A Tree Species Effect on Soil That Is Consistent Across the Species’ Range: The Case of Aspen and Soil Carbon in North America
Abstract
:1. Introduction
2. Material and Methods
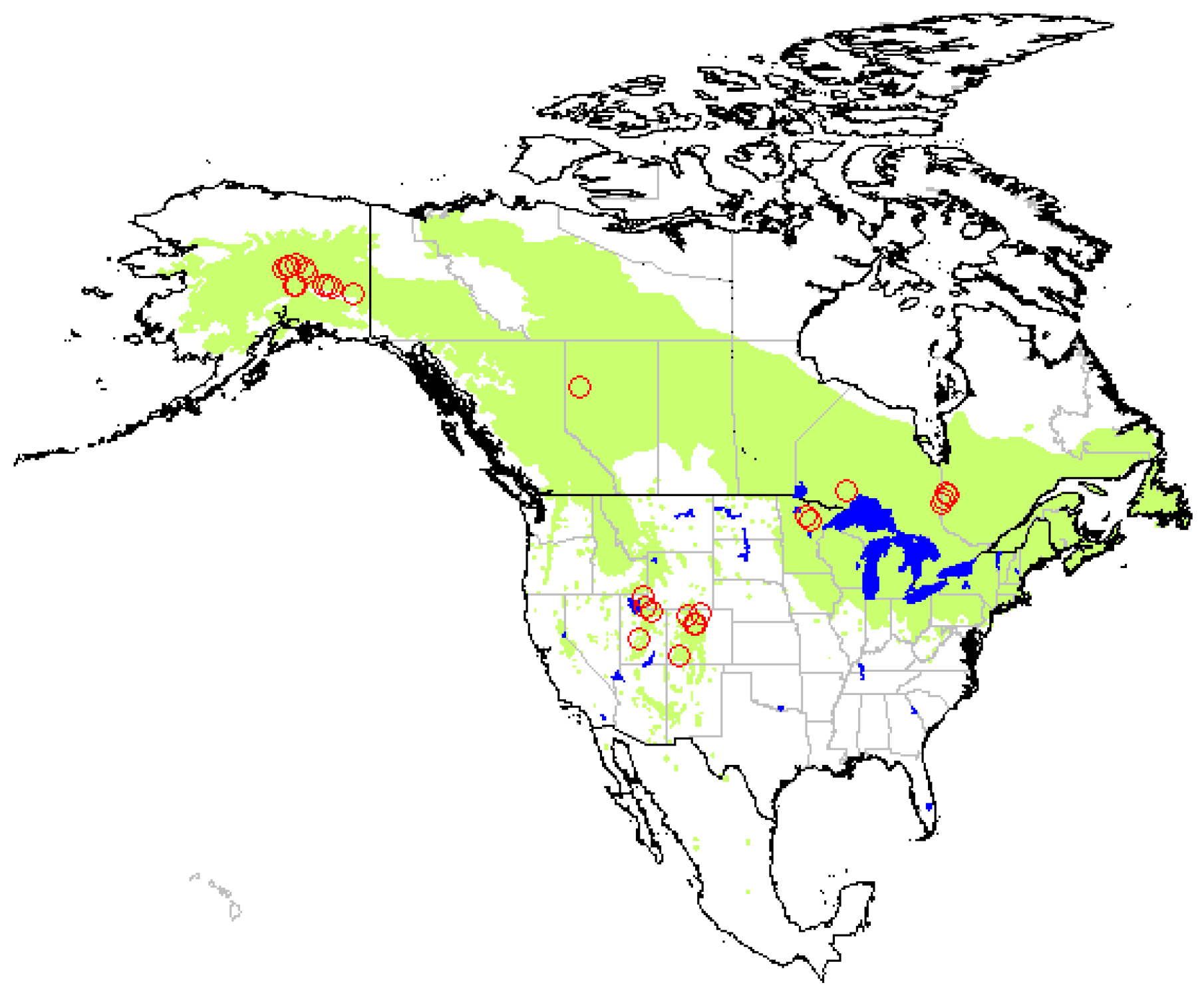
2.1. Study Area
2.2. Data Collection
3. Results
4. Discussion
4.1. Aspen Does Not Increase Soil C But Promotes Its Stability
4.2. Drivers of Aspen Effects on Soil Carbon Stability
4.2.1. Litter Quantity
4.2.2. Litter Quality
4.2.3. The Importance of Understory
4.2.4. Rooting Pattern and Rhizosphere Processes
4.3. Factors Responsible for Inconsistencies
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Reference | Location | Conifer Species 1 | Stand Age 2 (Year) | Sampling Depth (cm) | Soil Type | MAT 3 (°C) | MAP 4 (mm) | N | C Concentration | C Content | C Stability | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FF | MIN | FF | MIN | FF+MIN | FF | MIN | Indicators 5 | |||||||||
| Alban 1982 [68] | MN | Pg, Pr, Pb | 39–40, 41–49 | FF, 0–61, 0–80 | Alfisols | 4 | 610 | 1 | 0, -(5) | 0(2), -(4) | ||||||
| Alexander and Mack 2016 [102] | AK | Pm | 20–100 | FF, 0–10 | Gelisols to Inceptisols | n.a. | 286 | 2–14 | 0(3) | 0, -(2) | 0(3) | |||||
| Ayres et al. 2009 [54] | CO | Pc, Pe | n.a. | 0–10 | n.a. | n.a. | n.a. | 4 | 0(2) | 0(2) | ||||||
| Bauhus et al. 1998 [103] | QC | Ab+Pg | 50, 124 | FF, 0–10 | Spodosols, Alfisols | 0.6 | 823 | 1 | 0(4) | 0(4) | ||||||
| Boča and Van Miegroet 2017 [28] | UT | Ptm+Al+Ac+Pf | mature | FF, 0–50 | Alfisols, Mollisols | 3.2, 4.5 | 1031, 823 | 4 | - | + | 0 | +(3) | A, B, C | |||
| Buck and St. Clair 2012 [104] | UT | Al+Pe+Ptm | n.a. | FF(OA), 0–10 | n.a. | 10.1 | 201 | 10 | 0 | 0 | ||||||
| Côté et al. 2000 [31] | QC | Ab+Pg | 50, 124 | FF, 0–10 | Spodosols, Alfisols | 0.6 | 823 | 4 | 0 | 0 | 0 | 0 | 0 | D | ||
| Dobarco and Van Miegroet 2014 [38] | UT | Ptm+Al+Ac+Pf | mature | 0–15 | Alfisols, Mollisols | 3.2, 4.5 | 1197, 812 | 6, 5 | 0(2) | 0(2) | 0(4), +(2) | A, B, D | ||||
