Sydowia polyspora Dominates Fungal Communities Carried by Two Tomicus Species in Pine Plantations Threatened by Fusarium circinatum
Abstract
:1. Introduction
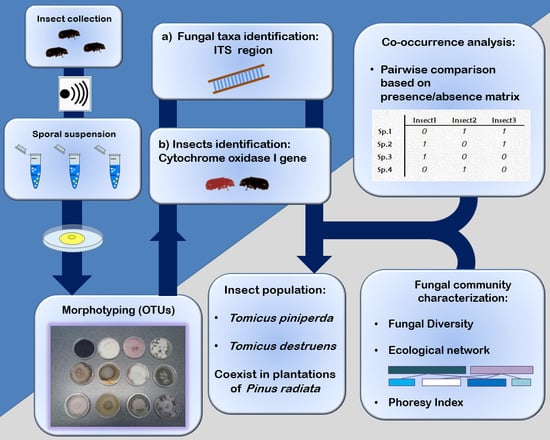
2. Materials and Methods
2.1. Samples Collection
2.2. Molecular Identification of Tomicus Species
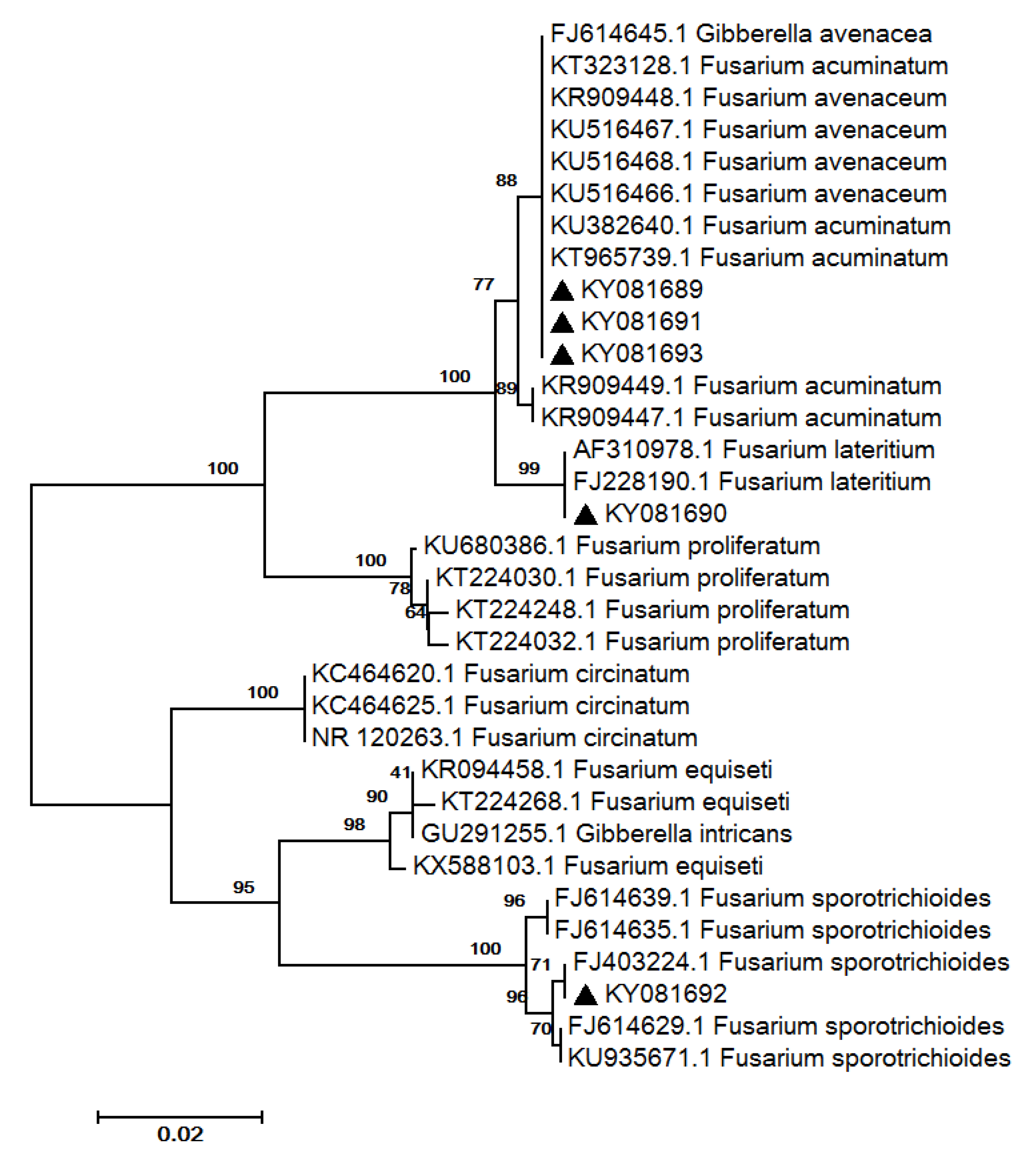
2.3. Fungal Isolation and Identification
2.4. Data Analysis
3. Results
3.1. Shoot Collection and Insect Identification
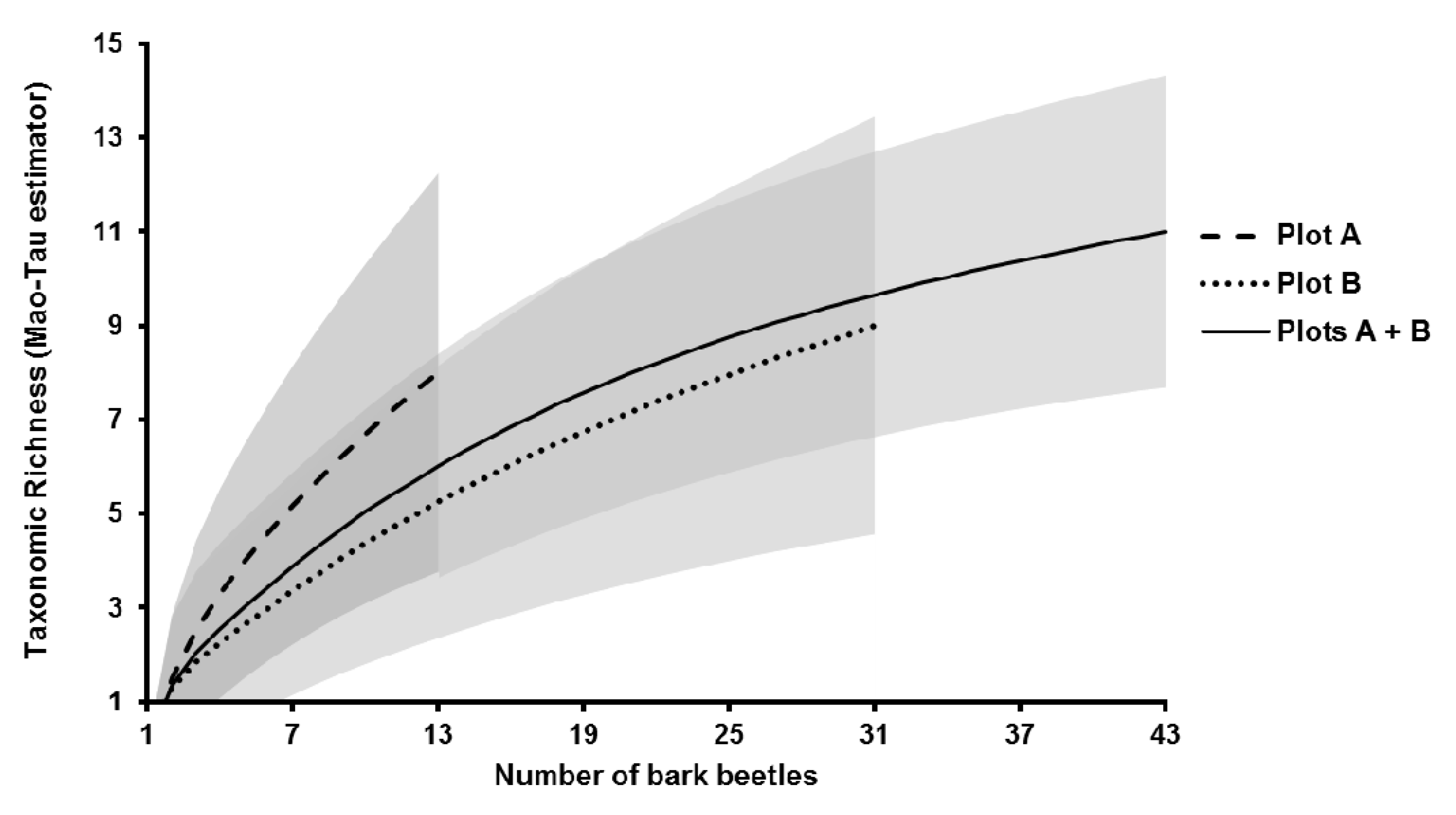
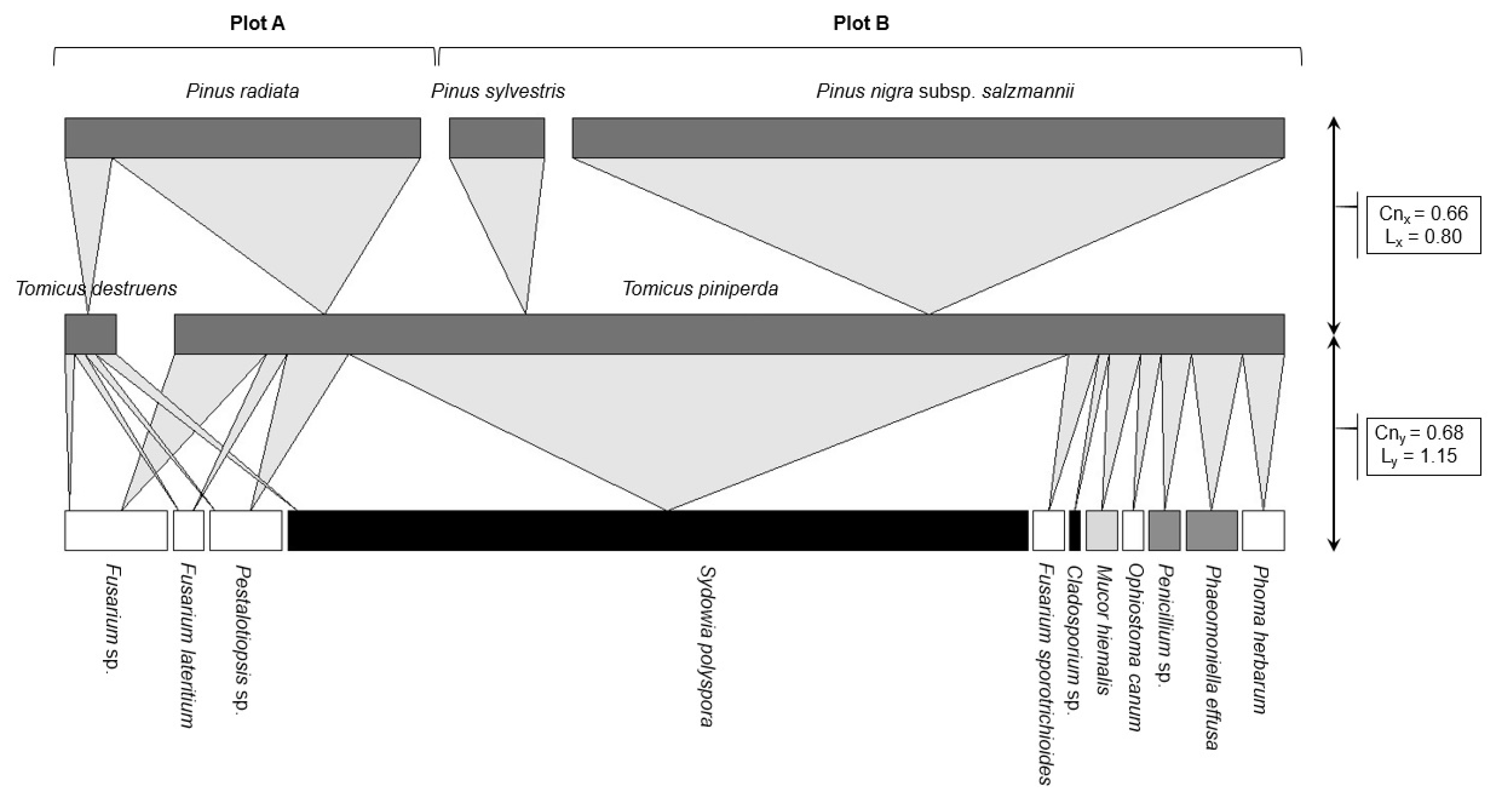
3.2. Fungal Community Characterisation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Six, D.L. Ecological and evolutionary determinants of bark beetle-Fungus symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Beer, D. Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China. Fungal Divers. 2009, 38, 133–145. [Google Scholar]
- Sallé, A.; Monclus, R.; Yart, A.; Garcia, J.; Romary, P.; Lieutier, F. Fungal flora associated with Ips typographus: Frequency, virulence, and ability to stimulate the host defence reaction in relation to insect population levels. Can. J. For. Res. 2005, 35, 365–373. [Google Scholar] [CrossRef]
- Storer, A.J.; Wood, D.L.; Gordon, T.R. Twig beetles, Pityophthorus spp. (Coleoptera: Scolytidae), as vectors of the pitch canker pathogen in California. Can. Entomol. 2004, 136, 685–693. [Google Scholar] [CrossRef]
- Santini, A.; Faccoli, M. Dutch elm disease and elm bark beetles: A century of association. IForest 2014, 8, 126–134. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Bruns, T.D. Fungal community ecology: A hybrid beast with a molecular master. Bioscience 2008, 58, 799–810. [Google Scholar] [CrossRef]
- Aegerter, B.J.; Gordon, T.R.; Storer, A.J.; Wood, D.L. Pitch Canker. A Technical Review; UCANR Publications: Oakland, CA, USA, 2003; p. 13. [Google Scholar]
