Mixed-Species Effects on Soil C and N Stocks, C/N Ratio and pH Using a Transboundary Approach in Adjacent Common Garden Douglas-Fir and Beech Stands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Descriptions
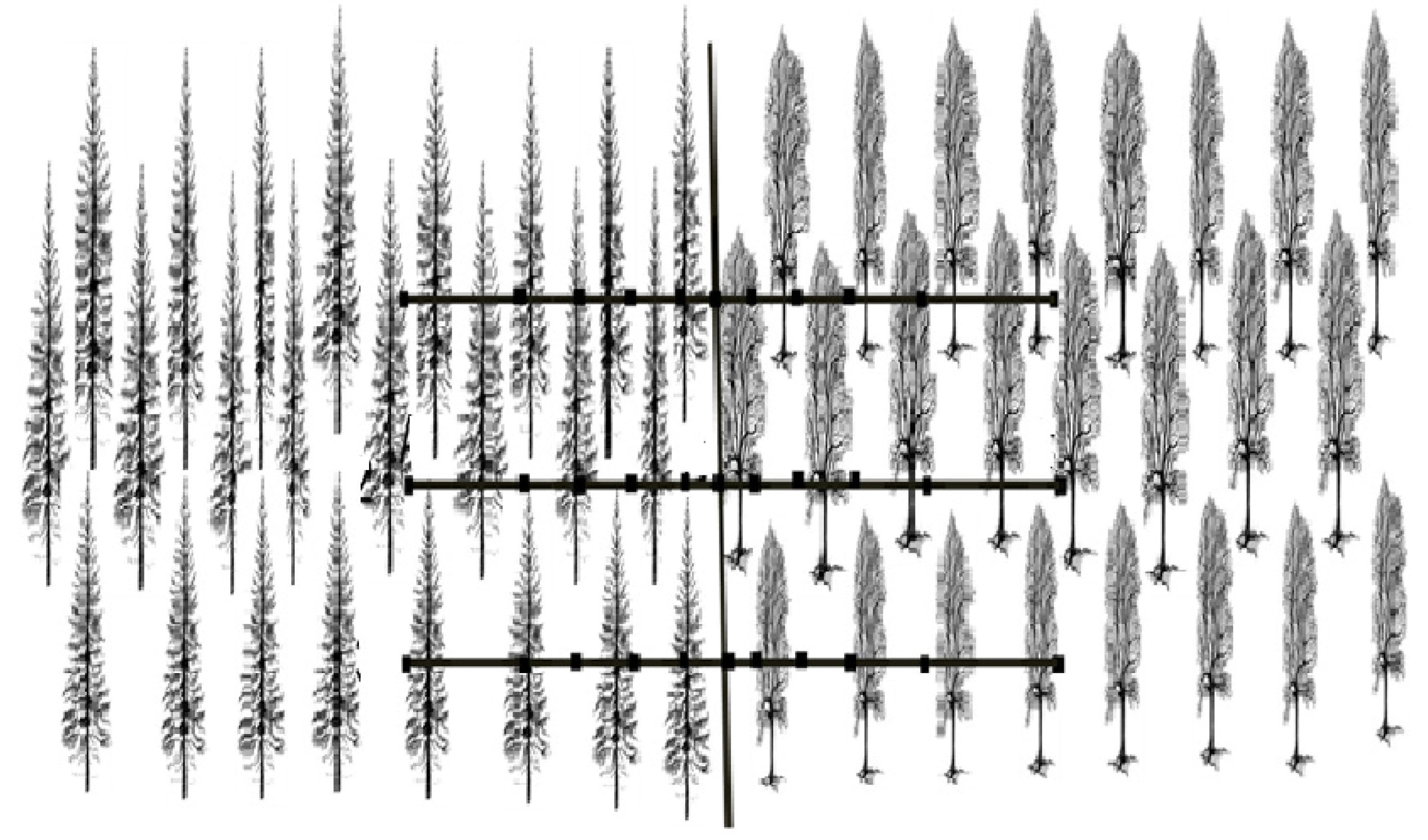
2.2. The ‘Transboundary Approach’ and Experimental Design
2.3. Sample Preparation and Laboratory Analyses
2.4. Calculations of Stocks and Statistical Analyses
3. Results
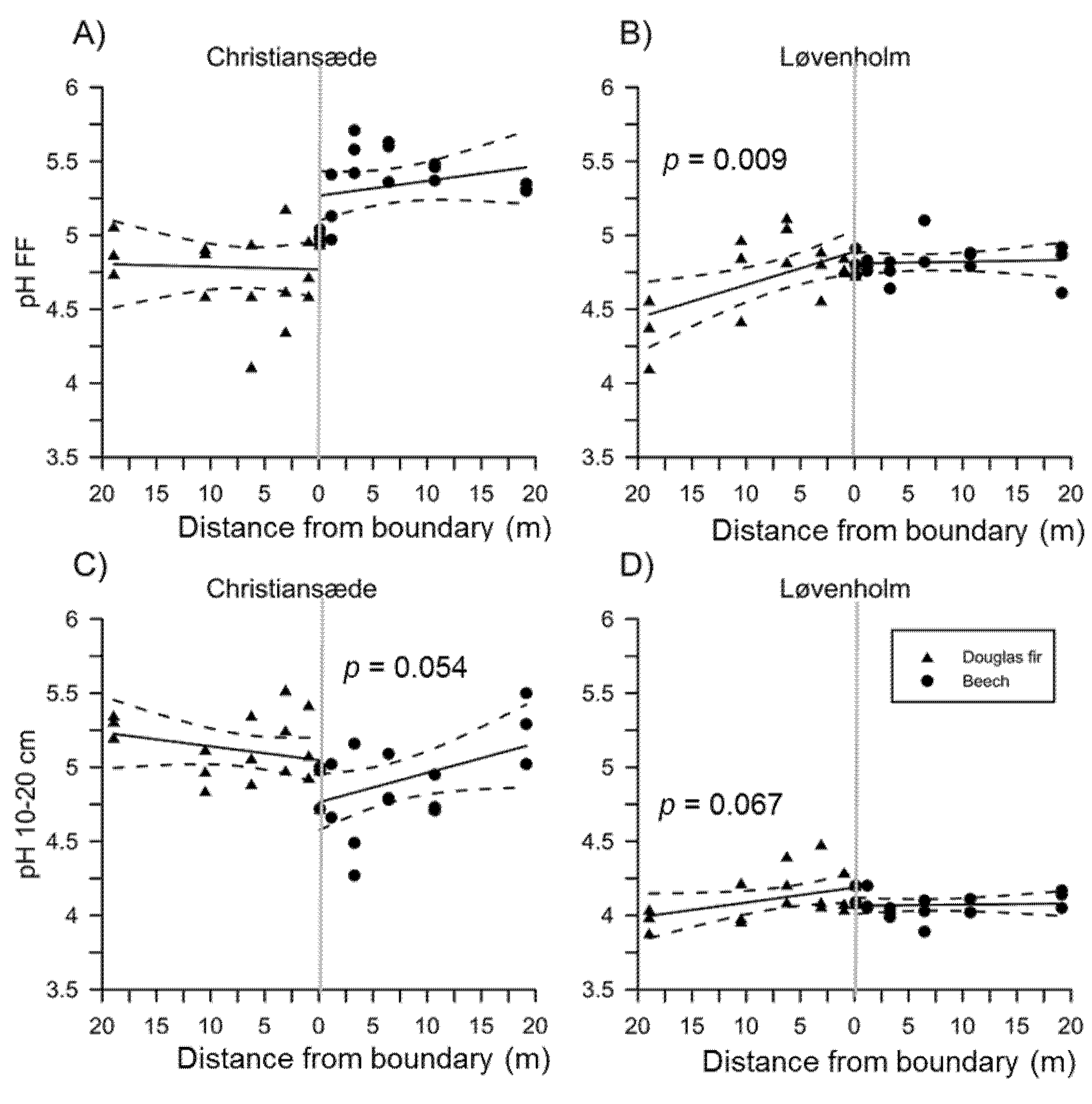
3.1. Effects on Soil Properties by Sites and Tree Species
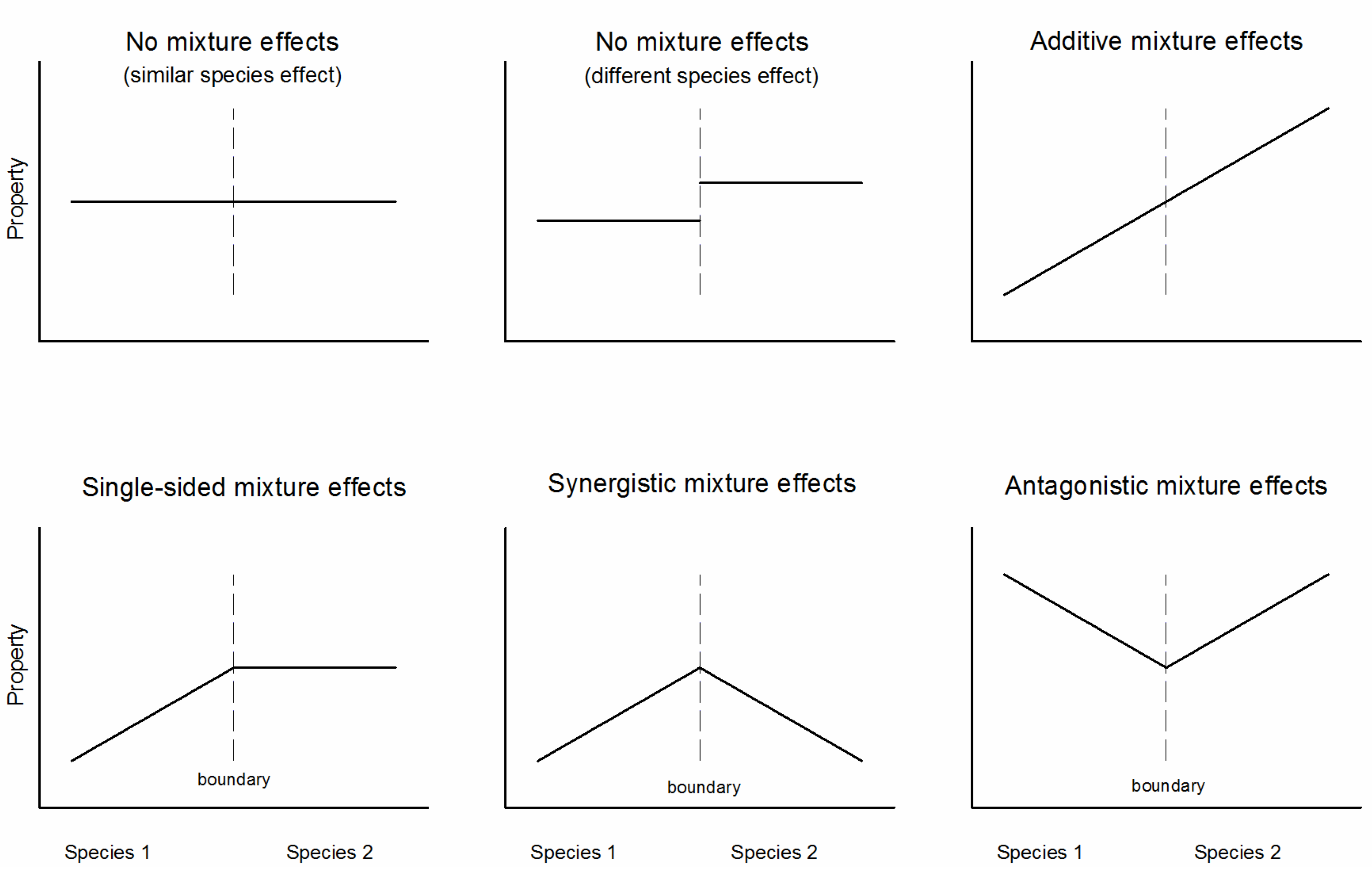
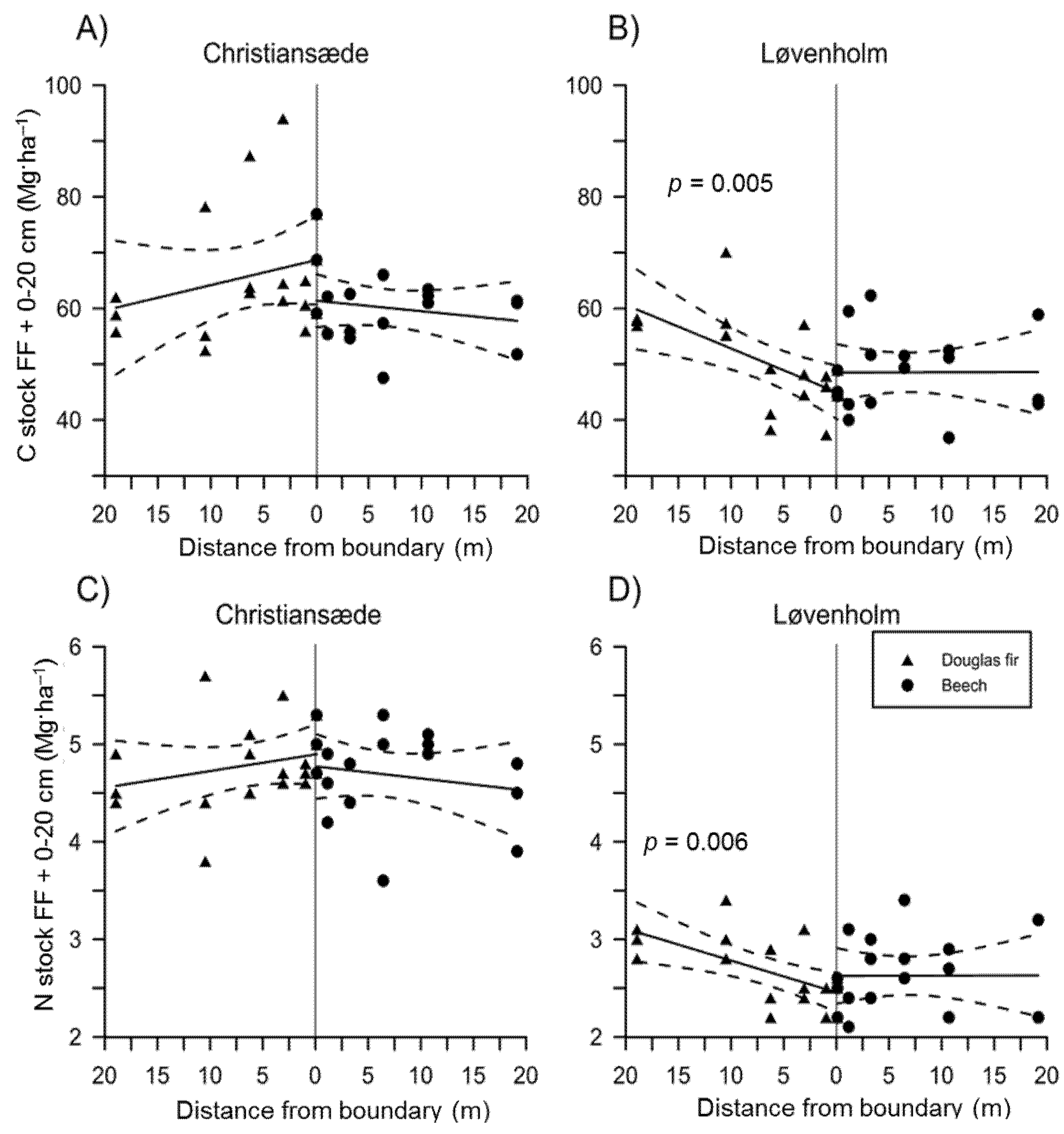
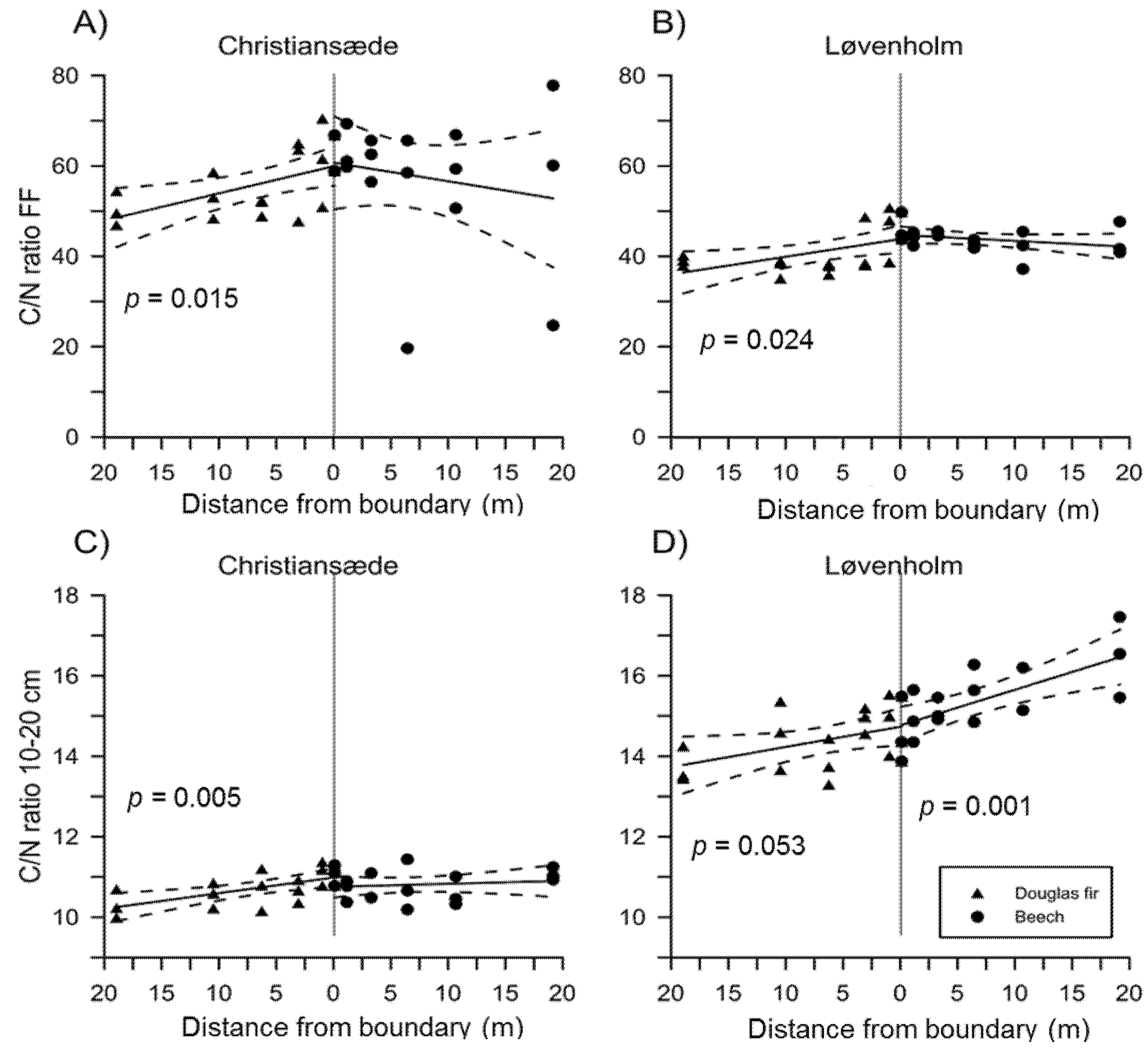
3.2. Effects of Species Mixture Gradients on Soil Properties
4. Discussion
4.1. Tree Species Effects
4.2. Effects of Beech and Douglas-Fir Mixtures
4.3. The Transboundary Approach
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Nadrowski, K.; Wirth, C.; Scherer-Lorenzen, M. Is forest diversity driving ecosystem function and service? Curr. Opin. Environ. Sustain. 2010, 2, 75–79. [Google Scholar] [CrossRef]
- Pretzsch, H. Diversity and productivity in forests: Evidence from long-term experimental plots. In Forest Diversity and Function; Scherer-Lorenzen, M., Körner, C., Schulze, E.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 176, pp. 41–64. [Google Scholar]
