Can Carbon Fluxes Explain Differences in Soil Organic Carbon Storage under Aspen and Conifer Forest Overstories?
Abstract
:1. Introduction
2. Materials and Methods
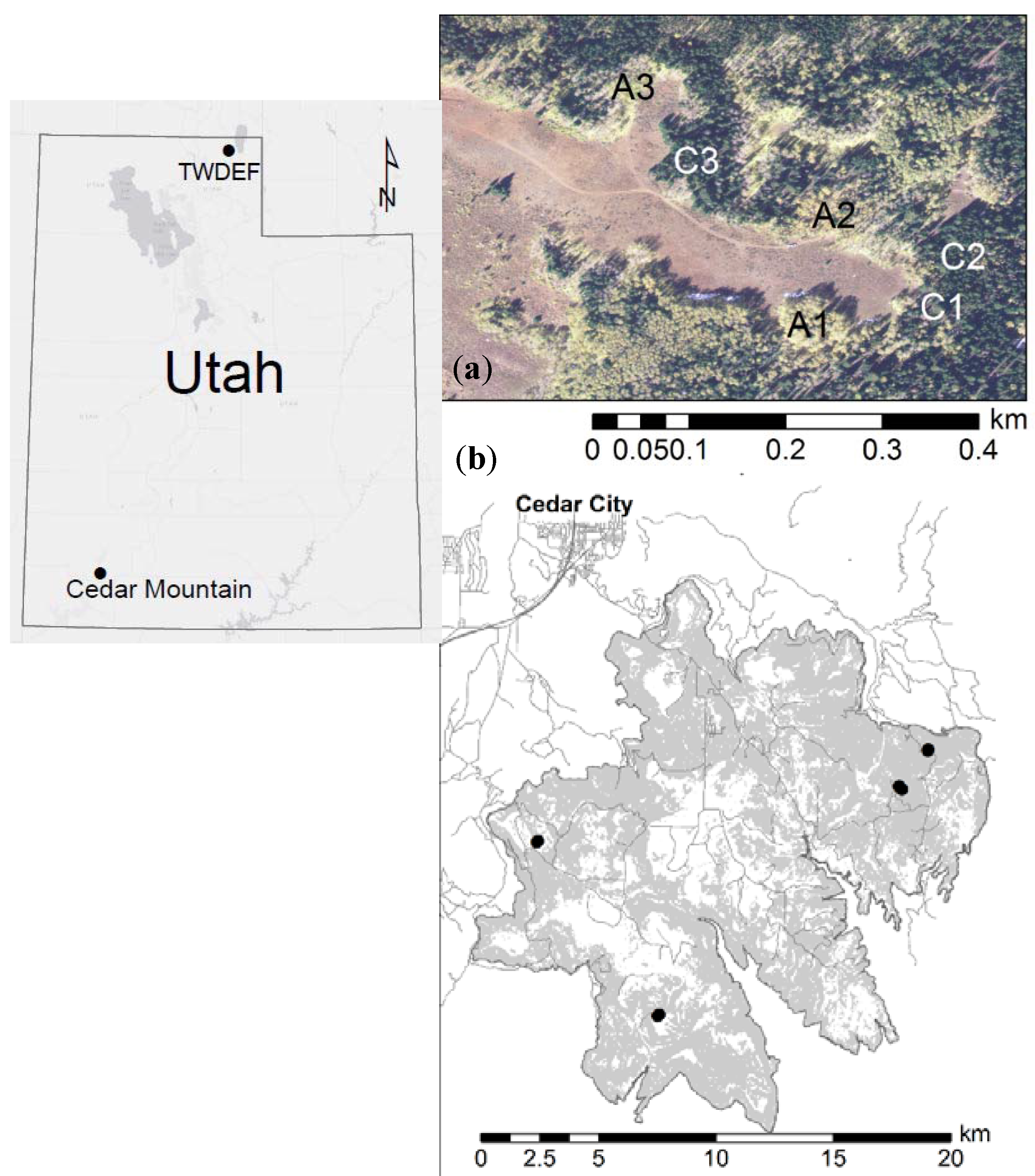
2.1. Site Description
2.2. Field Sampling
2.3. Laboratory Analyses
2.4. Carbon Fluxes
2.4.1. Aboveground C Input
2.4.2. Soil Solution Fluxes
2.4.3. Belowground C Input
2.5. Statistical Analysis
3. Results
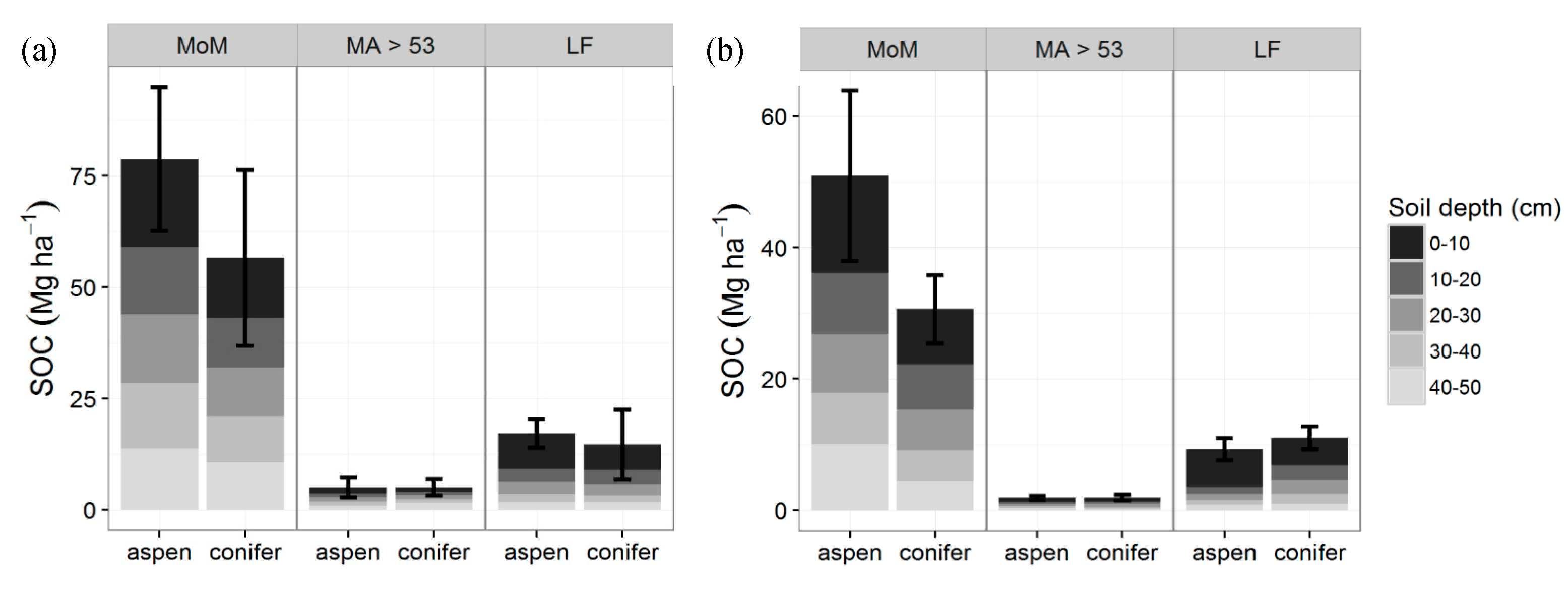
3.1. SOC Distribution under Aspen and Conifer Forest Stands
3.2. Relative Role of Foliage Inputs to SOC Storage
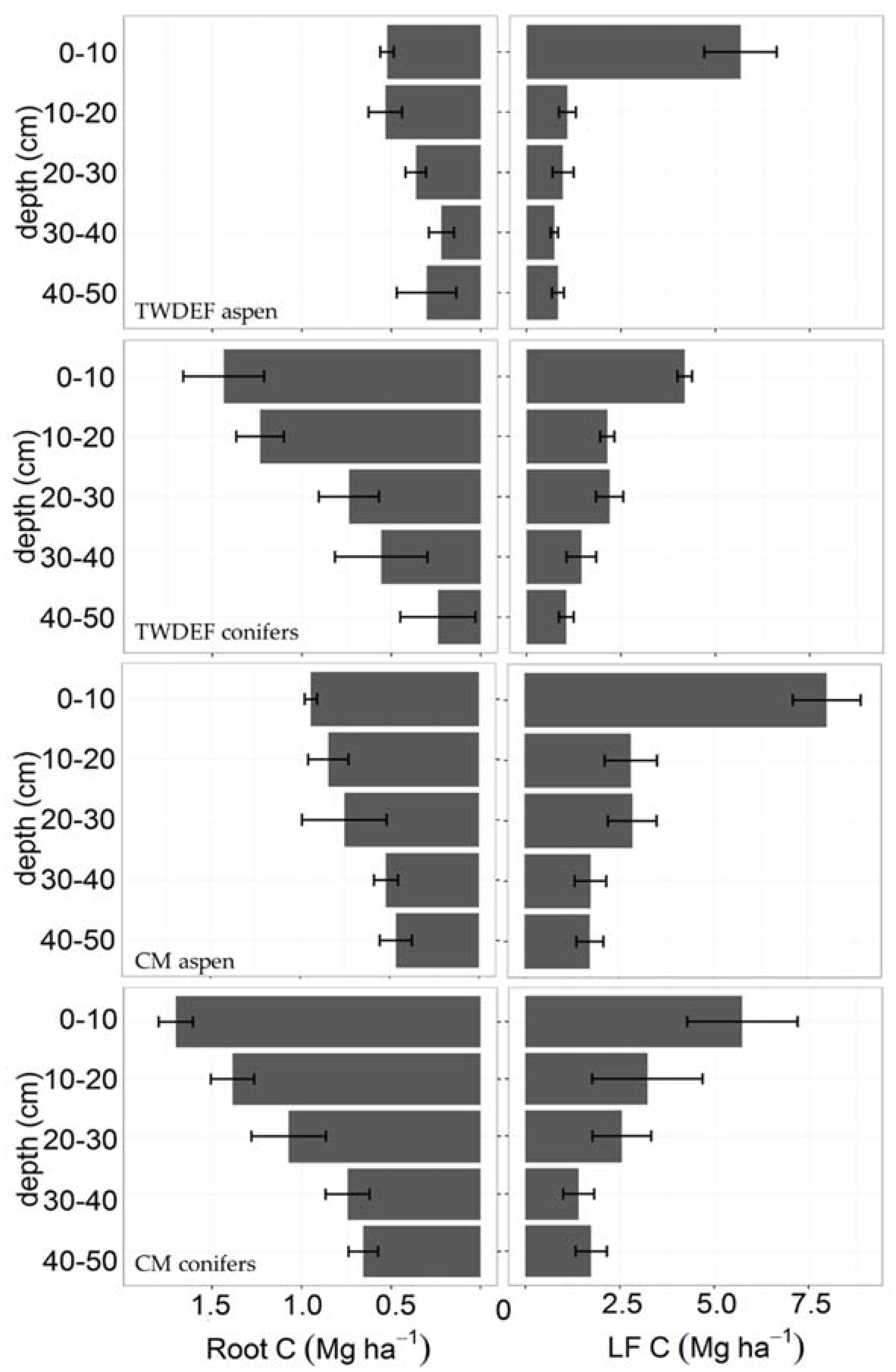
3.3. Relative Role of Root Inputs to SOC Storage
4. Discussion
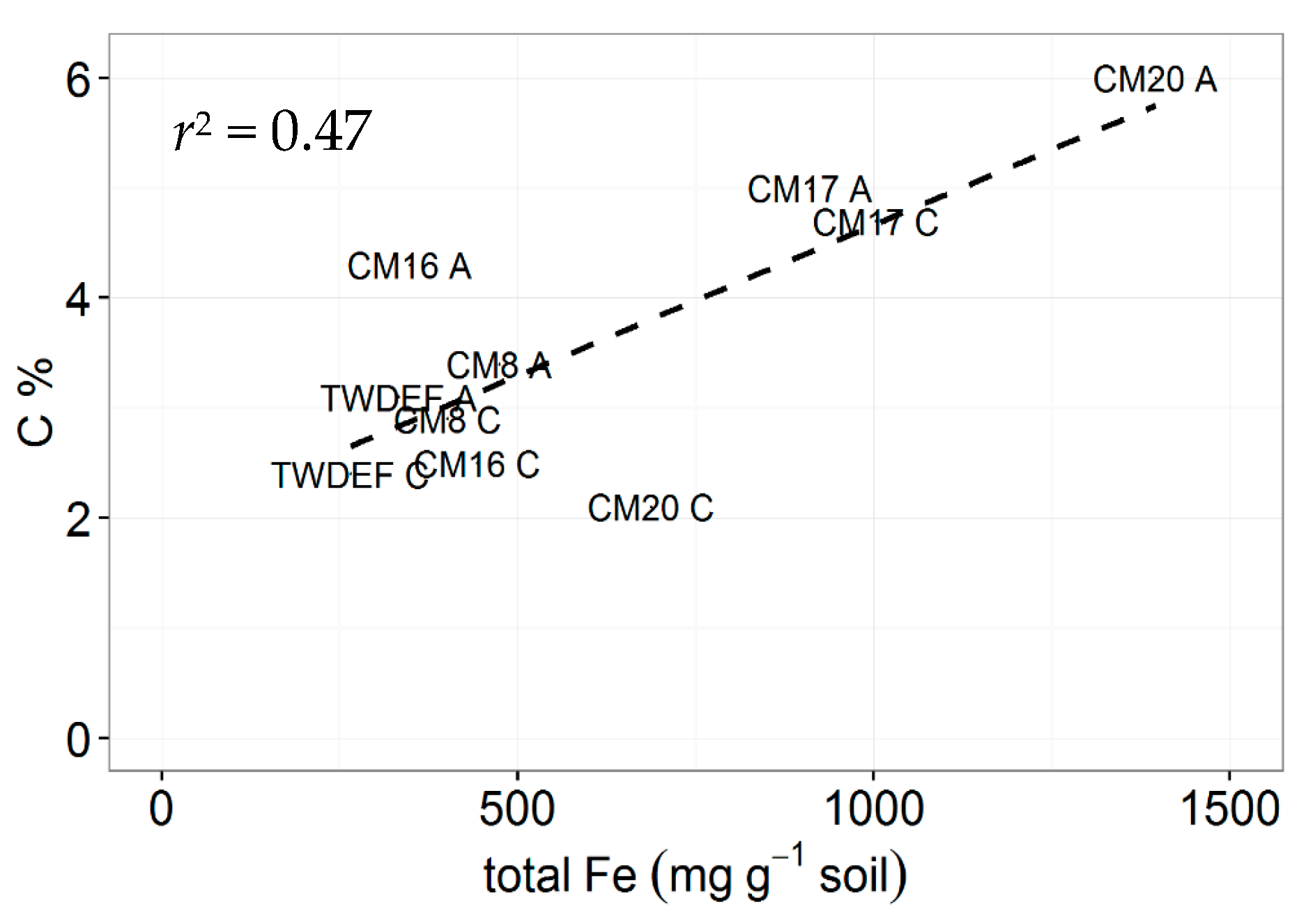
4.1. SOC Pools, and Biotic and Abiotic Controls on SOC and MoM
4.2. Aboveground C Input
4.3. Belowground C Input