- Storer, A.J.; Wood, D.L.; Gordon, T.R. Could biological control of wilding pines increase the potential for damage by the pitch canker pathogen? In Managing Wilding Conifers in New Zealand: Present and Future; Hill, R., Zydenbos, S., Bezar, C., Eds.; New Zealand Plant Protection Society: Christchurch, New Zealand, 2004; pp. 97–111. [Google Scholar]
- Brockerhoff, E.G.; Dick, M.; Ganley, R.; Roques, A.; Storer, A.J. Role of insect vectors in epidemiology and invasion risk of Fusarium circinatum, and risk assessment of biological control of invasive Pinus contorta. Biol. Invasions 2016, 18, 1177–1190. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Wright, J.; Heppe, E.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, M.J. A genetically homogenous population of Fusarium circinatum causes pitch canker of Pinus radiata in the Basque Country, Spain. Fungal Biol. 2011, 115, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Romón, P.; Iturrondobeitia, J.C.; Gibson, K.; Lindgren, B.S.; Goldarazena, A. Quantitative association of bark beetles with pitch canker fungus and effects of verbenone on their semiochemical communication in Monterey pine forests in Northern Spain. Environ. Entomol. 2007, 36, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Bezos, D.; Martínez-Álvarez, P.; Diez, J.J.; Fernández, M.M. The pine shoot beetle Tomicus piniperda as a plausible vector of Fusarium circinatum in northern Spain. Ann. For. Sci. 2015, 72, 1079–1088. [Google Scholar] [CrossRef]
- Annila, E.; Långström, B.; Varama, M.; Hiukka, R.; Niemelä, P. Susceptibility of defoliated Scots pine to spontaneous and induced attack by Tomicus piniperda and Tomicus minor. Silva Fenn. 1999, 33, 93–106. [Google Scholar] [CrossRef]
