Critical Habitat Elements, with an Emphasis on Coarse Woody Debris, Associated with Ant Presence or Absence in the Moist Cold Sub-Boreal Forests of the Interior of British Columbia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinitz, O.; Heller, J.; Tsoar, A.; Rotem, D.; Kadmon, R. Predicting regional patterns of similarity in species compositions for conservation planning. Conserv. Biol. 2005, 19, 1978–1988. [Google Scholar] [CrossRef]
- Vojta, C.D. Old dog, new tricks: Innovations with presence-Absence information. J. Wildl. Manag. 2005, 69, 845–848. [Google Scholar] [CrossRef]
- MacKenzie, D.I. What are the issues with presence-Absence data for wildlife managers? J. Wildl. Manag. 2005, 69, 849–860. [Google Scholar] [CrossRef]
- Littlewood, N.A.; Young, M.R. A habitat suitability model for the narrow-headed ant, Formica exsecta, evaluated against independent data. Insect Conserv. Divers. 2008, 1, 108–113. [Google Scholar] [CrossRef]
- Worner, S.P.; Gevrey, M. Modelling global insect pest assemblages to determine risk of invasion. J. Appl. Ecol. 2006, 43, 858–867. [Google Scholar] [CrossRef]
- Watts, M.J.; Worner, S.P. Comparing ensemble and cascaded neural networks that combine biotic and abiotic variables to predict insect species distributions. Ecol. Inform. 2008, 3, 354–366. [Google Scholar] [CrossRef]
- Gorb, S.N.; Gorb, E.V.; Punttila, P. Effects of redispersal of seeds by ants on the vegetation pattern in a deciduous forest: A case study. Acta Oecol. 2000, 21, 293–301. [Google Scholar] [CrossRef]
- Nkem, J.N.; de Bruyn, L.A.L.; Grant, C.D.; Hulugalle, N.R. The impact of ant bioturbation and foraging activities on surrounding soil properties. Pedobiologia 2000, 44, 609–621. [Google Scholar] [CrossRef]
- Risch, A.C.; Jurgensen, M.F.; Schutz, M.; Page-Dumroese, D.S. The contribution of red wood ants to soil C and N pools and CO2 emissions in subalpine forests. Ecology 2005, 86, 419–430. [Google Scholar] [CrossRef]
- Punttila, P.; Haila, Y.; Pajunen, T.; Tukia, H. Colonisation of clearcut forests by ants in the southern Finnish taiga: A quantitative survey. Oikos 1991, 61, 250–262. [Google Scholar] [CrossRef]
- Punttila, P.; Haila, Y.; Niemela, J.; Pajunen, T. 1994. Ant communites in fragments of old-growth tiaga and managed surroundings. Ann. Zool. Fennici 1994, 31, 131–144. [Google Scholar]
- Punttila, P.; Haila, Y. Colonisation of a burned forest by ants in the southern Finnish boreal forest. Silva Fennica 1996, 60, 421–435. [Google Scholar] [CrossRef]
- Seppä, P.; Sundström, L.; Punttila, P. Facultative polygyny and habitat succession in boreal ants. Biol. J. Linn. Soc. 1995, 56, 533–551. [Google Scholar] [CrossRef]
- Punttila, P. Succession, forest fragmentation, and the distribution of wood ants. Oikos 1996, 75, 291–298. [Google Scholar] [CrossRef]
- Savolainen, R.; Vepsäläinen, K.; Wuorenrinne, H. Ant assemblages in the tiaga biome: Testing the role of territorial wood ants. Oecologia 1989, 81, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Punttila, P.; Haila, Y.; Tukia, H. Ant communities in taiga clearcuts: Habitat effects and species interactions. Ecography 1996, 19, 16–28. [Google Scholar] [CrossRef]
- Finnegan, R.J. Ants as predators of forest pests. Entomophaga Mem. H. S. 1974, 7, 53–59. [Google Scholar]