- Rothe, A.; Binkley, D. Nutritional interactions in mixed species forests: A synthesis. Can. J. For. Res. 2001, 31, 1855–1870. [Google Scholar] [CrossRef]
- Kolström, M.; Lindner, M.; Vilén, T.; Maroschek, M.; Seidl, R.; Lexer, M.J.; Netherer, S.; Kremer, A.; Delzon, S.; Barbati, A.; et al. Reviewing the science and implementation of climate change adaptation measures in European forestry. Forests 2012, 2, 961–982. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Hermann, R.K.; Lavender, D.P. Pseudotsuga menzieslif (mirb). In Silvics of North America: 1. Conifers; 2. Hardwoods. Agriculture Handbook 654; Burns, R.M.H., Barbara, H., Eds.; Forest Service, United States Department of Agriculture: Washington, D.C., WA, USA, 1990; Volume 2, p. 877. [Google Scholar]
- Hermann, R.K. North American tree species in Europe. J. For. 1987, 85, 27–32. [Google Scholar]
- Isaac-Renton, M.G.; Roberts, D.R.; Hamann, A.; Spiecker, H. Douglas-fir plantations in Europe: A retrospective test of assisted migration to address climate change. Glob. Change Biol. 2014, 20, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Goßner, M.; Ammer, U. The effects of Douglas-fir on tree-specific arthropod communities in mixed species stands with European beech and Norway spruce. Eur. J. For. Res. 2006, 125, 221–235. [Google Scholar] [CrossRef]