- Fernández, M.M.; Costas, J.M.S. Susceptibility of fire-damaged pine trees (Pinus pinaster and Pinus nigra) to attacks by Ips sexdentatus and Tomicus piniperda (Coleoptera: Scolytidae). Entomol. Gen. 1999, 24, 105–114. [Google Scholar] [CrossRef]
- Haack, R.A.; Poland, T.M. Evolving management strategies for a recently discovered exotic forest pest: the pine shoot beetle, Tomicus piniperda (Coleoptera). Biol. Invasions 2001, 3, 307–322. [Google Scholar] [CrossRef]
- Gallego, D.; Cánovas, F.; Esteve, M.A.; Galián, J. Descriptive biogeography of Tomicus (Coleoptera: Scolytidae) species in Spain. J. Biogeogr. 2004, 31, 2011–2024. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Horn, A.; Lieutier, F.; Branco, M.; Kerdelhué, C. Distribution and population genetic structure of the Mediterranean pine shoot beetle Tomicus destruens in the Iberian Peninsula and Southern France. Agric. For. Entomol. 2006, 8, 103–111. [Google Scholar] [CrossRef]
- Horn, A.; Kerdelhué, C.; Lieutier, F.; Rossi, J.P. Predicting the distribution of the two bark beetles Tomicus destruens and Tomicus piniperda in Europe and the Mediterranean region. Agric. For. Entomol. 2012, 14, 358–366. [Google Scholar] [CrossRef]
- Horn, A.; Roux-Morabito, G.; Lieutier, F.; Kerdelhue, C. Phylogeographic structure and past history of the circum-Mediterranean species Tomicus destruens Woll. (Coleoptera: Scolytinae). Mol. Ecol. 2006, 15, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Gallego, D.; Galián, J. Hierarchical structure of mitochondrial lineages of Tomicus destruens (Coleoptera, Scolytidae) related to environmental variables. J. Zool. Syst. Evol. Res. 2008, 46, 331–339. [Google Scholar] [CrossRef]
- Ambourn, A.K.; Juzwik, J.; Eggers, J.E. Flight periodicities, phoresy rates, and levels of Pseudopityophthorus minutissimus branch colonization in oak wilt centers. For. Sci. 2006, 52, 243–250. [Google Scholar]