- Torgersen, T.R.; Mason, R.R. Predation on egg masses of Douglas-fir tussock moth. Environ. Entomol. 1987, 16, 90–93. [Google Scholar] [CrossRef]
- Way, M.J.; Khoo, K.C. Role of ants in pest management. Ann. Rev. Entomol. 1992, 37, 479–503. [Google Scholar] [CrossRef]
- Wagner, D.; Brown, M.J.F.; Gordon, D.M. Harvester ant nests, soil biota and soil chemistry. Oecologia 1997, 112, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Heithaus, E.R. Seed predation by rodents in three ant-dispersed plants. Ecology 1981, 62, 136–145. [Google Scholar] [CrossRef]
- Brown, J.H.; Reichman, O.J.; Davidson, D.W. Granivory in desert ecosystems. Annu. Rev. Ecol. Evol. Syst. 1979, 10, 201–227. [Google Scholar] [CrossRef]
- Haines, B.L. Element and energy flows through colonies of the leaf-cutting ant, Atta colombica, in Panama. Biotropica 1978, 10, 270–277. [Google Scholar] [CrossRef]
- Torgersen, T.R.; Bull, E.V. Down logs as habitat for forest dwelling ants—The primary prey of pileated woodpeckers in northeastern Oregon. Northwest Sci. 1995, 69, 294–303. [Google Scholar]
- Gyug, L.W.; Higgins, R.J.; Todd, M.A.; Meggs, J.M.; Lindgren, B.S. Dietary dependence of Williamson’s Sapsucker nestlings on ants associated with dead and decaying wood in British Columbia. Can. J. For. Res. 2014, 44, 628–637. [Google Scholar] [CrossRef]
- Noyce, K.V.; Kannowski, P.B.; Riggs, M.R. Black bears as ant-eaters: Seasonal associations between bear myrmecophagy and ant ecology in north-central Minnesota. Can. J. Zool. 1997, 75, 1671–1686. [Google Scholar] [CrossRef]
- Elgmork, K.; Unander, S. Brown bear use of ant mounds in Scandinavia. Ursus 1999, 10, 269–274. [Google Scholar]
- Swenson, J.E.; Jansson, A.; Riig, R.; Sandegren, F. Bears and ants: Myrmecophagy by brown bears in central Scandinavia. Can. J. Zool. 1999, 77, 551–561. [Google Scholar] [CrossRef]
- Lindgren, B.S.; MacIsaac, A.M. A preliminary study of ant diversity and of ant dependence on dead wood in central interior British Columbia. In Proceedings of the Symposium on the Ecology and Management of Dead Wood in Western Forests, Reno, NV, USA, 2–4 November 1999; Shea, P.J., Laudenslayer, W.F., Jr., Valentine, B., Weatherspoon, C.P., Lisle, T.E., Eds.; USDA Forest Service: Albany, CA, USA, 2002; pp. 111–119. [Google Scholar]
- Lafleur, B.; Parsons, W.F.J.; Bradley, R.L.; Francoeur, A. Ground-nesting ant assemblages and their relationships to habitat factors along a chronosequence of postfire-regenerated lichen-spruce woodland. Environ. Entomol. 2006, 35, 1515–1524. [Google Scholar]
- Meidinger, D.V.; Pojar, J. Ecosystems of British Columbia; BC Ministry of Forests: Victoria, BC, Canada, 1991; p. 330. [Google Scholar]
- Maser, C.; Anderson, R.G.; Cromack, K., Jr.; Williams, J.T.; Martin, R.E. Dead and down woody material. In Wildlife Habitats in Managed Forests: The Blue Mountains of Oregon and Washington; Thomas, J.W., Ed.; U.S. Department of Agriculture: Washington, DC, USA, 1979; pp. 78–95. [Google Scholar]
- Menard, S. Applied Logistic Regression Analysis, 2nd ed.; Sage Publications Inc.: Thousand Oaks, CA, USA, 2002; p. 111. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 4857, 1285–1293. [Google Scholar] [CrossRef]
- Bolton, B.; Alpert, G.; Ward, P.S.; Naskrecki, P. Bolton’s Catalogue of the Ants of the World. Available online: http://www.hup.harvard.edu/catalog.php?isbn=9780674021518 (accessed on 19 April 2017).