- Prietzel, J.; Bachmann, S. Changes in soil organic C and N stocks after forest transformation from Norway spruce and scots pine into Douglas fir, Douglas fir/spruce, or European beech stands at different sites in southern Germany. For. Ecol. Manag. 2012, 269, 134–148. [Google Scholar] [CrossRef]
- Reyer, C.; Lasch, P.; Mohren, G.M.J.; Sterck, F.J. Inter-specific competition in mixed forests of Douglas-fir (Pseudotsuga menziesii) and common beech (Fagus sylvatica) under climate change—A model-based analysis. Ann. For. Sci. 2010, 67, 805. [Google Scholar] [CrossRef]
- Larsen, J.B.; Nielsen, A.B. Nature-based forest management—where are we going? For. Ecol. Manag. 2007, 238, 107–117. [Google Scholar] [CrossRef]
- Rothe, A.; Ewald, J.; Hibbs, D.E. Effect of admixed broadleaves foliar on conifers foliar nutrient status. For. Ecol. Manag. 2003, 172, 327–338. [Google Scholar] [CrossRef]
- Cole, D.W.; Compton, J.E.; Edmonds, R.L.; Homann, P.S.; Van Miegroet, H. Comparison of carbon accumulation in Douglas fir and red alder forests. In Carbon Forms and Functions in Forest Soils; McFee, W.W., Kelly, J.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1995; pp. 527–546. [Google Scholar]