- Faccoli, M. Morphological separation of Tomicus piniperda and T. destruens (Coleoptera: Curculionidae: Scolytinae): New and old characters. Eur. J. Entomol. 2006, 103, 433–442. [Google Scholar] [CrossRef]
- Vainio, E.J.; Korhonen, K.; Hantula, J. Genetic variation in Phlebiopsis gigantea as detected with random amplified microsatellite (RAMS) markers. Mycol. Res. 1998, 102, 187–192. [Google Scholar] [CrossRef]
- Kohlmayr, B.; Riegler, M.; Wegensteiner, R.; Stauffer, C. Morphological and genetic identification of the three pine pests of the genus Tomicus (Coleoptera, Scolytidae) in Europe. Agric. For. Entomol. 2002, 4, 151–157. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Lacap, D.C.; Hyde, K.D.; Liew, E.C.Y. An evaluation of the fungal “morphotype” concept based on ribosomal DNA sequences. Fungal Divers. 2003, 12, 53–66. [Google Scholar]
- Stenlid, J.; Karlsson, J.-O.; Högberg, N. Intraspecific genetic variation in Heterobasidion annosum revealed by amplification of minisatellite DNA. Mycol. Res. 1994, 98, 57–63. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing Professional: Ames, IA, USA, 2006; p. 388. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zak, J.C.; Willing, M.R. Fungal Biodiversity Patterns. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier Academic Press: Boston, MA, USA, 2004; p. 777. [Google Scholar]
- Camargo, J.A. Must dominance increase with the number of subordinate species in competitive iteractions? J. Theor. Biol. 1993, 161, 537–542. [Google Scholar] [CrossRef]
- Colwell, R.K. Estimates: Statistical Estimation of Species Richness and Shared Species from Samples, Version 9 User’s Guide and Application. 2011. Available online: http://viceroy.colorado.edu/estimates/ (accessed on 30 March 2017).