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- McKenney, D.W.; Pedlar, J.H.; Lawrence, K.; Hutchinson, M.F.; Kenney, D.W.; Campbell, K. Potential impacts of climate change on the distribution of North American trees. Bioscience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- Jensen, V. Decomposition of Angiosperm Tree Leaf Litter. In Biology of Plant Litter Decomposition; Academic Press: London, UK, 1974; pp. 69–104. [Google Scholar]
- Millar, C.S. Decomposition of Coniferous Leaf Litter. In Biology of Plant Litter Decomposition; Academic Press: London, UK, 1974; pp. 105–128. [Google Scholar]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For. Ecol. Manag. 2011, 262, 2008–2023. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Leide, J.; Riedel, M.; Zemke, J.; Kögel-Knabner, I. Distribution of cutin and suberin biomarkers under forest trees with different root systems. Plant Soil 2014, 381, 95–110. [Google Scholar] [CrossRef]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Woldeselassie, M.; van Miegroet, H.; Gruselle, M.-C.; Hambly, N. Storage and stability of soil organic carbon in aspen and conifer forest soils of northern Utah. Soil Sci. Soc. Am. J. 2012, 76, 2230. [Google Scholar] [CrossRef]
- Rogers, P. Using Forest Health Monitoring to assess aspen forest cover change in the southern Rockies ecoregion. For. Ecol. Manag. 2002, 155, 223–236. [Google Scholar] [CrossRef]
- Kulakowski, D.; Veblen, T.T.; Drinkwater, S. The persistence of quaking aspen (Populus Tremuloides) in the Grand Mesa area, Colorado. Ecol. Appl. 2004, 14, 1603–1614. [Google Scholar] [CrossRef]
- Di Orio, A.P.; Callas, R.; Schaefer, R.J. Forty-eight year decline and fragmentation of aspen (Populus tremuloides) in the South Warner Mountains of California. For. Ecol. Manag. 2005, 206, 307–313. [Google Scholar] [CrossRef]
- Román Dobarco, M.; van Miegroet, H. Soil organic carbon storage and stability in the aspen-conifer ecotone in montane forests in Utah State, USA. Forests 2014, 5, 666–688. [Google Scholar] [CrossRef]
- Natural Resources Conservation Service. Snow Telemetry (SNOTEL) Precipitation and Air Temperature Data for T. W. Daniels Experimental Forest (Utah). Available online: http://wcc.sc.egov.usda.gov/nwcc/site?sitenum=1098 (accessed on 15 October 2016).
- Wadleigh, L.; Jenkins, M.J. Fire frequency and the vegetative mosaic of a spruce-fir forest in northern Utah. Gt. Basin Nat. 1996, 56, 28–37. [Google Scholar]
- Dover, J.H. Geologic Map of the Logan 30ʹ X 60ʹ Quadrangle, Cache and Rich Counties, Utah, and Lincoln and Uinta Counties, Wyoming; Geological Survey (U.S.): Denver, CO, USA, 1995.