- Ewers, B.; Binkley, D.; Bashkin, M. Influence of adjacent stand on spatial patterns of soil carbon and nitrogen in Eucalyptus and Albizia plantations. Can. J. For. Res. 1996, 26, 1501–1503. [Google Scholar] [CrossRef]
- Lavery, J.M.; Comeau, P.G.; Prescott, C.E. The influence of red alder patches on light, litterfall, and soil nutrients in adjacent conifer stands. Can. J. For. Res. 2004, 34, 56–64. [Google Scholar] [CrossRef]
- Malchair, S.; Carnol, M. Microbial biomass and C and N transformations in forest floors under European beech, sessile oak, Norway spruce and Douglas-fir at four temperate forest sites. Soil Biol. Biochem. 2009, 41, 831–839. [Google Scholar] [CrossRef]
- Vesterdal, L.; Raulund-Rasmussen, K. Forest floor chemistry under seven tree species along a soil fertility gradient. Can. J. For. Res. 1998, 28, 1636–1647. [Google Scholar] [CrossRef]
- Mareschal, L.; Bonnaud, P.; Turpault, M.P.; Ranger, J. Impact of common European tree species on the chemical and physicochemical properties of fine earth: An unusual pattern. Eur. J. Soil Sci. 2010, 61, 14–23. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Nabuurs, G.J.; Verburg, P.H.; de Waal, R.W. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. For. Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Rothe, A.; Kreutzer, K.; Küchenhoff, H. Influence of tree species composition on soil and soil solution properties in two mixed spruce-beech stands with contrasting history in Southern Germany. Plant Soil 2002, 240, 47–56. [Google Scholar] [CrossRef]
- Dawud, S.M.; Raulund-Rasmussen, K.; Finér, L.; Domisch, T.; Jaroszewicz, B.; Vesterdal, L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snall, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef] [PubMed]
- Dawud, S.M.; Raulund-Rasmussen, K.; Ratcliffe, S.; Domisch, T.; Finér, L.; Joly, F.X.; Hättenschwiler, S.; Vesterdal, L. Tree species functional group is a more important driver of soil properties than tree species diversity across major European forest types. Funct. Ecol. 2016, 1–10. [Google Scholar] [CrossRef]
- Binkley, D. The influence of tree species on the forest soil: Processes and patterns. In Proceedings of the Trees and Soil Workshop; Mead, D.J., Cornforth, I.S., Eds.; Lincoln University Press: Canterbury, New Zealand, 1995; pp. 1–34. [Google Scholar]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Raulund-Rasmussen, K.; Vejre, H. Effect of tree species and soil properties on nutrient immobilization in the forest floor. Plant Soil 1995, 168–169, 345–352. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; United States Department of Agriculture: Washington, D.C., WA, USA, 1999; Volume 436, p. 869.