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Ne’eman, G.; Dafni, A.; Potts, S.G. A new pollination probability index (PPI) for pollen load analysis as a measure for pollination effectiveness of bees. J. Apic. Res. 1999, 38, 19–23. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Volume 55, pp. 275–286. [Google Scholar]
- De Mendiburu, F. Una Herramienta de Análisis Estadístico para la Investigación Agrícola; Universidad Nacional de Ingeniería (UNI-PERU): Miraflores, Perú, 2009; p. 248. [Google Scholar]
- Signorell, A.; Aho, K.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Bolker, B.; Caeiro, F.; Champely, S.; Chessel, D.; et al. DescTools: Tools for Descriptive Statistics. 2015. Available online: https://cran.r-project.org/web/packages/DescTools/index.html (accessed on 30 March 2017).
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. R News 2008, 8, 8–11. [Google Scholar]
- Dunne, J.A.; Williams, R.J.; Martinez, N.D. Network structure and biodiversity loss in food webs: Robustness increases with connectance. Ecol. Lett. 2002, 5, 558–567. [Google Scholar] [CrossRef]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Robert, V.; Stegehuis, G.; Stalpers, J. The MycoBank Engine and Related Databases. 2005. Available online: http://www.mycobank.org (accessed on 5 March 2017).
- Terhonen, E.; Marco, T.; Sun, H.; Jalkanen, R.; Kasanen, R.; Vuorinen, M.; Asiegbu, F. The effect of latitude, season and needle-age on the mycota of Scots pine (Pinus sylvestris) in Finland. Silva Fenn. 2011, 45, 301–317. [Google Scholar] [CrossRef]
- Romón, P.; Troya, M.; de Gamarra, M.E.F.; Eguzkitza, A.; Iturrondobeitia, J.C.; Goldarazena, A. Fungal communities associated with pitch canker disease of Pinus radiata caused by Fusarium circinatum in northern Spain: Association with insects and pathogen-saprophyte antagonistic interactions. Can. J. Plant Pathol. 2008, 30, 241–253. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W.; Kim, J.; Lu, M.; Breuil, C. Determining fungal diversity on Dendroctonus ponderosae and Ips pini affecting lodgepole pine using cultural and molecular methods. Fungal Divers. 2006, 19, 1560–2745. [Google Scholar]
- Botella, L.; Diez, J.J. Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers. 2011, 47, 9–18. [Google Scholar] [CrossRef]
- Giordano, L.; Garbelotto, M.; Nicolotti, G.; Gonthier, P. Characterization of fungal communities associated with the bark beetle Ips typographus varies depending on detection method, location, and beetle population levels. Mycol. Prog. 2013, 12, 127–140. [Google Scholar] [CrossRef]
- Sanz-Ros, A.V.; Müller, M.M.; San Martín, R.; Diez, J.J. Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fungal Biol. 2015, 119, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Romón, P.; Zhou, X.; Iturrondobeitia, J.C.; Wingfield, M.J.; Goldarazena, A. Ophiostoma species (Ascomycetes: Ophiostomatales) associated with bark beetles (Coleoptera: Scolytinae) colonizing Pinus radiata in northern Spain. Can. J. Microbiol. 2007, 53, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Romón, P.; De Beer, Z.W.; Fernández, M.; Diez, J.; Wingfield, B.D.; Wingfield, M.J. Ophiostomatoid fungi including two new fungal species associated with pine root-feeding beetles in northern Spain. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2014, 106, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Davydenko, K.; Vasaitis, R.; Meshkova, V.; Menkis, A. Fungi associated with the red-haired bark beetle, Hylurgus ligniperda (Coleoptera: Curculionidae) in the forest-steppe zone in eastern Ukraine. Eur. J. Entomol. 2014, 111, 561–565. [Google Scholar] [CrossRef]
- Jankowiak, R.; Bilański, P. Fungal flora associated with Tomicus piniperda L. in an area close to a timber yard in southern Poland. J. Appl. Entomol. 2007, 131, 579–584. [Google Scholar] [CrossRef]