| Giardina et al. 2001 [29] | CO | Pc | 40–250 | 0–15 | n.a. | 0.5 | 700–850 | 6 | + | D | ||||||
| Hannam et al. 2004 [105] | AB | Pg | 80–140 | FF | Alfisols | −0.6 | 433 | 3 | - | 0 | ||||||
| Hannam et al. 2005 [106] | AB | Pg | 80–140 | FF | Alfisols | −0.6 | 433 | 3 | - | 0 | ||||||
| Jerabkova et al. 2006 [107] | AB | Pg mainly | 70–125 | FF, 0–7 | Alfisols | −0.6 | 433 | 3 | 0 | 0 | 0 | 0 | ||||
| Kishchuk et al. 2014 [108] | AB | Pg mainly | 62–124 | 10–17 | Alfisols | −0.6 | 431 | 3 | 0 | |||||||
| Laganière et al. 2009 [73] | QC | Pm | 79–89 | FF | Alfisols | 0.8 | 890 | 3 | - | |||||||
| Laganière et al. 2011 [39] | QC | Pm | 90 | 0–15 | Alfisols | 0.7 | 890 | 8 | + | A | ||||||
| Laganière et al. 2012 [33] | QC | Pm | 90 | n/a | Alfisols | 0.7 | 890 | 8 | + | E | ||||||
| Laganière et al. 2013 [30] | QC, ON | Pm, Pb | 90, 83 | FF, 0–55 | Alfisols, Inseptisols | 0.7, 2.5 | 890, 712 | 8, 4 | 0, - | 0(2), - | 0(2) | +(2) | +(2) | C, D | ||
| Lamarche et al. 2004 [109] | QC | Pg+Pm+Ab | 57–131 | FF | Alfisols, Spodosols | 0.8 | 857 | 18 | 0 | |||||||
| Olsen and Van Miegroet 2010 [32] | UT | Al+Pe | mature | 0–30 | Alfisols | 7 | 950 | 3 | 0 | 0 | D | |||||
| Paré and Bergeron 1996 [4] | QC | Pg | 49–123 | FF, 0–10 | Alfisols | 0.6 | 823 | 8 | - | 0 | ||||||
| Ste-Marie et al. 2007 [69] | QC | Pb | 59–89 | FF, 0–20 | Alfisols, Spodosols | 0.7 | 890 | 3 | 0, + | 0(2) | ||||||
| Weishampel et al. 2009 [110] | MN | Pb+Pr+Ab | 20–58 | FF, 0–40 | Alfisols | 3 | 785 | 3–15 | - | 0 | 0 | |||||
| Woldeselassie et al. 2012 [3] | UT | Ptm+Pe+Al+Pc | mature | FF, 0–60 | variable | 4.5 | 890–950 | 6 | - | 0, + | + | +(3) | A, C, D | |||
References
- Burns, R.; Honkala, B. Silvics of North America: 2. Hardwoods. Agriculture Handbook 654.; Department of Agriculture, Forest Service: Washington, DC, USA, 1990.
