geology environment and hydro-ecological problem of karst region. Land Res. South China 2003, 1, 21–25. [Google Scholar]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [PubMed]
- Cheng, J.Z.; Lee, X.Q.; Theng, B.K.G.; Zhang, L.K.; Fang, B.; Li, F.S. Biomass accumulation and carbon sequestration in an age-sequence of Zanthoxylum bungeanum plantations under the Grain for Green Program in karst regions, Guizhou province. Agric. For. Meteorol. 2015, 203, 88–95. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Qiu, G.Y.; Mo, D.K.; Lin, H.; Sun, H.; Wang, Q.X.; Zhao, S.H.; Yin, J. Rocky desertification and its causes in karst areas: A case study in Yongshun county, Hunan province, China. Environ. Geol. 2009, 57, 1481–1488. [Google Scholar] [CrossRef]
- Peng, W.X.; Wang, K.L.; Song, T.Q.; Zeng, F.P.; Wang, J.R. Controlling and restoration models of complex degradation vulnerable Karst ecosystem. Acta Ecol. Sin. 2008, 28, 811–820. [Google Scholar]
- Du, H.; Wang, K.L.; Peng, W.X.; Zeng, F.P.; Song, T.Q.; Zhang, H.; Lu, S.Y. Spatial heterogeneity of soil mineral oxide components in depression between karst hills, Southwest China. Chin. Geogr. Sci. 2014, 24, 163–179. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Study of the main constituents of some authentic walnut oils. J. Agric. Food Chem. 2005, 53, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Labuckas, D.O.; Maestri, D.M.; Perello, M.; Martınez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Xie, W.D.; Xie, X.P.; Jiang, G.X.; Lai, J.Y.; Wei, X.M. Vegetational diversity and biomass in Juglans regia forest under conversion cultivated land into forests model. Guizhou Agric. Sci. 2010, 38, 177–179. [Google Scholar]
- Javid, A.D.; Somaiah, S. Effect of Land Use Change on Carbon Sequestration: A Case Study in Shahmirzad Walnut Orchad, Semnan, Iran. Environ. Monit. Assess. 2015, 187, 55. [Google Scholar]
- Tan, Q.J.; Qin, Z.S.; Wang, X.Y.; Zhang, H.; Zeng, F.P.; Chen, H.S.; Wang, W.L. Relationships between biomass and soil nutrients in Juglans regia of different ages in depression between karst hills. Carsol. Sin. 2016, 35, 226–232. [Google Scholar]
- Condit, R. Research in large, long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Cote, L.; Brown, S.; Pare, D.; Fyles, J.; Bauhus, J. Dynamics of carbon acid nitrogen mineralization in relation to stand type, stand age and soil texture in the boreal mixedwood. Soil Biol. Biochem. 2000, 32, 1079–1090. [Google Scholar] [CrossRef]