- Silva, X.; Terhonen, E.; Sun, H.; Kasanen, R.; Heliövaara, K.; Jalkanen, R.; Asiegbu, F.O. Comparative analyses of fungal biota carried by the pine shoot beetle (Tomicus piniperda L.) in northern and southern Finland. Scand. J. For. Res. 2015, 30, 497–506. [Google Scholar] [CrossRef]
- Millberg, H.; Boberg, J.; Stenlid, J. Changes in fungal community of Scots pine (Pinus sylvestris) needles along a latitudinal gradient in Sweden. Fungal Ecol. 2015, 17, 126–139. [Google Scholar] [CrossRef]
- Jankowiak, R. Fungi associated with Tomicus piniperda in Poland and assessment of their virulence using Scots pine seedlings. Ann. For. Sci. 2006, 63, 801–808. [Google Scholar] [CrossRef]
- Jankowiak, R. Fungi associated with Ips typographus on Picea abies in southern Poland and their succession into the phloem and sapwood of beetle-infested trees and logs. For. Pathol. 2005, 35, 37–55. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Alves-Santos, F.M.; Diez, J.J. In vitro and in vivo Interactions between Trichoderma viride and Fusarium circinatum. Silva Fenn. 2012, 46, 303–316. [Google Scholar] [CrossRef]
- Bezos, D.; Lomba, J.; Martinez-Álvarez, P.; Fernandez, M.; Diez, J. Effects of pruning in monterrey pine plantations affected by Fusarium circinatum. For. Syst. 2012, 21, 481–488. [Google Scholar]
- Bateman, C.; Skelton, J.; Bateman, C.; Sigut, M.; Skelton, J.; Smith, K.E. Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environ. Entomol. 2016, 45, 883–890. [Google Scholar]
- Lygis, V.; Vasiliauskaite, I.; Matelis, A.; Pliūra, A.; Vasaitis, R. Fungi in living and dead stems and stumps of Pinus mugo on coastal dunes of the Baltic Sea. Plant Prot. Sci. 2014, 50, 221–226. [Google Scholar]
- Pirttilä, A.M.; Pospiech, H.; Laukkanen, H.; Myllylä, R.; Hohtola, A. Two endophytic fungi in different tissues of Scots pine buds (Pinus sylvestris L.). Microb. Ecol. 2003, 45, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.; Tyszkiewicz, Z.; Piroznikow, E. Fungal succession in relation to volatile organic compounds emissions from Scots pine and Norway spruce leaf litter-decomposing fungi. Atmos. Environ. 2016, 131, 301–306. [Google Scholar] [CrossRef]
- Talgø, V.; Chastagner, G.; Thomsen, I.M.; Cech, T.; Riley, K.; Lange, K.; Klemsdal, S.S.; Stensvand, A. Sydowia polyspora associated with current season needle necrosis (CSNN) on true fir (Abies spp.). Fungal Biol. 2010, 114, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Kurek, M. The early stages of fungal succession in Pinus sylvestris phloem and sapwood infested by Tomicus piniperda. Dendrobiology 2006, 56, 27–36. [Google Scholar]
- Boberg, J.B.; Ihrmark, K.; Lindahl, B.D. Decomposing capacity of fungi commonly detected in Pinus sylvestris needle litter. Fungal Ecol. 2011, 4, 110–114. [Google Scholar] [CrossRef]
- Hu, H.L.; Jeewon, R.; Zhou, D.Q.; Zhou, T.X.; Hyde, K.D. Phylogenetic diversity of endophytic Pestalotiopsis species in Pinus armandii and Ribes spp.: Evidence from rDNA and β-tubulin gene phylogenies. Fungal Divers. 2007, 24, 1–22. [Google Scholar]
- Zamora, P.; Martínez-Ruiz, C.; Diez, J.J. Fungi in needles and twigs of pine plantations from northern Spain. Fungal Divers. 2008, 30, 171–184. [Google Scholar]
- Giordano, L.; Gonthier, P.; Varese, G.C.; Miserere, L.; Nicolotti, G. Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers. 2009, 38, 69–83. [Google Scholar]