- Bartos, D.L.; Amacher, M.C. Soil Properties Associated with Aspen to Conifer Succession. Rangelands 1998, 20, 25–28. [Google Scholar]
- Woldeselassie, M.; Van Miegroet, H.; Gruselle, M.-C.; Hambly, N. Storage and Stability of Soil Organic Carbon in Aspen and Conifer Forest Soils of Northern Utah. Soil Sci. Soc. Am. J. 2012, 76, 2230. [Google Scholar] [CrossRef]
- Paré, D.; Bergeron, Y. Effect of colonizing tree species on soil nutrient availability in clay soil of the boreal mixedwood. Can. J. For. Res. 1996, 26, 1022–1031. [Google Scholar] [CrossRef]
- Reich, P.B.; Bakken, P.; Carlson, D.; Frelich, L.E.; Friedman, S.K.; Grigal, D.F. Influence of Logging, Fire, and Forest Type on Biodiversity and Productivity in Southern Boreal Forests. Ecology 2001, 82, 2731–2748. [Google Scholar] [CrossRef]
- Laganière, J.; Cavard, X.; Brassard, B.W.; Paré, D.; Bergeron, Y.; Chen, H.Y.H. The influence of boreal tree species mixtures on ecosystem carbon storage and fluxes. For. Ecol. Manag. 2015, 354, 119–129. [Google Scholar] [CrossRef]
- Kuhn, T.J.; Safford, H.D.; Jones, B.E.; Tate, K.W. Aspen (Populus tremuloides) stands and their contribution to plant diversity in a semiarid coniferous landscape. Plant Ecol. 2011, 212, 1451–1463. [Google Scholar] [CrossRef]
- DeByle, N.V.; Winokur, R.P. Aspen: Ecology and Management in the Western United States. General Technical Report RM-119. USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1985; Volume 119. [Google Scholar]
- Griffis-Kyle, K.L.; Beier, P. Small isolated aspen stands enrich bird communities in southwestern ponderosa pine forests. Biol. Conserv. 2003, 110, 375–385. [Google Scholar] [CrossRef]
- LaMalfa, E.M.; Ryle, R. Differential Snowpack Accumulation and Water Dynamics in Aspen and Conifer Communities: Implications for Water Yield and Ecosystem Function. Ecosystems 2008, 11, 569–581. [Google Scholar] [CrossRef]
- Long, J.N.; Mock, K. Changing perspectives on regeneration ecology and genetic diversity in western quaking aspen: Implications for silviculture. Can. J. For. Res. 2012, 42, 2011–2021. [Google Scholar] [CrossRef]
- Baker, F.S. Aspen in the Central Rocky Mountain Region. U. S. Dep. Agric. Bull. 1925, 1291. [Google Scholar] [CrossRef]
- Packard, F.M. Wildlife and Aspen in Rocky Mountain National Park, Colorado. Ecology 1942, 23, 478–482. [Google Scholar] [CrossRef]
- Bartos, D.L. Landscape dynamics of aspen and conifer forests. In Sustaining Aspen in Western Landscapes: Symposium Proceedings; Shepperd, W.D., Binkley, D., Bartos, D.L., Stohlgren, T.J., Eskew, L.G., Eds.; USDA Forest Service., Rocky Mountain Research Station: Fort Collins, CO, USA, 2001; pp. 5–14. [Google Scholar]
- Rogers, P. Using Forest Health Monitoring to assess aspen forest cover change in the southern Rockies ecoregion. For. Ecol. Manag. 2002, 155, 223–236. [Google Scholar] [CrossRef]
- Di Orio, A.P.; Callas, R.; Schaefer, R.J. Forty-eight year decline and fragmentation of aspen (Populus tremuloides) in the South Warner Mountains of California. For. Ecol. Manag. 2005, 206, 307–313. [Google Scholar] [CrossRef]
- Kulakowski, D.; Veblen, T.T.; Drinkwater, S. The Persistence of Quaking Aspen (Populus tremuloides) in the Grand Mesa Area, Colorado. Ecol. Appl. 2004, 14, 1603–1614. [Google Scholar] [CrossRef]
- Worrall, J.J.; Rehfeldt, G.E.; Hamann, A.; Hogg, E.H.; Marchetti, S.B.; Michaelian, M.; Gray, L.K. Recent declines of Populus tremuloides in North America linked to climate. For. Ecol. Manag. 2013, 299, 35–51. [Google Scholar] [CrossRef]