- Van Miegroet, H.; Boettinger, J.L.; Baker, M.A.; Nielsen, J.; Evans, D.; Stum, A. Soil carbon distribution and quality in a montane rangeland-forest mosaic in northern Utah. For. Ecol. Manag. 2005, 220, 284–299. [Google Scholar] [CrossRef]
- Schimpf, D.J.; Henderson, J.A.; MacMahon, J.A. Some aspects of succession in the spruce-fir forest zone of northern Utah. Gt. Basin Nat. 1980, 40, 1–26. [Google Scholar]
- Natural Resources Conservation Service. Snow Telemetry (SNOTEL) Precipitation and Air Temperature Data for Kolob (Utah). Available online: https://wcc.sc.egov.usda.gov/nwcc/site?sitenum=561 (accessed on 15 October 2016).
- Evans, D. A Spatiotemporal Analysis of Aspen Decline in Southern Utah’s Cedar Mountain, Using Remote Sensing and Geographic Information Systems. Master’s Thesis, Utah State University, Logan, UT, USA, 2010. [Google Scholar]
- McNab, W.H.; Avers, P.E. Ecological Subregions of the United States, Section Descriptions; USDA Forest Service: Washington, DC, USA, 1994; p. 267.
- DeRose, R.J.; Long, J.N. Disturbance, structure, and composition: Spruce beetle and Engelmann spruce forests on the Markagunt Plateau, Utah. For. Ecol. Manag. 2007, 244, 16–23. [Google Scholar] [CrossRef]
- Bowns, J.E.; Bagley, C.F. Vegetation responses to long-term sheep grazing on mountain ranges. J. Range Manag. 1986, 39, 431–434. [Google Scholar] [CrossRef]
- Kaiser, M.; Ellerbrock, R.H.; Sommer, M. Separation of coarse organic particles from bulk surface soil samples by electrostatic attraction. Soil Sci. Soc. Am. J. 2009, 73, 2118–2130. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Bu, X.; Wang, L.; Ma, W.; Yu, X.; McDowell, W.H.; Ruan, H. Spectroscopic characterization of hot-water extractable organic matter from soils under four different vegetation types along an elevation gradient in the Wuyi Mountains. Geoderma 2010, 159, 139–146. [Google Scholar] [CrossRef]
- Smucker, A.J.M.; McBurney, S.L.; Srivastava, A.K. Quantitative separation of roots from compacted soil profiles by the hydropneumatic elutriation system. Agron. J. 1982, 74, 500. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Dilling, J.; Kaiser, K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry. Water Res. 2002, 36, 5037–5044. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Raich, J.W.; Nadelhoffer, K.J. Belowground carbon allocation in forest ecosystems: Global trends. Ecology 1989, 70, 1346–1354. [Google Scholar] [CrossRef]
- Olsen, H.R.; van Miegroet, H. Factors affecting carbon dioxide release from forest and rangeland soils in northern Utah. Soil Sci. Soc. Am. J. 2010, 74, 282. [Google Scholar] [CrossRef]
- Zak, D.R.; Grigal, D.F.; Ohmann, L.F. Kinetics of microbial respiration and nitrogen mineralization in great lakes forests. Soil Sci. Soc. Am. J. 1993, 57, 1100. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garrten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A Review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Subke, J.A.; Inglima, I.; Cotrufo, M.F. Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Glob. Chang. Biol. 2006, 12, 921–943. [Google Scholar] [CrossRef]
- Johnson, M.G.; Tingey, D.T.; Phillips, D.L.; Storm, M.J. Advancing fine root research with minirhizotrons. Environ. Exp. Bot. 2001, 45, 263–289. [Google Scholar] [CrossRef]