- Callesen, I. Transfer functions for carbon sequestration, nitrogen retention and nutrient release capability in forest soils based on soil texture classification. University of Copenhagen: Copenhagen, Denmark, 2003. [Google Scholar]
- Holmsgaard, E.; Bang, C. Et træartsforsøg med nåletræer, bøg og eg; de første 10 år. Forstlige Forsøgsvaesen 1977, 35, 159–196. (In Denish) [Google Scholar]
- Westman, C.J. A simple device for sampling of volumetric forest soil cores. Silva Fennica 1995, 29, 247–251. [Google Scholar] [CrossRef]
- Matejovic, I. Determination of carbon, hydrogen, and nitrogen in soils by automated elemental analysis (dry combustion method). Commun. Soil Sci. Plant Anal. 1993, 24, 2213–2222. [Google Scholar] [CrossRef]
- Skjemstad, J.; Baldock, J.A. Total and organic carbon. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Volume 3, pp. 225–238. [Google Scholar]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (Toc) in Soils and Sediments; United States Environmental Protection Agency, Office of Research and Development, National Exposure Research Lab Environmental Sciences Division: Washington, D.C., WA, USA, 2002; p. 25.
- Crawley, M.J. Analysis of covariance. In The r Book; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 537–556. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Lenth, R.V.; Hervé, M. Ismeans: Least-squares means. R Package Version 2.16. 2015. [Google Scholar]
- Grömping, U. Relative importance for linear regression in r: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- Lindeman, R.H.; Merenda, P.F.; Gold, R.Z. Introduction to bivariate and multivariate analysis; Scott, Foresman: Glenview, IL, USA, 1980. [Google Scholar]
- Cremer, M.; Kern, N.V.; Prietzel, J. Soil organic carbon and nitrogen stocks under pure and mixed stands of European beech, Douglas fir and Norway spruce. For. Ecol. Manag. 2016, 367, 30–40. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Hansen, K.; Vesterdal, L.; Schmidt, I.K.; Gundersen, P.; Sevel, L.; Bastrup-Birk, A.; Pedersen, L.B.; Bille-Hansen, J. Litterfall and nutrient return in five tree species in a common garden experiment. For. Ecol. Manag. 2009, 257, 2133–2144. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Reich, P.B.; Oleksyn, J.; Ogdahl, M.; Zytkowiak, R.; Hale, C.; Karolewski, P. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 2006, 87, 2288–2297. [Google Scholar] [CrossRef]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Guckland, A.; Jacob, M.; Flessa, H.; Thomas, F.M.; Leuschner, C. Acidity, nutrient stocks, and organic-matter content in soils of a temperate deciduous forest with different abundance of European beech (Fagus sylvatica L.). J. Plant Nutr. Soil Sci. 2009, 172, 500–511. [Google Scholar] [CrossRef]
- De Schrijver, A.; De Frenne, P.; Staelens, J.; Verstraeten, G.; Muys, B.; Vesterdal, L.; Wuyts, K.; van Nevel, L.; Schelfhout, S.; De Neve, S.; et al. Tree species traits cause divergence in soil acidification during four decades of postagricultural forest development. Glob. Change Biol. 2012, 18, 1127–1140. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Vogel, C.; Heister, K.; Buegger, F.; Tanuwidjaja, I.; Haug, S.; Schloter, M.; Koegel-Knabner, I. Clay mineral composition modifies decomposition and sequestration of organic carbon and nitrogen in fine soil fractions. Biol. Fertil. Soils 2015, 51, 427–442. [Google Scholar] [CrossRef]