- Boulanger, Y.; Gauthier, S.; Gray, D.R.; Le Goff, H.; Lefort, P.; Morissette, J. Fire regime zonation under current and future climate over eastern Canada. Ecol. Appl. 2013, 23, 904–923. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.H.; Vasiliauskas, S.; Kayahara, G.J.; Ilisson, T. Wildfire promotes broadleaves and species mixture in boreal forest. For. Ecol. Manag. 2009, 257, 343–350. [Google Scholar] [CrossRef]
- Lafleur, B.; Cazal, A.; Leduc, A.; Bergeron, Y. Soil organic layer thickness influences the establishment and growth of trembling aspen (Populus tremuloides) in boreal forests. For. Ecol. Manag. 2015, 347, 209–216. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Goetz, S.J.; Mack, M.C.; Alexander, H.D.; Jin, Y.; Randerson, J.T.; Loranty, M.M. The impacts and implications of an intensifying fire regime on Alaskan boreal forest composition and albedo. Glob. Chang. Biol. 2011, 17, 2853–2866. [Google Scholar] [CrossRef]
- Kulakowski, D.; Matthews, C.; Jarvis, D.; Veblen, T.T. Compounded disturbances in sub-alpine forests in western Colorado favour future dominance by quaking aspen (Populus tremuloides). J. Veg. Sci. 2013, 24, 168–176. [Google Scholar] [CrossRef]
- Ilisson, T.; Chen, H.Y.H. Response of six boreal tree species to stand replacing fire and clearcutting. Ecosystems 2009, 12, 820–829. [Google Scholar] [CrossRef]
- Boucher, Y.; Auger, I.; Noël, J.; Grondin, P.; Arseneault, D. Fire is a stronger driver of forest composition than logging in the boreal forest of eastern Canada. J. Veg. Sci. 2017, 28, 57–68. [Google Scholar] [CrossRef]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. Camb. Philos. Soc. 2015, 9, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 1–15. [Google Scholar] [CrossRef]
- Boča, A.; Van Miegroet, H. Can carbon fluxes explain differences in soil organic carbon storage under aspen and conifer forest overstories? Forests 2017. accepted. [Google Scholar]
- Giardina, C.P.; Ryan, M.G.; Hubbard, R.M.; Binkley, D. Tree Species and Soil Textural Controls on Carbon and Nitrogen Mineralization Rates. Soil Sci. Soc. Am. J. 2001, 65, 1272–1279. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bergeron, Y.; Chen, H.Y.H.; Brassard, B.W.; Cavard, X. Stability of Soil Carbon Stocks Varies with Forest Composition in the Canadian Boreal Biome. Ecosystems 2013, 16, 852–865. [Google Scholar] [CrossRef]
- Côté, L.; Brown, S.; Paré, D.; Fyles, J.; Bauhus, J. Dynamics of carbon and nitrogen mineralization in relation to stand type, stand age and soil texture in the boreal mixedwood. Soil Biol. Biochem. 2000, 32, 1079–1090. [Google Scholar] [CrossRef]
- Olsen, H.R.; Van Miegroet, H. Factors Affecting Carbon Dioxide Release from Forest and Rangeland Soils in Northern Utah. Soil Sci. Soc. Am. J. 2010, 74, 282. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bergeron, Y.; Chen, H.Y.H. The effect of boreal forest composition on soil respiration is mediated through variations in soil temperature and C quality. Soil Biol. Biochem. 2012, 53, 18–27. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of Soil Organic Matter: Association with Minerals or Chemical Recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Von Lützow, M.V.; Kogel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Román Dobarco, M.; Van Miegroet, H. Soil Organic Carbon Storage and Stability in the Aspen-Conifer Ecotone in Montane Forests in Utah State, USA. Forests 2014, 5, 666–688. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D.; Bergeron, Y.; Chen, H.Y.H. Black Spruce Soils Accumulate More Uncomplexed Organic Matter than Aspen Soils. Soil Sci. Soc. Am. J. 2011, 75, 1125. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Fire effects on temperate forest soil C and N storage. Ecol. Appl. 2011, 21, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest impacts on soil carbon storage in temperate forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Kishchuk, B.E.; Morris, D.M.; Lorente, M.; Keddy, T.; Sidders, D.; Quideau, S.; Thiffault, E.; Kwiaton, M.; Maynard, D. Disturbance intensity and dominant cover type influence rate of boreal soil carbon change: A Canadian multi-regional analysis. For. Ecol. Manag. 2016, 381, 48–62. [Google Scholar] [CrossRef]