- Song, T.Q.; Peng, W.X.; Zeng, F.P.; Wang, K.L.; Ouyang, Z.W. Vegetation succession rule and regeneration strategies in disturbed karst area, northwest Guangxi. J. Mt. Sci. 2008, 26, 597–604. [Google Scholar]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta-analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Miles, L.; Kapos, V. Reducing greenhouse gas emissions from deforestation and forest degradation: Global land-use implications. Science 2008, 320, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, W.; Benayas, J.M.R.; Alice, F.E. Carbon accumulation in the biomass and soil of different aged secondary forests in the humid tropics of Costa Rica. For. Ecol. Manag. 2011, 262, 1400–1408. [Google Scholar] [CrossRef]
- Tian, D.L.; Wang, X.K.; Fang, X.; Yan, W.D.; Ning, X.B.; Wang, G.J. Carbon storage and spatial distribution in different vegetation restoration patterns in karst areas, Guizhou province. Sci. Silvae Sin. 2011, 47, 7–14. [Google Scholar]
- Wang, F.M.; Xu, X.; Zou, B.; Guo, Z.H.; Li, Z.A.; Zhu, W.X. Biomass accumulation and carbon sequestration in four different aged Casuarina equisetifolia coastal shelterbelt plantations in South China. PLoS ONE 2013, 8, e77449. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Baker, T.R.; Phillips, O.L.; Almeida, S.; Alvarez, E.; Arroyo, L.; Chave, J.; Czimczik, C.I.; Di Fiore, A.; Higuchi, N.; et al. The above-ground coarse wood productivity of 104 Neotropical forest plots. Glob. Chang. Biol. 2004, 10, 563–591. [Google Scholar] [CrossRef]
- Redondo-Brenes, A. Growth, carbon sequestration, and management of native tree plantations in humid regions of Costa Rica. New For. 2007, 34, 253–268. [Google Scholar] [CrossRef]
- Lasco, R.D.; Pulhin, F.B.; Visco, R.G.; Racelis, D.A.; Guillermo, I.Q.; Sales, R.F. Carbon stocks assessment of Philippine forest ecosystems. Presented at the Science-Policy Workshop on Terrestrial Carbon Assessment for Possible Carbon Trading, Bogor, Indonesia, 28–29 February 2000. [Google Scholar]
- Romberger, J.A.; Hejnowicz, Z.; Hill, J.F. Plant Structure: Function and Development; The Blackburn Press: Caldwel, NJ, USA, 2004. [Google Scholar]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, A.A. Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- Farley, K.A.; Kelly, E.F.; Hofstede, R.G.M. Soil organic carbon and water retention following conversion of grasslands to pine plantations in the Ecuadoran Andes. Ecosystems 2004, 7, 729–739. [Google Scholar] [CrossRef]
- Seely, B.; Welham, C.; Blanco, J.A. Towards the application of soil organic matter as an indicator of forest ecosystem productivity: Deriving thresholds, developing monitoring systems, and evaluating practices. Ecol. Indic. 2010, 10, 999–1008. [Google Scholar] [CrossRef]
- Xu, W.; Yin, Y.; Zhou, S. Social and economic impacts of carbon sequestration and land use change on peasant households in rural China: A case study of Liping, Guizhou province. J. Environ. Manag. 2007, 85, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Schmid, I.; Kazda, M. Root distribution of Norway spruce in monospecific and mixed stands on different soils. For. Ecol. Manag. 2002, 159, 37–47. [Google Scholar] [CrossRef]