- Schmid, I.; Kazda, M. Vertical distribution and radial growth of coarse roots in pure and mixed stands of Fagus sylvatica and Picea abies. Can. J. For. Res. 2001, 31, 539–548. [Google Scholar] [CrossRef]
- Grossiord, C.; Gessler, A.; Granier, A.; Berger, S.; Bréchet, C.; Hentschel, R.; Hommel, R.; Scherer-Lorenzen, M.; Bonal, D. Impact of interspecific interactions on the soil water uptake depth in a young temperate mixed species plantation. J. Hydrol. 2014, 519, 3511–3519. [Google Scholar] [CrossRef]
- Göransson, H.; Bambrick, M.T.; Godbold, D.L. Overyielding of temperate deciduous tree mixtures is maintained under throughfall reduction. Plant Soil 2016, 408, 285–298. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M.; Schulze, E.D.; Don, A.; Schumacher, J.; Weller, E. Exploring the functional significance of forest diversity: A new long-term experiment with temperate tree species (biotree). Perspect. Plant Ecol. Evol. Syst. 2007, 9, 53–70. [Google Scholar] [CrossRef]
- Setiawan, N.N.; Vanhellemont, M.; Baeten, L.; Van de Peer, T.; Ampoorter, E.; Ponette, Q.; Verheyen, K. Local neighbourhood effects on sapling growth in a young experimental forest. For. Ecol. Manag. 2017, 384, 424–443. [Google Scholar] [CrossRef]
- Baeten, L.; Verheyen, K.; Wirth, C.; Bruelheide, H.; Bussotti, F.; Finér, L.; Jaroszewicz, B.; Selvi, F.; Valladares, F.; Allan, E.; et al. A novel comparative research platform designed to determine the functional significance of tree species diversity in European forests. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 281–291. [Google Scholar] [CrossRef]
| Horizons | Depth (cm) | pH | Clay (%) | Silt (%) | Fine Sand (%) | Coarse Sand (%) | Total C (%) | Total N (%) | Extractable P (mg·kg–1) | Exchangeable Cations (cmol·kg–1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | K+ | ||||||||||
| CHR, Mollic Hapludalf, loam | ||||||||||||
| A1 | 0–5 | 3.8 | 12.0 | 10.0 | 49.1 | 28.9 | 2.8 | 2.1 | 110 | 4.60 | 0.60 | 0.12 |
| A2 | 5–25 | 5.2 | 12.0 | 11.0 | 50.2 | 26.8 | 1.5 | 1.7 | 110 | 9.30 | 0.40 | 0.10 |
| Bt | 25–50 | 6.2 | 15.0 | 18.0 | 43.6 | 23.4 | 0.4 | 0.5 | 240 | 12.20 | 0.60 | 0.18 |
| Btg | 50–73 | 7.5 | 18.0 | 20.0 | 38.5 | 23.5 | - | - | 380 | 21.70 | 0.50 | 0.13 |
| Ckg | 73–110 | 7.7 | 9.0 | 16.0 | 52.6 | 22.4 | - | - | - | - | 0.30 | 0.07 |
| LØV, Typic Haplumbrept, loamy sand | ||||||||||||
| Ap | 0–27 | 4.1 | 3.5 | 12.0 | 36.8 | 47.7 | 0.9 | 0.7 | 119 | 0.47 | 0.06 | 0.06 |
| Bw | 27–55 | 4.8 | 2.5 | 6.5 | 49.0 | 42.0 | 0.4 | 0.3 | 161 | 0.48 | 0.05 | 0.04 |
| BC | 55–70 | 4.7 | 4.0 | 9.0 | 48.7 | 38.3 | 0.1 | 0.2 | 223 | 0.21 | 0.03 | 0.04 |
| Cg | 70– | 4.5 | 3.5 | 10.5 | 43.6 | 42.4 | 0.1 | - | 123 | 0.78 | 0.07 | 0.07 |
| Species | Stem Number (N·ha–1) | Height100 (m) | Basal Area (m2·ha–1) | Volume (m3·ha–1) | MAI (m3·ha–1·year–1) |
|---|---|---|---|---|---|
| CHR | |||||
| Beech | 362 | 24.2 | 23 | 324 | 12 |
| Douglas-fir | 583 | 30.2 | 57 | 725 | 19 |
| LØV | |||||
| Beech | 275 | 25.3 | 21 | 304 | 10 |
| Douglas-fir | 334 | 31.2 | 43 | 570 | 23 |
| Site | Soil Layer | N | C Stock (Mg·ha–1) | N Stock (Mg·ha–1) | C/N Ratio | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| CHR | FF | 36 | 6.3 b | 1.4 | 0.1 b | 0 | 56.9 a | 1.9 | 5.1 a | 0.1 |
| 0–5cm | 36 | 20.8 a | 0.7 | 1.4 a | 0 | 14.7 b | 0.2 | 4.4 a | 0.1 | |
| 5–10cm | 36 | 13.6 a | 0.4 | 1.1 a | 0 | 12.1 b | 0.2 | 4.5 a | 0 | |