- Harrison, R.B.; Footen, P.W.; Strahm, B.D. Deep Soil Horizons: Contribution and Importance to Soil Carbon Pools and in Assessing Whole-Ecosystem Response to Management and Global Change. For. Sci. 2011, 57, 67–76. [Google Scholar]
- Boča, A.; Van Miegroet, H.; Gruselle, M.-C. Forest Overstory Effect on Soil Organic Carbon Storage: A Meta-analysis. Soil Sci. Soc. Am. J. 2014, 78, S35–S47. [Google Scholar] [CrossRef]
- Gower, S.T.; Hunter, A.; Campbell, J.; Vogel, J.; Veldhuis, H.; Harden, J.; Trumbore, S.; Norman, J.M.; Kucharik, C.J. Nutrient dynamics of the southern and northern BOREAS boreal forests. Ecoscience 2000, 7, 481–490. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Cyle, K.T.; Hill, N.; Young, K.; Jenkins, T.; Hancock, D.; Schroeder, P.A.; Thompson, A. Substrate quality influences organic matter accumulation in the soil silt and clay fraction. Soil Biol. Biochem. 2016, 103, 138–148. [Google Scholar] [CrossRef]
- Lajtha, K.; Bowden, R.D.; Nadelhoffer, K. Litter and Root Manipulations Provide Insights into Soil Organic Matter Dynamics and Stability. Soil Sci. Soc. Am. J. 2014, 78, S261. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y. Influence of Environmental Variability on Root Dynamics in Northern Forests. CRC Crit. Rev. Plant Sci. 2009, 28, 179–197. [Google Scholar] [CrossRef]
- Hajek, P.; Hertel, D.; Leuschner, C. Intraspecific variation in root and leaf traits and leaf-root trait linkages in eight aspen demes (Populus tremula and P. tremuloides). Front. Plant Sci. 2013, 4, 415. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.J.; Gower, S.T.; Vogel, J.G.; Norman, J.M. Root mass, net primary production and turnover in aspen, jack pine and black spruce forests in Saskatchewan and Manitoba, Canada. Tree Physiol. 1997, 17, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y.; Paré, D. Differences in fine root productivity between mixed- and single-species stands. Funct. Ecol. 2011, 25, 238–246. [Google Scholar] [CrossRef]
- Bauhus, J.; Messier, C. Soil exploitation strategies of fine roots in different tree species of the southern boreal forest of eastern Canada. Can. J. For. Res. 1999, 29, 260–273. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Berg, S.; Wallenstein, M.D.; Simmons, B.L.; Wall, D.H. Tree species traits influence soil physical, chemical, and biological properties in high elevation forests. PLoS ONE 2009, 4, e5964. [Google Scholar] [CrossRef] [PubMed]
- Finér, L.; Messier, C.; De Grandpré, L. Fine-root dynamics in mixed boreal conifer - broad-leafed forest stands at different successional stages after fire. Can. J. For. Res. 1997, 27, 304–314. [Google Scholar] [CrossRef]
- Block, R.M.A.; Van Rees, K.C.J.; Knight, J.D. A Review of Fine Root Dynamics in Populus Plantations. Agrofor. Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Fyles, J.; Titus, B.D. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 2006, 9, 46–62. [Google Scholar] [CrossRef]