- Wheeler, G.C.; Wheeler, J. The Ants of North Dakota; The University of North Dakota Press: Grand Forks, ND, USA, 1963; p. 326. [Google Scholar]
- Wheeler, G.C.; Wheeler, J. The Ants of Nevada; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1986; p. 138. [Google Scholar]
- Francoeur, A. Révision taxonomique des espèces néarctiques du group fusca, genre Formica (Formicidae: Hymenoptera). Mém. Soc. Entomol. Québec 1973, 3, 1–316. [Google Scholar]
- Naumann, K.; Preston, W.B.; Ayre, G.L. An annotated checklist of the ants (Hymenoptera: Formicidae) of British Columbia. J. Entomol. Soc. BC 1999, 96, 29–68. [Google Scholar]
- Hansen, L.D.; Klotz, J.H. Carpenter Ants of The United States and Canada; Comstock Publishing Associates: Ithaca, NY, USA, 2005; p. 204. [Google Scholar]
- Higgins, R.J.; Lindgren, B.S. Seral changes in ant (Hymenoptera: Formicidae) assemblages in the sub-boreal forests of British Columbia. Insect Conserv. Divers. 2015, 8, 337–347. [Google Scholar] [CrossRef]
- Kéry, M.; Schmidt, B.R. Imperfect detection and its consequences for conservation. Community Ecol. 2008, 9, 207–216. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990; p. 732. [Google Scholar]
- Higgins, R.J.; Lindgren, B.S. The effect of manipulated shading on the colony abundance of two species of ants, Formica aserva and Leptothorax muscorum, in dead wood. Entomol. Exp. Appl. 2012, 143, 292–300. [Google Scholar] [CrossRef]
- Shane, C.F.; Steyaert, S.M.J.G.; Swenson, J.E.; Storch, I.; Kindberg, J.; Barck, H.; Zedrosser, A. A “clearcut” case? Brown bear selection of coarse woody debris and carpenter ants on clearcuts. For. Ecol. Manag. 2015, 348, 164–173. [Google Scholar]
- Savolainen, R.; Seppä, P. Genetic relatedness among worker nestmates of three formicine slave-making ants. Insect Soc. 1996, 43, 31–36. [Google Scholar] [CrossRef]
- Francoeur, A. The ant fauna near the tree-line in northern Quebec. Nordicana 1983, 47, 177–180. [Google Scholar]
- Billeck, I. Density dependence and colony growth in the ant species Formica neorufibarbis. J. Anim. Ecol. 2001, 70, 895–905. [Google Scholar] [CrossRef]
- Billeck, I. Worker demography in the ant Formica neorufibarbis. Ecol. Entomol. 2003, 28, 139–144. [Google Scholar] [CrossRef]
- Elmes, G.W.; Keller, L. Distribution and Ecology of Queen Numbers in Ants of the Genus Myrmica. In Queen Number and Sociality in Insects; Keller, L., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 294–307. [Google Scholar]