| 10–20cm | 36 | 22.2 a | 0.4 | 2.1 a | 0 | 10.8 b | 0.1 | 5.0 a | 0 | |
| FF + 0–20cm | 36 | 62.9 a | 1.6 | 4.7 a | 0.1 | 13.3 b | 0.2 | - | - | |
| LØV | FF | 36 | 11.8 a | 0.7 | 0.3 a | 0 | 42.5 b | 0.7 | 4.8 b | 0 |
| 0–5cm | 36 | 15.4 a | 0.6 | 0.9 b | 0 | 17.3 a | 0.2 | 3.9 b | 0 | |
| 5–10cm | 36 | 8.2 b | 0.2 | 0.5 b | 0 | 15.3 a | 0.3 | 3.9 b | 0 | |
| 10–20cm | 36 | 13.9 b | 0.4 | 0.9 b | 0 | 14.9 a | 0.2 | 4.1 b | 0 | |
| FF + 0–20cm | 36 | 49.3 b | 1.3 | 2.7 b | 0.1 | 18.6 a | 0.2 | - | - | |
| Species | Soil Layer | n | C Stock (Mg·ha–1) | N Stock (Mg·ha–1) | C/N Ratio | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Beech | FF | 30 | 7.4 b | 0.8 | 0.2 b | 0 | 50.3 a | 2.4 | 5.1 a | 0.1 |
| 0–5cm | 30 | 17.6 b | 0.6 | 1.2 a | 0.1 | 15.4 b | 0.3 | 4.3 a | 0.1 | |
| 5–10cm | 30 | 10.8 a | 0.5 | 0.9 a | 0.1 | 13.6 a | 0.5 | 4.2 a | 0 | |
| 10–20cm | 30 | 18.0 a | 0.8 | 1.5 a | 0.1 | 13.1 a | 0.5 | 4.5 b | 0.1 | |
| FF + 0–20cm | 30 | 53.8 a | 1.4 | 3.6 a | 0.2 | 15.5 a | 0.6 | - | - | |
| Douglas-fir | FF | 30 | 11.3 a | 1.7 | 0.3 a | 0 | 47.6 a | 1.7 | 4.7 b | 0 |
| 0–5cm | 30 | 18.4 a | 0.8 | 1.1 a | 0.1 | 16.5 a | 0.4 | 3.9 a | 0 | |
| 5–10cm | 30 | 10.3 a | 0.6 | 0.8 a | 0.1 | 13.4 a | 0.4 | 4.2 a | 0 | |
| 10–20cm | 30 | 18.1 a | 0.8 | 1.5 a | 0.1 | 12.5 a | 0.4 | 4.6 a | 0 | |
| FF + 0–20cm | 30 | 58.1 a | 2.3 | 3.7 a | 0.2 | 16.2 a | 0.5 | - | - | |
| Soil Layers | Variables | R2 | |||
|---|---|---|---|---|---|
| C Stock | N Stock | C/N Ratio | Soil pH | ||
| Forest Floor | Site | 87% | 88% | 83% | 37% |
| Species | 8% | 6% | 2% | 48% | |
| Distance | 2% | 4% | 10% | 0% | |
| Transect | 2% | 3% | 5% | 4% | |
| Site: Distance | - | - | - | 6% | |
| Species: Distance | - | - | - | 5% | |
| 0–5 cm | Site | 94% | 99% | 84% | 65% |
| Species | 1% | 0% | 9% | 17% | |
| Distance | 0% | 0% | 0% | 3% | |
| Transect | 3% | 0% | 3% | 9% | |
| Site: Distance | 2% | - | 3% | - | |
| Species: Distance | - | - | - | 6% | |
| 5–10 cm | Site | 92% | 99% | 97% | 93% |
| Species | 1% | 0% | 0% | 0% | |
| Distance | 3% | 0% | 1% | 0% | |
| Transect | 1% | 1% | 2% | 6% | |
| Site: Distance | 3% | - | - | - | |
| 10–20 cm | Site | 96% | 99% | 96% | 94% |
| Species | 0% | 0% | 2% | 2% | |
| Distance | 0% | 0% | 0% | 0% | |
| Transect | 1% | 0% | 1% | 1% | |
| Site: Distance | 3% | - | 0% | 2% | |
| Species: Distance | - | - | 2% | - | |
| FF + 0–20 cm | Site | 82% | 98% | 98% | - |
| Species | 4% | 0% | 1% | - | |
| Distance | 0% | 0% | 0% | - | |
| Transect | 4% | 1% | 0% | - | |
| Site: Distance | 9% | 1% | - | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawud, S.M.; Vesterdal, L.; Raulund-Rasmussen, K. Mixed-Species Effects on Soil C and N Stocks, C/N Ratio and pH Using a Transboundary Approach in Adjacent Common Garden Douglas-Fir and Beech Stands. Forests 2017, 8, 95. https://doi.org/10.3390/f8040095
Dawud SM, Vesterdal L, Raulund-Rasmussen K. Mixed-Species Effects on Soil C and N Stocks, C/N Ratio and pH Using a Transboundary Approach in Adjacent Common Garden Douglas-Fir and Beech Stands. Forests. 2017; 8(4):95. https://doi.org/10.3390/f8040095
Chicago/Turabian StyleDawud, Seid Muhie, Lars Vesterdal, and Karsten Raulund-Rasmussen. 2017. "Mixed-Species Effects on Soil C and N Stocks, C/N Ratio and pH Using a Transboundary Approach in Adjacent Common Garden Douglas-Fir and Beech Stands" Forests 8, no. 4: 95. https://doi.org/10.3390/f8040095