- Strukelj, M.; Brais, S.; Quideau, S.A.; Oh, S.-W. Chemical transformations of deadwood and foliar litter of mixed boreal species during decomposition. Can. J. For. Res. 2012, 42, 772–788. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bradley, R.L. How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can. J. For. Res. 2010, 40, 465–475. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y. Carbon:nitrogen stoichiometry in forest ecosystems during stand development. Glob. Ecol. Biogeogr. 2011, 20, 354–361. [Google Scholar] [CrossRef]
- Preston, C.M.; Bhatti, J.S.; Flanagan, L.B.; Norris, C. Stocks, chemistry, and sensitivity to climate change of dead organic matter along the Canadian boreal forest transect case study. Clim. Chang. 2006, 74, 233–251. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Gower, S.T.; Vogel, J.G.; Norman, M.; Kucharik, C.J.; Steele, S.J. Carbon distribution and aboveground net primary production in aspen, jack pine, and black spruce stands in Saskatchewan and Manitoba, Canada. J. Geophys. Res. Biogeosci. 1997, 102, 29029–29041. [Google Scholar] [CrossRef]
- Smith, J.L.; Bell, J.M.; Bolton, H.; Bailey, V.L. The initial rate of C substrate utilization and longer-term soil C storage. Biol. Fertil. Soils 2007, 44, 315–320. [Google Scholar] [CrossRef]
- Yang, H.S.; Janssen, B.H. Relationship between substrate initial reactivity and residues ageing speed in carbon mineralization. Plant Soil 2002, 239, 215–224. [Google Scholar] [CrossRef]
- Légaré, S.; Paré, D.; Bergeron, Y. Influence of aspen on forest floor properties in black spruce-dominated stands. Plant Soil 2005, 275, 207–220. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Houle, D. Forest floor gross and net nitrogen mineralization in three forest types in Quebec, Canada. Soil Biol. Biochem. 2006, 38, 2135–2143. [Google Scholar] [CrossRef]
- Alban, D.H. Effects of Nutrient Accumulation by Aspen, Spruce, and Pine on Soil Properties. Soil Sci. Soc. Am. J. 1982, 46, 853–861. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Paré, D.; Gagnon, D. The contrasting effects of aspen and jack pine on soil nutritional properties depend on parent material. Ecosystems 2007, 10, 1299–1310. [Google Scholar] [CrossRef]
- Wuddivira, M.N.; Camps-Roach, G. Effects of organic matter and calcium on soil structural stability. Eur. J. Soil Sci. 2007, 58, 722–727. [Google Scholar] [CrossRef]
- Baldock, J.; Skjemstad, J. Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org. Geochem. 2000, 31, 697–710. [Google Scholar] [CrossRef]
- Whittinghill, K.A.; Hobbie, S.E. Effects of pH and calcium on soil organic matter dynamics in Alaskan tundra. Biogeochemistry 2012, 111, 569–581. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bradley, R.L. Linking the abundance of aspen with soil faunal communities and rates of belowground processes within single stands of mixed aspen–black spruce. Appl. Soil Ecol. 2009, 41, 19–28. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of Carbon Sequestration in Soil Aggregates. CRC Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Bossuyt, H.; Six, J.; Hendrix, P.F. Protection of soil carbon by microaggregates within earthworm casts. Soil Biol. Biochem. 2005, 37, 251–258. [Google Scholar] [CrossRef]
- Lubbers, I.M.; van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; van Groenigen, J.W. Greenhouse-gas emissions from soils increased by earthworms. Nat. Clim. Chang. 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Munroe, J.S.; Attwood, E.C.; O’Keefe, S.S.; Quackenbush, P.J.M. Eolian deposition in the alpine zone of the Uinta Mountains, Utah, USA. Catena 2015, 124, 119–129. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Stam, B.R.; Malechek, J.C.; Bartos, D.L.; Bowns, J.E.; Godfrey, E.B. Effect of Conifer Encroachment Into Aspen Stands on Understory Biomass. Rangel. Ecol. Manag. 2008, 61, 93–97. [Google Scholar] [CrossRef]