- Heinze, J. Habitat structure, dispersal strategies and queen number in two boreal Leptothorax species. Oecologia 1993, 96, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Stahl, M.; Hölldobler, B. Ecophysiology of hibernation in boreal Leptothorax ants (Hymenoptera: Formicidae). Ecoscience 1996, 3, 429–435. [Google Scholar] [CrossRef]
- Stille, M. Queen/worker thorax volume ratios and nest-founding strategies in ants. Oecologia 1996, 105, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, R.; Sundström, L.; Fortelius, W. Monogyny and polygyny in Formica ants: The result of alternative dispersal tactics. In Queen Number and Sociality in Insects; Keller, L., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 308–333. [Google Scholar]
- Cherix, D.; Chautems, D.; Fletcher, D.J.C.; Fortelius, W.; Gris, G.; Keller, L.; Passera, L.; Rosengren, R.; Vargo, E.L.; Walter, F. Alternative reproductive strategies in Formica lugubris Zett. Ethol. Ecol. Evol. 1991, 1, 61–66. [Google Scholar]
- Heinze, J.; Hölldobler, B. Ants in the cold. Memorabilia Zool. 1994, 48, 99–108. [Google Scholar]
- Akre, R.D.; Hansen, L.D.; Myhre, E.A. Colony size and polygyny in carpenter ants (Hymenoptera: Formicidae). J. Kans. Entomol. Soc. 1994, 67, 1–9. [Google Scholar]
- Wilson, E.O.; Hunt, G.L.J. Habitat selection by the queens of two field-dwelling species of ants. Ecology 1966, 47, 485–487. [Google Scholar] [CrossRef]
- Punttila, P.; Autio, O.; Kotiaho, J.S.; Kotze, D.J.; Loukola, O.J.; Noreika, N.; Vuori, A.; Vepsäläinen, K. The effects of drainage and restoration of pine mires on habitat structure, vegetation and ants. Silva. Fennica. 2016, 50, 1462. [Google Scholar] [CrossRef]
- Savolainen, R.; Vepsäläinen, K. A competition hierarchy among boreal ants: Impact on resource partitioning and community structure. Oikos 1988, 51, 135–155. [Google Scholar] [CrossRef]
- Cerdá, X.; Retana, J.; Cros, S. Thermal disruption of transitive hierarchies in Mediterranean ant communities. J. Anim. Ecol. 1997, 66, 363–374. [Google Scholar] [CrossRef]
- Jonsell, M.; Weslien, J.; Ehnström, B. Substrate requirements of red-listed saproxylic invertebrates in Sweden. Biodivers. Conserv. 1998, 7, 749–764. [Google Scholar] [CrossRef]
- Warren, R.J.; Bradford, M.A. Ant colonization and coarse woody debris decomposition in temperate forests. Insect Soc. 2012, 59, 215–221. [Google Scholar] [CrossRef]