- Linnakoski, R.; Mahilainen, S.; Harrington, A.; Vanhanen, H.; Eriksson, M.; Mehtätalo, L.; Pappinen, A.; Wingfield, M.J. Seasonal succession of fungi associated with Ips typographus Beetles and their phoretic mites in an outbreak region of Finland. PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hausner, G.; Iranpour, M.; Kim, J.J.; Breuil, C.; Davis, C.N.; Gibb, E.A.; Reid, J.; Loewen, P.C.; Hopkin, A.A. Fungi vectored by the introduced bark beetle Tomicus piniperda in Ontario, Canada, and comments on the taxonomy of Leptographium lundbergii, Leptographium terebrantis, Leptographium truncatum, and Leptographium wingfieldii. Can. J. Bot. 2005, 83, 1222–1237. [Google Scholar] [CrossRef]
- Peverieri, G.S.; Capretti, P.; Tiberi, R. Associations between Tomicus destruens and Leptographium spp. in Pinus pinea and P. pinaster stands in Tuscany, central Italy. For. Pathol. 2006, 36, 14–20. [Google Scholar] [CrossRef]
- Linnakoski, R.; De Beer, Z.W.; Duong, T.A.; Niemelä, P.; Pappinen, A.; Wingfield, M.J. Grosmannia and Leptographium spp. associated with conifer-infesting bark beetles in Finland and Russia, including Leptographium taigense sp. nov. Antonie van Leeuwenhoek 2012, 102, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.M.; García, A.E.; Lieutier, F. Effects of various densities of Ophiostoma ips inoculations on Pinus sylvestris in north-western Spain. For. Pathol. 2004, 34, 1–11. [Google Scholar]
- Solheim, H.; Krokene, P.; Langström, B. Effects of growth and virulence of associated blue-stain fungi on host colonization behaviour of the pine shoot beetles Tomicus minor and T. piniperda. Plant Pathol. 2001, 50, 111–116. [Google Scholar] [CrossRef]
- Masuya, H.; Yamaoka, Y.; Kaneko, S.; Yamaura, Y. Ophiostomatoid fungi isolated from Japanese red pine and their relationships with bark beetles. Mycoscience 2009, 50, 212–223. [Google Scholar] [CrossRef]
- Jankowiak, R.; Rossa, R. Associations between Pityogenes bidentatus and fungi in young managed Scots pine stands in Poland. For. Pathol. 2008, 38, 169–177. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F.; Abdel-Salam, A.H. Natural incidence of Cladosporium spp. as a bio-control agent against whiteflies and aphids in Egypt. J. Appl. Entomol. 2003, 127, 228–235. [Google Scholar] [CrossRef]
- Eken, C.; Hayat, R. Preliminary evaluation of Cladosporium cladosporioides (Fresen.) de Vries in laboratory conditions, as a potential candidate for biocontrol of Tetranychus urticae Koch. World J. Microbiol. Biotechnol. 2009, 25, 489–492. [Google Scholar] [CrossRef]
- Campanile, G.; Ruscelli, A.; Luisi, N. Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. Eur. J. Plant Pathol. 2007, 117, 237–246. [Google Scholar] [CrossRef]
- Jensen, B.D.; Knorr, K.; Nicolaisen, M. In vitro competition between Fusarium graminearum and Epicoccum nigrum on media and wheat grains. Eur. J. Plant Pathol. 2016. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Fernández-González, R.A.; Sanz-Ros, A.V.; Pando, V.; Diez, J.J. Two fungal endophytes reduce the severity of pitch canker disease in Pinus radiata seedlings. Biol. Control 2016, 94, 1–10. [Google Scholar] [CrossRef]
- Meskens, C.; McKenna, D.; Hance, T.; Windsor, D. Host plant taxonomy and phenotype influence the structure of a Neotropical host plant–hispine beetle food web. Ecol. Entomol. 2011, 36, 480–489. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Savage, J.; Bullock, J.M.; Nowakowski, M.; Orr, R.; Tallowin, J.R.B.; Pywell, R.F. Agriculture, ecosystems and environment enhancing beetle and spider communities in agricultural grasslands: The roles of seed addition and habitat management. Agric. Ecosyst. Environ. 2013, 167, 79–85. [Google Scholar] [CrossRef]
- Schigel, D.S. Fungus–beetle food web patterns in boreal forests. Russ. Entomol. J. 2011, 20, 141–150. [Google Scholar]
- Lieutier, F.; Langström, B.; Faccoli, M. The Genus Tomicus. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier Academic Press: Boston, MA, USA, 2015; Volume 1, p. 640. [Google Scholar]