- Bisbee, K.E.; Gower, S.T.; Norman, J.M.; Nordheim, E.V. Environmental controls on ground cover species composition and productivity in a boreal black spruce forest. Oecologia 2001, 129, 261–270. [Google Scholar] [CrossRef]
- O’Connell, K.E.B.; Gower, S.T.; Norman, J.M. Net ecosystem production of two contrasting boreal black spruce forest communities. Ecosystems 2003, 6, 248–260. [Google Scholar] [CrossRef]
- Bona, K.A.; Shaw, C.H.; Fyles, J.W.; Kurz, W.A. Modelling moss-derived carbon in upland black spruce forests. Can. J. For. Res. 2016, 534, 520–534. [Google Scholar] [CrossRef]
- Gornall, J.L.; Jónsdóttir, I.S.; Woodin, S.J.; Van Der Wal, R. Arctic mosses govern below-ground environment and ecosystem processes. Oecologia 2007, 153, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.I.; Cornelissen, J.H.C.; Klahn, T.; Van Logtestijn, R.S.P.; Broekman, R.; Schweikert, W.; Aerts, R. An experimental comparison of chemical traits and litter decomposition rates in a diverse range of subarctic bryophyte, lichen and vascular plant species. J. Ecol. 2009, 97, 886–900. [Google Scholar] [CrossRef]
- Royer-Tardif, S.; Bradley, R.L. Forest floor properties across sharp compositional boundaries separating trembling aspen and jack pine stands in the southern boreal forest. Plant Soil 2011, 345, 353–364. [Google Scholar] [CrossRef]
- Harden, J.W.; O’Neill, K.P.; Trumbore, S.E.; Veldhuis, H.; Stocks, B.J. Moss and soil contributions to the annual net carbon flux of a maturing boreal forest. J. Geophys. Res. 1997, 102, 28805–28816. [Google Scholar] [CrossRef]
- Wickland, K.P.; Neff, J.C. Decomposition of soil organic matter from boreal black spruce forest: Environmental and chemical controls. Biogeochemistry 2008, 87, 29–47. [Google Scholar] [CrossRef]
- Fenton, N.J.; Bergeron, Y.; Paré, D. Decomposition rates of bryophytes in managed boreal forests: Influence of bryophyte species and forest harvesting. Plant Soil 2010, 336, 499–508. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Strong, W.L.; La Roi, G.H. Root-system morphology of common boreal forest trees in Alberta, Canada. Can. J. For. Res. 1983, 13, 1164–1173. [Google Scholar] [CrossRef]
- Pinno, B.D.; Wilson, S.D.; Steinaker, D.F.; Rees, K.C.J.; McDonald, S.A. Fine root dynamics of trembling aspen in boreal forest and aspen parkland in central Canada. Ann. For. Sci. 2010, 67, 710. [Google Scholar] [CrossRef]
- Yanai, R.D.; McFarlane, K.J.; Lucash, M.S.; Kulpa, S.E.; Wood, D.M. Similarity of nutrient uptake and root dimensions of Engelmann spruce and subalpine fir at two contrasting sites in Colorado. For. Ecol. Manag. 2009, 258, 2233–2241. [Google Scholar] [CrossRef]
- Eissenstat, D.M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Langley, A.J.; Chapman, S.K.; Hungate, B.A. Ectomycorrhizal colonization slows root decomposition: The post-mortem fungal legacy. Ecol. Lett. 2006, 9, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.; Tessier, J.; Morrison, I.; Scarratt, J.; Canning, B.; Klironomos, J. Soil depth distribution of ecto- and arbuscular mycorrhizal fungi associated with Populus tremuloides within a 3-year-old boreal forest clear-cut. Appl. Soil Ecol. 2002, 19, 209–216. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Jenny, H. Factors of Soil Formation. A System of Quantitative Pedology; Dover Publications: New York, NY, USA, 1994. [Google Scholar]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity—Evidence since the middle of the 20th century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Strahm, B.D.; Harrison, R.B. Controls on the Sorption, Desorption and Mineralization of Low-Molecular-Weight Organic Acids in Variable-Charge Soils. Soil Sci. Soc. Am. J. 2008, 72, 1653. [Google Scholar] [CrossRef]