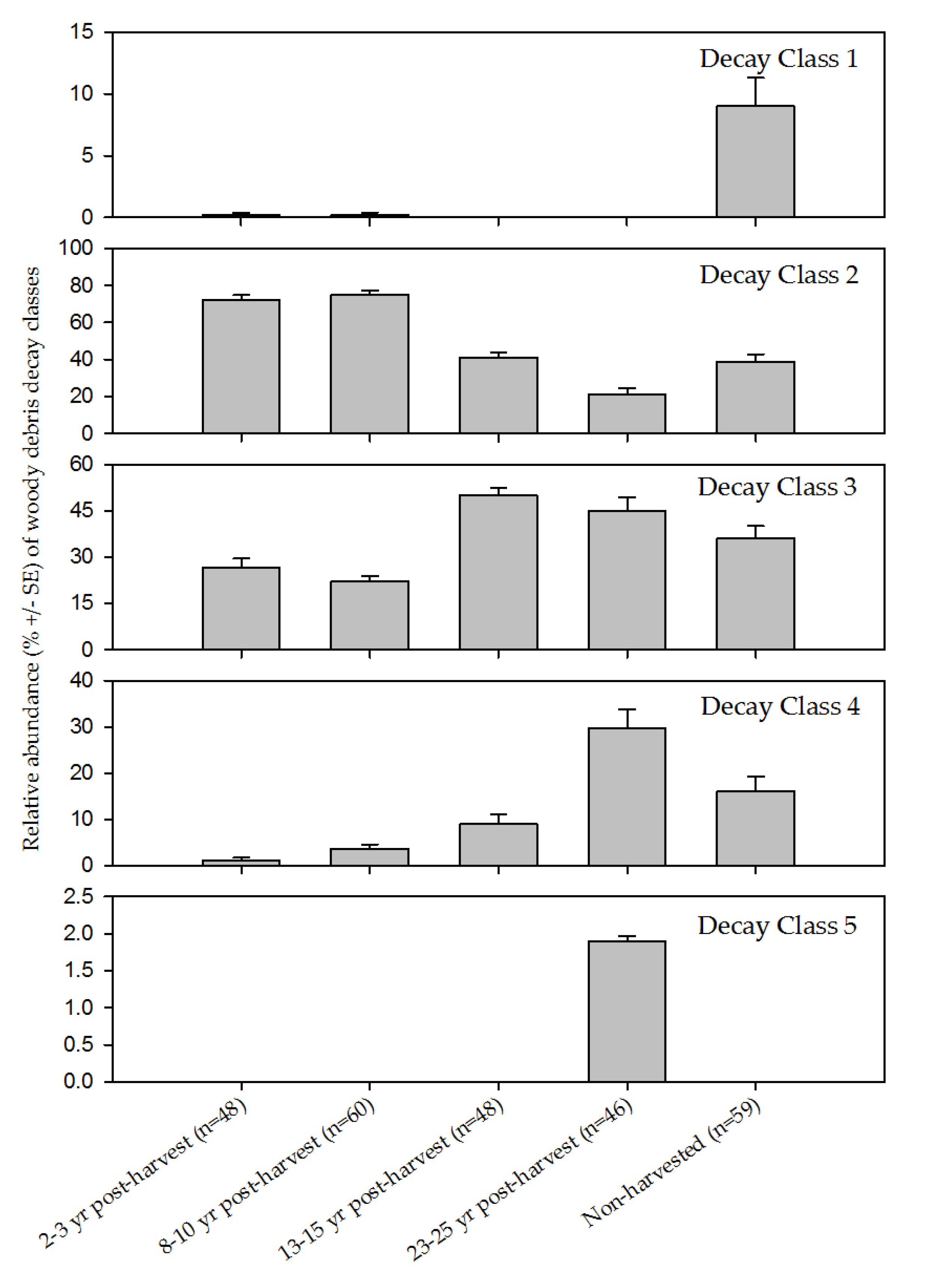
| Model Parameter | All Ants | CAHER | FOASE | FONEO | LECAN | MYALA |
|---|---|---|---|---|---|---|
| Nest Location | ||||||
| Downed Woody Debris | −0.15 (0.05) p = 0.006 | −0.83 (0.16) p < 0.001 | −0.29 (0.08) p < 0.001 | |||
| Stumps | 0.15 (0.05) p = 0.006 | 0.83 (0.16) p < 0.001 | 0.29 (0.08) p < 0.001 | |||
| CWD surface area of piece (m2). | 0.25 (0.12) [1.28] p = 0.038 | 0.79 (0.19) [2.2] p < 0.001 | 0.73 (0.28) [2.08] p = 0.008 | 0.63 (0.15) [1.89] p < 0.001 | ||
| CWD surface area within 50-m2 sample (m2) | −0.05 (0.02) [0.95] p = 0.02 | −1.11 (0.04) [0.89] p = 0.002 | ||||
| Other ant colonies of the same species within 50-m2 sub-section | a | 0.87 (0.10) [0.39] p < 0.001 | 1.11 (0.14) [3.03] p < 0.001 | 0.84 (0.11) [2.31] p < 0.001 | 0.17 (0.02) [1.19] p < 0.001 | 0.44 (0.03) [1.55] p < 0.001 |
| Other colonies of different species with 50-m2 sub-section | a | −0.05 (0.03) [0.95] p = 0.04 | −0.89 (0.25) [0.41] p < 0.001 | |||
| Other colonies of different species in same piece of CWD | a | −0.93 (0.24) [0.41] p < 0.001 | −0.26 (0.11) [0.77] p = 0.02 | −0.41 (0.13) [0.66] p = 0.002 | ||
| Percentage Bark (%) | 0.01 (0.00) [1.01] p = 0.4 | |||||
| Coarse Woody Debris | ||||||
| Decay Class 2 | −0.38 (0.08) p < 0.001 | −0.79 (0.22) p < 0.001 | −0.51 (0.13) p < 0.001 | |||
| Decay Class 3 | 0.20 (0.08) p < 0.001 | 0.10 (0.21) p < 0.001 | 0.15 (0.11) p < 0.001 | |||
| Decay Class 4 | 0.18 (0.12) p = 0.13 | 0.69 (0.30) p = 0.02 | 0.36 (0.16) p = 0.02 | |||
| Seral Age Class | ||||||
| Seral Age 1 (2–3 years post-harvest) | −1.48 (0.16) p < 0.001 | −1.02 (0.35) p = 0.003 | b | b | −1.41 (0.21) p < 0.001 | −1.43 (0.35) p = 0.01 |
| Seral Age 2 (8–10 years post-harvest) | 1.19 (0.10) p < 0.001 | −0.26 (0.22) p = 0.24 | 0.33 (0.11) p = 0.003 | 0.04 (0.17) p = 0.80 | ||
| Seral Age 3 (13–15 years post-harvest) | 1.63 (0.11) p < 0.001 | 0.56 (0.24) p = 0.02 | 0.88 (0.11) p < 0.000 | 0.06 (0.18) p = 0.70 | ||
| Seral Age 4 (23–25 years post-harvest) | 0.63 (0.12) p < 0.001 | 0.72 (0.22) p = 0.001 | b | 0.20 (0.15) p = 0.17 | 1.04 (0.18) p < 0.001 | |
| Seral Age 5 (Non-harvested) | −1.97 (0.24) p < 0.001 | b | b | b | b | 0.27 (0.25) p = 0.27 |
| Receiver Operating Characteristic | 0.79 | 0.81 | 0.86 | 0.72 | 0.78 | 0.88 |
| Ant Species | Seral Age (year) | |||||