| OTU | GenBank Accession Number of Best Matches | Id (%)/Qc (%) | Description of GenBank Best Match | Suggested Name for OTU | Accession Number |
|---|---|---|---|---|---|
| 1 | HG008754.1 | 100/100 | Sydowia polyspora (Bref. & Tavel) E. Müll. | S. polyspora | KY081694 |
| 2 | KM199339.1 | 99/97 | Pestalotiopsis hawaiiensis Maharachch., K.D. Hyde & Crous | Pestalotiopsis sp. | KY081696 |
| 3 | JX421725.1 | 100/100 | Phoma herbarum Westend | P. herbarum | KY081697 |
| 4 | KU182497.1 | 100/100 | Cladosporium cladosporioides (Fresen.) G.A. de Vries | Cladosporium sp. | KY081699 |
| 5 | JN617665.1 | 100/99 | Penicillium westlingii K.M. Zalessky | Penicillium sp. | KY081700 |
| 6 | JX421733.1 | 99/100 | Phaeomoniella effusa Damm & Crous (synonym: Aequabiliella effusa (Damm & Crous) Crous) | P. effusa | KY081695 |
| 7 | KU184424.1 | 99/100 | Ophiostoma canum (Münch) Syd. & P. Syd. | O. canum | KY081698 |
| 8 | AJ876490.1 | 99/100 | Mucor hiemali Wehmer | M. hiemalis | KY081701 |
| 9 | FJ403224.1 | 99/100 | Fusarium sporotrichioides Sherb. | F. sporotrichioides * | KY081692 |
| 10 | AF310978.1 | 100/100 | Fusarium lateritium Nees | F. lateritium * | KY081690 |
| 11 | KU516466.1 | 100/100 | Fusarium avenaceum (Fr.) Sacc. | Fusarium sp. * | KY081689 |
| 12 | KU516468.1 | 100/100 | F. avenaceum | Fusarium sp. * | KY081691 |
| 13 | KU516467.1 | 100/100 | F. avenaceum | Fusarium sp. * | KY081693 |
| Fungal Taxa | Relative Abundance | Total | PI (%) ± SE | |
|---|---|---|---|---|
| Plot A | Plot B | |||
| Cladosporium sp. | 1 (3.23%) | 0 (0.00%) | 1 (0.88%) | 8.33 × 10−3 ± 8.33 × 10−3 a |
| Fusarium lateritium | 1 (3.23%) | 2 (2.44%) | 3 (2.65%) | 0.15 ± 0.08 a |
| Fusarium sporotrichioides | 0 (0.00%) | 3 (3.66%) | 3 (2.65%) | 0.04 ± 0.04 a |
| Fusarium sp. | 2 (6.45%) | 8 (9.76%) | 10 (8.85%) | 0.74 ± 0.35 a |
| Mucor hiemalis | 1 (3.23%) | 2 (2.44%) | 3 (2.65%) | 0.11 ± 0.09 a |
| Ophiostoma canum | 0 (0.00%) | 2 (2.44%) | 2 (1.77%) | 0.04 ± 0.04 a |
| Penicillium sp. | 3 (9.68%) | 0 (0.00%) | 3 (2.65%) | 0.04 ± 0.04 a |
| Pestalotiopsis sp. | 6 (19.35%) | 1 (1.22%) | 7 (6.19%) | 0.29 ± 0.16 a |
| Phaeomoniella effusa | 2 (6.45%) | 3 (3.66%) | 5 (4.42%) | 0.15 ± 0.10 a |
| Phoma herbarum | 0 (0.00%) | 4 (4.88%) | 4 (3.54%) | 0.15 ± 0.08 a |
| Sydowia polyspora | 15 (48.39%) | 57 (69.51%) | 72 (63.72%) | 37.84 ± 4.30 b |
| Subtotal | 31 | 82 | 113 | |
| Total number of bark beetles | 49 | |||
| Total uncolonised samples | 7 (14.28%) | |||
| Ecological Index | Value |
|---|---|
| Taxonomic richness (Sobs) (plot A/plot B/total) | 8/9/11 |
| ICE | 13.78 |
| Chao1 | 11.00 |
| Chao2 | 12.95 |
| Jack1 | 14.90 |
| Jack2 | 16.86 |
| Shannon (H) | 1.43 |
| Simpson (D) | 0.42 |
| Shannon evenness (J) | 0.59 |
| Simpson evenness (E) | 0.21 |
| Sorensen index (I) | 0.70 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Adalia, E.J.; Sanz-Ros, A.V.; Flores-Pacheco, J.A.; Hantula, J.; Diez, J.J.; Vainio, E.J.; Fernández, M. Sydowia polyspora Dominates Fungal Communities Carried by Two Tomicus Species in Pine Plantations Threatened by Fusarium circinatum. Forests 2017, 8, 127. https://doi.org/10.3390/f8040127
Muñoz-Adalia EJ, Sanz-Ros AV, Flores-Pacheco JA, Hantula J, Diez JJ, Vainio EJ, Fernández M. Sydowia polyspora Dominates Fungal Communities Carried by Two Tomicus Species in Pine Plantations Threatened by Fusarium circinatum. Forests. 2017; 8(4):127. https://doi.org/10.3390/f8040127
Chicago/Turabian StyleMuñoz-Adalia, E. Jordán, Antonio V. Sanz-Ros, Juan A. Flores-Pacheco, Jarkko Hantula, Julio J. Diez, Eeva J. Vainio, and Mercedes Fernández. 2017. "Sydowia polyspora Dominates Fungal Communities Carried by Two Tomicus Species in Pine Plantations Threatened by Fusarium circinatum" Forests 8, no. 4: 127. https://doi.org/10.3390/f8040127