- Kenzo, T.; Ichie, T.; Hattori, D.; Kendawang, J.J.; Sakurai, K.; Ninomiya, I. Changes in above- and belowground biomass in early successional tropical secondary forests after shifting cultivation in Sarawak, Malaysia. For. Ecol. Manag. 2010, 260, 875–882. [Google Scholar] [CrossRef]
- Abdipour, M.; Hoseini, S.M.; Kaboli, H.; Kianian, M.K. Effect of Land Use Change on Carbon Sequestration: A Case Study in Shahmirzad Walnut Orchad, Semnan, Iran. Res. J. Soil. Biol. 2015, 7, 1–12. [Google Scholar]
| Characteristics Parameters | 1-Year-Old | 5-Year-Old | 9-Year-Old | 13-Year-Old |
|---|---|---|---|---|
| Site characteristics | ||||
| Altitude (m) | 605a ± 12 | 750b ± 19 | 790c ± 14 | 755b ± 20 |
| Slope (°) | 27a ± 3.6 | 29a ± 4.1 | 25a ± 3.4 | 26a ± 3.9 |
| Soil depth (cm) | 68a ± 5.9 | 65a ± 6.2 | 74a ± 6.0 | 70a ± 4.5 |
| Soil pH | 7.0a ± 0.2 | 6.8a ± 0.3 | 7.1a ± 0.3 | 7.2a ± 0.2 |
| Soil texture | Silt | Silt | Silt | Silt |
| Cation exchange capacity (cmol/kg) | 31.3a ± 3.2 | 32.5a ± 2.6 | 29.9a ± 2.9 | 32.1a ± 2.5 |
| Bulk density (g/cm3) | 1.26a ± 0.20 | 1.72a ± 0.29 | 1.31a ± 0.22 | 1.22a ± 0.19 |
| Coarse rock fragment (%) | 20.1a ± 3.8 | 16.8a ± 3.3 | 13.7a ± 4.2 | 16.2a ± 3.2 |
| Percentage of covered soil (%) | 57.8ab ± 3.0 | 60.1b ± 2.2 | 51.4a ± 3.8 | 68.9c ± 3.4 |
| Stand characteristics | ||||
| Mean DBH (cm) | 1.8a ± 0.2 | 6.4b ± 1.1 | 13.8c ± 2.4 | 17.5c ± 2.9 |
| Mean height (m) | 1.7a ± 0.4 | 3.7b ± 1.2 | 5.2bc ± 1.5 | 7.9c ± 1.8 |
| Stand density (plant/ha) | 380a ± 11 | 378a ± 13 | 370a ± 10 | 325b ± 15 |
| Component | 1-Year-Old | 5-Year-Old | 9-Year-Old | 13-Year-Old |
|---|---|---|---|---|
| Tree | ||||
| Foliage | 0.07a ± 0.02 | 0.26b ± 0.09 | 1.63c ± 0.17 | 2.28d ± 0.24 |
| Branch | - | 0.39a ± 0.12 | 4.95b ± 1.02 | 6.57b ± 1.54 |
| Stem | 0.11a ± 0.03 | 1.03b ± 0.27 | 6.46c ± 1.79 | 9.25c ± 1.95 |
| Fruit | - | - | 0.87a ± 0.18 | 2.91b ± 0.33 |
| Aboveground biomass (AGB) | 0.18a ± 0.06 | 1.68b ± 0.49 | 13.91c ± 2.49 | 21.01d ± 4.04 |
| Roots (BGB) | 0.08a ± 0.03 | 0.61b ± 0.12 | 4.24c ± 1.06 | 5.63c ± 1.19 |
| BGB/AGB | 0.44 | 0.36 | 0.30 | 0.27 |
| Total tree biomass (TTB) | 0.26a ± 0.07 | 2.29b ± 0.62 | 18.15c ± 3.52 | 26.64c ± 5.28 |
| Understory vegetation | ||||
| Shrub | - | - | 2.94a ± 0.43 | 2.48a ± 0.51 |
| Herb | - | - | 4.33a ± 1.40 | 3.92a ± 1.23 |
| Total understory vegetation | - | - | 7.27a ± 1.53 | 6.40a ± 1.75 |
| Litter | - | - | 0.59a ± 0.23 | 0.72a ± 0.38 |
| Total biomass | 0.26a ± 0.07 | 2.29b ± 0.62 | 26.01c ± 5.25 | 33.76c ± 7.12 |
| Component | 1-Year-Old | 5-Year-Old | 9-Year-Old | 13-Year-Old | Mean |
|---|---|---|---|---|---|
| Tree | |||||