- Woldeselassie, M.K. Soil Organic Carbon and Site Characteristics in Aspen and Evaluation of the Potential Effects of Conifer Encroachment on Soil Properties in Northern Utah, Master’s Thesis, Utah State University, Logan, UT, USA, 2009. [Google Scholar]
- Alexander, H.D.; Mack, M.C. A Canopy Shift in Interior Alaskan Boreal Forests: Consequences for Above- and Belowground Carbon and Nitrogen Pools during Post-fire Succession. Ecosystems 2016, 19, 98–114. [Google Scholar] [CrossRef]
- Bauhus, J.; Paré, D.; Côté, L. Effects of tree species, stand age and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil Biol. Biochem. 1998, 30, 1077–1089. [Google Scholar] [CrossRef]
- Buck, J.R.; St. Clair, S.B. Aspen Increase Soil Moisture, Nutrients, Organic Matter and Respiration in Rocky Mountain Forest Communities. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Hannam, K.D.; Quideau, S.A.; Oh, S.-W.; Kishchuk, B.E.; Wasylishen, R.E. Forest Floor Composition in Aspen- and Spruce-Dominated Stands of the Boreal Mixedwood Forest. Soil Sci. Soc. Am. J. 2004, 68, 1735. [Google Scholar] [CrossRef]
- Hannam, K.D.; Quideau, S.A; Kishchuk, B.E.; Oh, S.-W.; Wasylishen, R.E. Forest-floor chemical properties are altered by clear-cutting in boreal mixedwood forest stands dominated by trembling aspen and white spruce. Can. J. For. Res. 2005, 35, 2457–2468. [Google Scholar] [CrossRef]
- Jerabkova, L.; Prescott, C.E.; Kishchuk, B.E. Effect of variable-retention harvesting on soil nitrogen availability in boreal mixedwood forests. Can. J. For. Res. 2006, 36, 3029–3038. [Google Scholar] [CrossRef]
- Kishchuk, B.E.; Quideau, S.; Wang, Y.; Prescott, C. Long-term soil response to variable-retention harvesting in the EMEND (Ecosystem Management Emulating Natural Disturbance) experiment, northwestern Alberta. Can. J. Soil Sci. 2014, 94, 263–279. [Google Scholar] [CrossRef]
- Lamarche, J.; Bradley, R.L.; Pare, D.; Legare, S.; Bergeron, Y. Soil parent material may control forest floor properties more than stand type or stand age in mixedwood boreal forests. Ecoscience 2004, 11, 228–237. [Google Scholar] [CrossRef]
- Weishampel, P.; Kolka, R.; King, J.Y. Carbon pools and productivity in a 1-km2 heterogeneous forest and peatland mosaic in Minnesota, USA. For. Ecol. Manag. 2009, 257, 747–754. [Google Scholar] [CrossRef]
| Effect | C Concentration | C Content | C Stability | ||||
|---|---|---|---|---|---|---|---|
| FF | MIN | FF | MIN | FF+MIN | FF | MIN | |
| Increase | 0 | 0 | 1 | 2 | 1 | 2 | 13 |
| Decrease | 1 | 0 | 14 | 5 | 0 | 0 | 0 |
| No effect | 10 | 12 | 6 | 18 | 5 | 3 | 6 |
| Total number of cases | 11 | 12 | 21 | 25 | 6 | 5 | 19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laganière, J.; Boča, A.; Van Miegroet, H.; Paré, D. A Tree Species Effect on Soil That Is Consistent Across the Species’ Range: The Case of Aspen and Soil Carbon in North America. Forests 2017, 8, 113. https://doi.org/10.3390/f8040113
Laganière J, Boča A, Van Miegroet H, Paré D. A Tree Species Effect on Soil That Is Consistent Across the Species’ Range: The Case of Aspen and Soil Carbon in North America. Forests. 2017; 8(4):113. https://doi.org/10.3390/f8040113
Chicago/Turabian StyleLaganière, Jérôme, Antra Boča, Helga Van Miegroet, and David Paré. 2017. "A Tree Species Effect on Soil That Is Consistent Across the Species’ Range: The Case of Aspen and Soil Carbon in North America" Forests 8, no. 4: 113. https://doi.org/10.3390/f8040113