- Steele, S.J.; Gower, S.T.; Vogel, J.G.; Norman, J.M. Root mass, net primary production and turnover in aspen, jack pine and black spruce forests in Saskatchewan and Manitoba, Canada. Tree Physiol. 1997, 17, 577–587. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Volume 1. [Google Scholar]
- Champely, S. pwr: Basic Functions for Power Analysis. R Package Version 1.1.3. Available online: http://CRAN.R-project.org/package=pwr (accessed on 20 January 2017).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2015. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Mueggler, W.F. Age distribution and reproduction of intermountain aspen stands. West. J. Appl. For. 1989, 4, 41–45. [Google Scholar]
- Strahm, B.D.; Harrison, R.B. Controls on the sorption, desorption and mineralization of low-molecular-weight organic acids in variable-charge soils. Soil Sci. Soc. Am. J. 2008, 72, 1653. [Google Scholar] [CrossRef]
- Kaiser, K.; Zech, W. Sorption of dissolved organic nitrogen by acid subsoil horizons and individual mineral phases. Eur. J. Soil Sci. 2000, 51, 403–411. [Google Scholar] [CrossRef]
- Woldeselassie, M.K. Soil Organic Carbon and Site Characteristics in Aspen and Evaluation of the Potential Effects of Conifer Encroachment on Soil Properties in Northern Utah. Master’s Thesis, Utah State University, Logan, UT, USA, 2009. [Google Scholar]
- Alban, D.H.; Perala, D.A.; Schlaegel, B.E. Biomass and nutrient distribution in aspen, pine, and spruce stands on the same soil type in Minnesota. Can. J. For. Res. 1978, 8, 290–299. [Google Scholar] [CrossRef]
- Weishampel, P.; Kolka, R.; King, J.Y. Carbon pools and productivity in a 1-km2 heterogeneous forest and peatland mosaic in Minnesota, USA. For. Ecol. Manag. 2009, 257, 747–754. [Google Scholar] [CrossRef]
- Gower, S.T.; Vogel, J.G.; Norman, M.; Kucharik, C.J.; Steele, S.J. Carbon distribution and aboveground net primary production in aspen, jack pine, and black spruce stands in Saskatchewan and Manitoba, Canada. J. Geophys. Res. Biogeosci. 1997, 102, 29029–29041. [Google Scholar] [CrossRef]
- Laganiére, J.; Paré, D.; Bergeron, Y.; Chen, H.Y.H.; Brassard, B.W.; Cavard, X. Stability of soil carbon stocks varies with forest composition in the Canadian boreal biome. Ecosystems 2013, 16, 852–865. [Google Scholar] [CrossRef]
- Laganière, J.; Boča, A.; van Miegroet, H.; Paré, D. A tree species effect on soil that is consistent across the species’ range: The case of aspen and soil carbon in north America. Forests 2017, 8. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D.; Bergeron, Y.; Chen, H.Y.H. Black spruce soils accumulate more uncomplexed organic matter than aspen soils. Soil Sci. Soc. Am. J. 2011, 75, 1125. [Google Scholar] [CrossRef]
- Giardina, C.P.; Rhoades, C.C. Clear cutting and burning affect nitrogen supply, phosphorus fractions and seedling growth in soils from a Wyoming lodgepole pine forest. For. Ecol. Manag. 2001, 140, 19–28. [Google Scholar] [CrossRef]
- Boča, A.; van Miegroet, H.; Gruselle, M.-C. Forest overstory effect on soil organic carbon storage: A meta-analysis. Soil Sci. Soc. Am. J. 2014, 78, S35. [Google Scholar] [CrossRef]