| Foliage | 457.8ab ± 9.5 | 476.3b ± 7.6 | 439.0a ± 9.3 | 485.7b ± 8.2 | 464.7 |
| Branch | - | 430.7a ± 10.2 | 459.2b ± 8.4 | 421.6a ± 11.5 | 437.2 |
| Stem | 449.5a ± 7.3 | 478.1b ± 12.1 | 464.6ab ± 10.7 | 452.9a ± 9.5 | 461.3 |
| Fruit | - | - | 478.7a ± 8.1 | 494.1a ± 9.3 | 486.4 |
| Roots | 448.3a ± 9.3 | 461.6a ± 10.2 | 442.9a ± 9.6 | 456.7a ± 9.9 | 452.3 |
| Understory vegetation | |||||
| Shrub | - | - | 434.9a ± 10.4 | 484.2b ± 11.5 | 459.6 |
| Herb | - | - | 456.3a ± 9.8 | 492.3b ± 13.2 | 474.3 |
| Litter | - | - | 469.5a ± 12.3 | 482.9a ± 9.8 | 476.2 |
| Component | 1-Year-Old | 5-Year-Old | 9-Year-Old | 13-Year-Old |
|---|---|---|---|---|
| Tree | ||||
| Foliage | 0.03 a ± 0.01 | 0.12b ± 0.04 | 0.72c ± 0.27 | 1.11c ± 0.25 |
| Branch | - | 0.17a ± 0.07 | 2.27b ± 0.42 | 2.77b ± 0.54 |
| Stem | 0.05a ± 0.02 | 0.49b ± 0.13 | 3.01c ± 0.89 | 4.19c ± 1.05 |
| Fruit | - | - | 0.42a ± 0.08 | 1.44b ± 0.16 |
| Aboveground biomass (AGB) | 0.08a ± 0.03 | 0.78b ± 0.29 | 6.42c ± 1.30 | 9.51c ± 2.21 |
| Roots (BGB) | 0.04a ± 0.02 | 0.28b ± 0.06 | 1.88c ± 0.54 | 2.57c ± 0.58 |
| BGB/AGB | 0.50 | 0.36 | 0.29 | 0.27 |
| Total tree biomass (TTB) | 0.12a ± 0.04 | 1.06b ± 0.36 | 9.30c ± 1.75 | 12.08c ± 2.78 |
| Understory vegetation | ||||
| Shrub | - | - | 1.28a ± 0.25 | 1.20a ± 0.27 |
| Herb | - | - | 1.98a ± 0.77 | 1.93a ± 0.63 |
| Total understory vegetation | - | - | 3.26a ± 0.78 | 3.13a ± 0.81 |
| Litter | - | - | 0.28a ± 0.11 | 0.35a ± 0.16 |
| Total biomass | 0.12a ± 0.04 | 1.06b ± 0.36 | 12.84c ± 2.67 | 15.56c ± 3.51 |
| Soil | Depth (cm) | 1-Year-Old | 5-Year-Old | 9-Year-Old | 13-Year-Old |
|---|---|---|---|---|---|
| Carbon storage (Mg ha−1) | 0–10 | 9.75a ± 1.12 | 13.85b ± 2.07 | 9.05a ± 1.52 | 14.17b ± 2.38 |
| 10–20 | 8.33ab ± 0.81 | 11.50b ± 2.34 | 6.53a ± 1.08 | 10.49b ± 2.63 | |
| 20–30 | 5.48a ± 1.20 | 8.34b ± 1.12 | 5.09a ± 1.06 | 8.21b ± 1.65 | |
| 30–50 | 6.49a ± 1.04 | 12.33b ± 1.43 | 6.00a ± 1.21 | 11.14b ± 1.06 | |
| 50–100 | 3.32a ± 0.59 | 7.13c ± 0.68 | 6.89c ± 0.70 | 5.77b ± 0.54 | |
| 0–100 | 33.37a ± 4.18 | 53.15b ± 6.46 | 33.56a ± 6.39 | 49.78b ± 5.81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, K.; Zeng, Z.; Du, H.; Zeng, F. Biomass and Carbon Sequestration by Juglans regia Plantations in the Karst Regions of Southwest China. Forests 2017, 8, 103. https://doi.org/10.3390/f8040103
Zhang H, Wang K, Zeng Z, Du H, Zeng F. Biomass and Carbon Sequestration by Juglans regia Plantations in the Karst Regions of Southwest China. Forests. 2017; 8(4):103. https://doi.org/10.3390/f8040103
Chicago/Turabian StyleZhang, Hao, Kelin Wang, Zhaoxia Zeng, Hu Du, and Fuping Zeng. 2017. "Biomass and Carbon Sequestration by Juglans regia Plantations in the Karst Regions of Southwest China" Forests 8, no. 4: 103. https://doi.org/10.3390/f8040103