|---|---|---|---|---|---|---|
| 2–3 | 8–10 | 13–15 | 23–25 | Non-Harvested | ||
| Camponotus herculeanus | absent | 467 | 736 | 545 | 279 | 298 |
| present | 6 (1.3) | 20 (2.6) | 24 (4.2) | 13 (4.3) | 0 (0) | |
| Formica aserva | absent | 473 | 730 | 540 | 301 | 298 |
| present | 0 (0) | 26 (3.4) | 29 (5.1) | 1 (0.3) | 0 (0) | |
| Formica neorufibarbis | absent | 470 | 709 | 566 | 292 | 298 |
| present | 3 (0.6) | 47 (6.2) | 3 (0.5) | 10 (3.3) | 0 (0) | |
| Leptothorax muscorum | absent | 456 | 537 | 387 | 277 | 297 |
| present | 17 (3.6) | 219 (29.0) | 182 (32.0) | 25 (8.3) | 1 (0.3) | |
| Myrmica alaskensis | absent | 467 | 687 | 434 | 228 | 283 |
| present | 6 (1.3) | 69 (9.1) | 135 (23.7) | 74 (24.5) | 15 (5.0) | |
| Ant Species | Decay Class | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Camponotus herculeanus | absent | 31 | 1258 | 815 | 192 | 8 |
| present | 0 (0) | 20 (1.6) | 32 (3.8) | 7 (3.5) | 0 (0) | |
| Formica aserva | absent | 31 | 1260 | 815 | 194 | 8 |
| present | 0 (0) | 18 (1.4) | 32 (3.8) | 5 (2.5) | 0 (0) | |
| Formica neorufibarbis | absent | 31 | 1248 | 826 | 192 | 7 |
| present | 0 (0) | 30 (2.3) | 21 (2.5) | 7 (3.5) | 1 (12.5) | |
| Leptothorax muscorum | absent | 31 | 1018 | 687 | 183 | 7 |
| present | 0 (0) | 260 (20.3) | 160 (18.9) | 16 (8.0) | 1 (12.5) | |
| Myrmica alaskensis | absent | 31 | 1182 | 699 | 152 | 6 |
| present | 0 (0) | 96 (7.5) | 148 (17.5) | 47 (2.4) | 2 (22.2) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgins, R.J.; Gillingham, M.G.; Lindgren, B.S. Critical Habitat Elements, with an Emphasis on Coarse Woody Debris, Associated with Ant Presence or Absence in the Moist Cold Sub-Boreal Forests of the Interior of British Columbia. Forests 2017, 8, 129. https://doi.org/10.3390/f8040129
Higgins RJ, Gillingham MG, Lindgren BS. Critical Habitat Elements, with an Emphasis on Coarse Woody Debris, Associated with Ant Presence or Absence in the Moist Cold Sub-Boreal Forests of the Interior of British Columbia. Forests. 2017; 8(4):129. https://doi.org/10.3390/f8040129
Chicago/Turabian StyleHiggins, Robert J., Michael G. Gillingham, and B. Staffan Lindgren. 2017. "Critical Habitat Elements, with an Emphasis on Coarse Woody Debris, Associated with Ant Presence or Absence in the Moist Cold Sub-Boreal Forests of the Interior of British Columbia" Forests 8, no. 4: 129. https://doi.org/10.3390/f8040129