- Vogt, K.A.; Grier, C.C.; Vogt, D.J. Production, turnover, and nutrient dynamics of above- and belowground detritus of world forests. Adv. Ecol. Res. 1986, 15, 303–377. [Google Scholar]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Fire effects on temperate forest soil C and N storage. Ecol. Appl. 2011, 21, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Van Miegroet, H.; Olsson, M. Ecosystem disturbance and soil organic carbon—A review. In Soil Carbon in Sensitive European Ecosystems: From Science to Land Management; Jandl, R., Rodeghiero, M., Olsson, M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 85–117. [Google Scholar]
- Bartos, D.L.; Debyle, N.V. Quantity, decomposition, and nutrient dynamics of aspen litterfall in Utah. For. Sci. 1981, 27, 381–390. [Google Scholar]
- Gower, S.T.; Hunter, A.; Campbell, J.; Vogel, J.; Veldhuis, H.; Harden, J.; Trumbore, S.; Norman, J.M.; Kucharik, C.J. Nutrient dynamics of the southern and northern BOREAS boreal forests. Ecoscience 2000, 7, 481–490. [Google Scholar] [CrossRef]
- Fröberg, M.; Hansson, K.; Kleja, D.B.; Alavi, G. Dissolved organic carbon and nitrogen leaching from Scots pine, Norway spruce and silver birch stands in southern Sweden. For. Ecol. Manag. 2011, 262, 1742–1747. [Google Scholar] [CrossRef]
- Fröberg, M.; Jardine, P.M.; Hanson, P.J.; Swanston, C.W.; Todd, D.E.; Tarver, J.R.; Garten, C.T. Low dissolved organic carbon input from fresh litter to deep mineral soils. Soil Sci. Soc. Am. J. 2007, 71, 347. [Google Scholar] [CrossRef]
- Leppälammi-Kujansuu, J.; Aro, L.; Salemaa, M.; Hansson, K.; Kleja, D.B.; Helmisaari, H.-S. Fine root longevity and carbon input into soil from below- and aboveground litter in climatically contrasting forests. For. Ecol. Manag. 2014, 326, 79–90. [Google Scholar] [CrossRef]
- Hansson, K.; Helmisaari, H.-S.; Sah, S.P.; Lange, H. Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For. Ecol. Manag. 2013, 309, 58–65. [Google Scholar] [CrossRef]
- Kuptz, D.; Fleischmann, F.; Matyssek, R.; Grams, T.E.E. Seasonal patterns of carbon allocation to respiratory pools in 60-year-old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytol. 2011, 191, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.; Tessier, J.; Morrison, I.; Scarratt, J.; Canning, B.; Klironomos, J. Soil depth distribution of ecto- and arbuscular mycorrhizal fungi associated with Populus tremuloides within a 3-year-old boreal forest clear-cut. Appl. Soil Ecol. 2002, 19, 209–216. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Clemente, J.S.; Simpson, A.J.; Simpson, M.J. Association of specific organic matter compounds in size fractions of soils under different environmental controls. Org. Geochem. 2011, 42, 1169–1180. [Google Scholar] [CrossRef]
- Dümig, A.; Häusler, W.; Steffens, M.; Kögel-Knabner, I. Clay fractions from a soil chronosequence after glacier retreat reveal the initial evolution of organo–mineral associations. Geochim. Cosmochim. Acta 2012, 85, 1–18. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Kalbitz, K. Cycling downwards—Dissolved organic matter in soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Michalzik, B.; Kalbitz, K.; Park, J.; Solinger, S. Fluxes and concentrations of dissolved organic carbon and nitrogen—A synthesis for temperate forests. Biogeochemistry 2001, 52, 173–205. [Google Scholar] [CrossRef]
- Hansson, K.; Kleja, D.B.; Kalbitz, K.; Larsson, H. Amounts of carbon mineralised and leached as DOC during decomposition of Norway spruce needles and fine roots. Soil Biol. Biochem. 2010, 42, 178–185. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Högberg, P.; Read, D.J. Towards a more plant physiological perspective on soil ecology. Trends Ecol. Evol. 2006, 21, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.P.; Fahey, T.J. Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 2006, 87, 1302–1313. [Google Scholar] [CrossRef]
- Sommer, J.; Dippold, M.A.; Flessa, H.; Kuzyakov, Y. Allocation and dynamics of C and N within plant-soil system of ash and beech. J. Plant Nutr. Soil Sci. 2016, 179, 376–387. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 2003, 23, 375–396. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Román Dobarco, M.; Jacobson, A.R.; Miegroet, H.V. Chemical composition of soil organic carbon from mixed aspen-conifer forests characterized with Fourier transform infrared spectroscopy. Forests 2014, 5, 666–688. [Google Scholar]

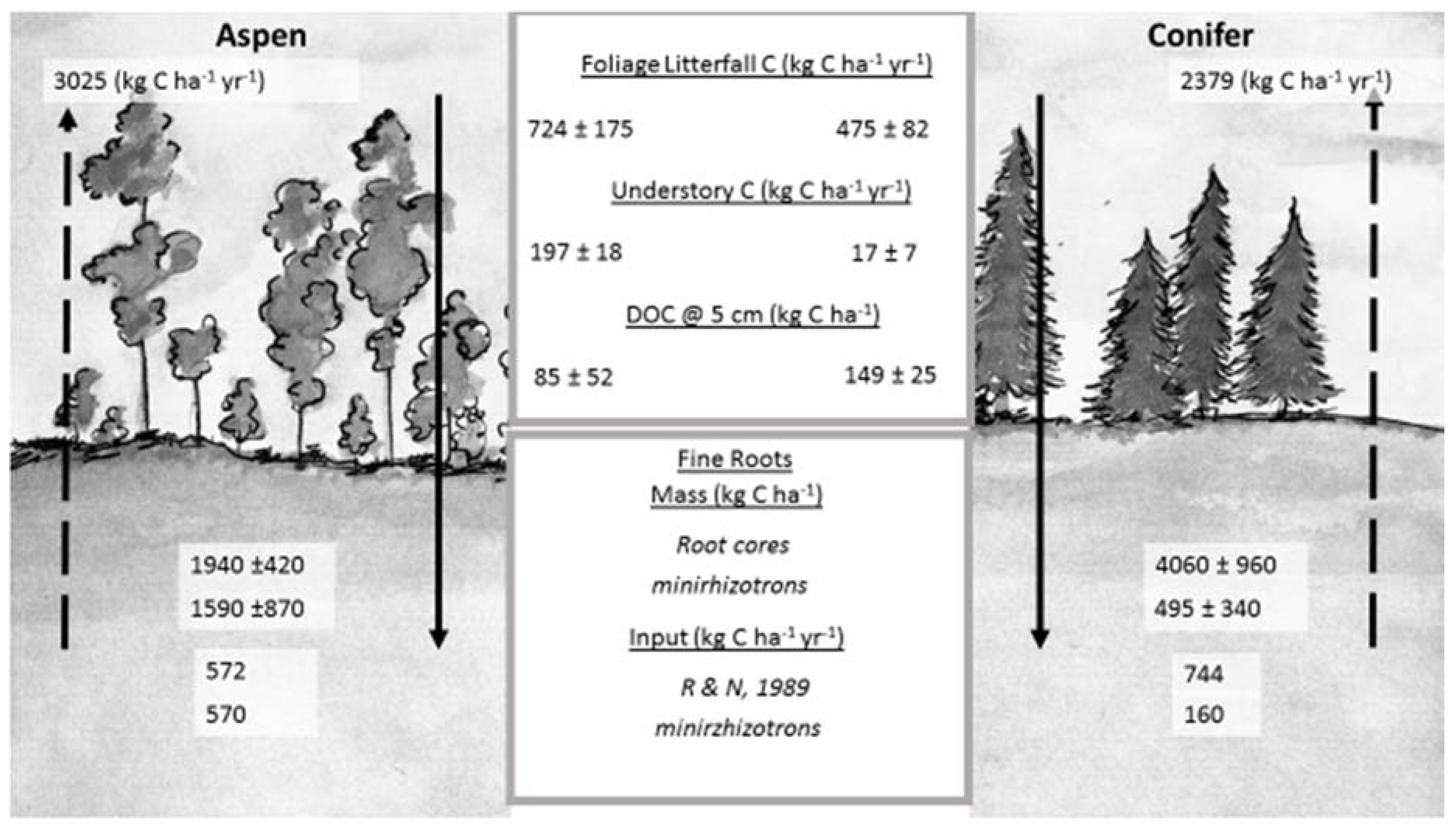
| Aspen | Conifer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | UTM Coordinates | Elev. (m) | Slope (%) | Aspect | LBA (m2·ha−1) | Stems (ha−1) | Soil Texture | UTM Coordinates | Elev. (m) | Slope (%) | Aspect | LBA (m2·ha−1) | stems (ha−1) | Soil Texture |
| CM8 | X: 320149 Y: 4150010 | 2703 | 23 | NW | 54.4 | 639 | Loam | X: 320206 Y: 4150075 | 2699 | 23 | NW | 65.7 | 526 | Loam, clay loam |
| CM16 | X: 3316696 Y: 4161467 | 2680 | 11 | N | 19.7 | 529 | Sandy loam | X: 331651 Y: 4161417 | 2702 | 8 | N | 45.9 | 1298 | Loam |
| CM17 | X: 315048 Y: 4157533 | 2724 | 4 | NW | 19.8 | 2396 | Loam | X: 315004 Y: 4157475 | 2714 | 9 | N | 34.6 | 1403 | Loam |
| CM20 | X: 330427 Y: 4159551 | 2896 | 11 | W | 34.7 | 1057 | Sandy loam | X: 330542 Y: 4159749 | 2892 | 15 | N | 45.6 | 1569 | Sandy loam |
| TWDEF * | X: 0457840 Y: 4634963 | 2634–2649 | 1–11 | SSE–SE | 48.7 | 1949 | Loam, clay loam | X: 0457952 Y: 4634897 | 2636–2659 | 1–9 | SSE–SE | 56.4 | 3138 | Loam, clay loam |
| Year | DOC | |||
|---|---|---|---|---|
| Aspen 5 cm | Aspen 45 cm | Conifer 5 cm | Conifer 45 cm | |
| 2014 | 56.26 ± 2.35 | 49.20 ± 4.56 | 177.61 ± 152.82 | 82.11 ± 97.43 |
| 2015 | 52.81 ± 10.19 | 24.67 ± 5.91 | 137.96 ± 33.14 | 65.11 ± 11.91 |
| 2016 | 145.44 ± 49.23 | 46.66 ± 9.13 | 130.49 ± 27.35 | 67.47 ± 38.39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boča, A.; Van Miegroet, H. Can Carbon Fluxes Explain Differences in Soil Organic Carbon Storage under Aspen and Conifer Forest Overstories? Forests 2017, 8, 118. https://doi.org/10.3390/f8040118
Boča A, Van Miegroet H. Can Carbon Fluxes Explain Differences in Soil Organic Carbon Storage under Aspen and Conifer Forest Overstories? Forests. 2017; 8(4):118. https://doi.org/10.3390/f8040118
Chicago/Turabian StyleBoča, Antra, and Helga Van Miegroet. 2017. "Can Carbon Fluxes Explain Differences in Soil Organic Carbon Storage under Aspen and Conifer Forest Overstories?" Forests 8, no. 4: 118. https://doi.org/10.3390/f8040118





