Regeneration Dynamics of Coast Redwood, a Sprouting Conifer Species: A Review with Implications for Management and Restoration
Abstract
:1. Introduction
- (1)
- review the regeneration dynamics of coast redwood after a variety of disturbance events;
- (2)
- review the dynamics of stand development and density management in even-aged and multiaged stands;
- (3)
- discuss the implications for management of stands of different structures and managing for different objectives including restoration; and
- (4)
- discuss the implications for management with climate change and the presence of invasive organisms.
2. Environment and Species Characteristics
2.1. Disturbance History
2.2. Site Productivity
2.3. Regeneration—Redwood and Other Species
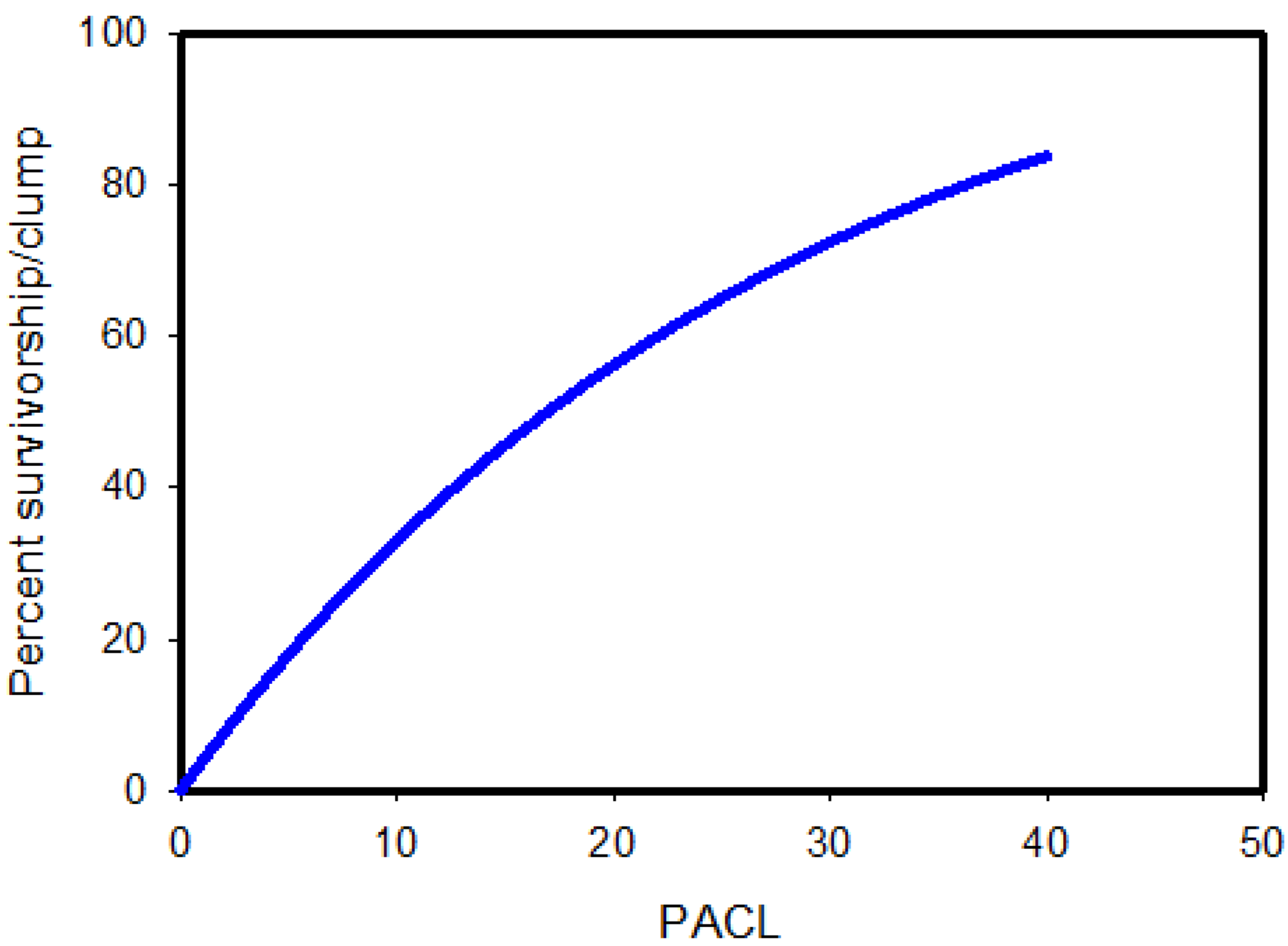
2.3.1. Sprouts
2.3.2. Seedlings
2.4. Spatial and Clonal Patterns
3. Stand Dynamics
3.1. Even-Aged Stands
3.2. Multiaged Stands
4. Management
4.1. Young Redwood Stand Management
4.2. Older Redwood Stand Management
4.3. Restoration
5. Climate Change and Genetic Diversity
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fox, L. Current status and distribution of coast redwood. In Coast Redwood Forest Ecology and Management; LeBlanc, J., Ed.; University of California: Berkeley, CA, USA, 1996; pp. 18–19. [Google Scholar]
- Hartley, R.K. Redwood forest conservation: Where do we go from here? In Proceedings of Coast Redwood Forests in a Changing California: A Symposium for Scientists and Managers; Standiford, R.B., Weller, T.J., Piirto, D.D., Stuart, J.D., Eds.; PSW-GTR-238; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2012; pp. 3–10. [Google Scholar]
- California State Park and Recreation Commission; National Park Service. Redwood State and National Parks General Management Plan; Redwood National and State Parks: Humboldt/Del Norte Counties, CA, USA, 2000.
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Busing, R.T.; Fujimori, T. Dynamics of composition and structure in an old Sequoia sempervirens forest. J. Veg. Sci. 2002, 13, 785–792. [Google Scholar] [CrossRef]
- Van Pelt, R.; Sillett, S.C.; Kruse, W.A.; Freund, J.A.; Kramer, R.D. Emergent crowns and light-use complementarity lead to global maximum biomass and leaf area in Sequoia sempervirens forests. For. Ecol. Manag. 2016, 375, 279–308. [Google Scholar] [CrossRef]
- Jones, D.A.; O’Hara, K.L. Carbon density in managed coast redwood stands: Implications for forest carbon estimation. Forestry 2012, 85, 99–110. [Google Scholar] [CrossRef]
- Noss, R.F. The Redwood Forest: History, Ecology and Conservation of the Coast Redwoods; Island Press: Washington, DC, USA, 2000. [Google Scholar]
- Anekonda, T.S. A Genetic Architecture Study of Coast Redwood; University of California: Berkeley, CA, USA, 1992. [Google Scholar]
- Sillett, S.C.; Van Pelt, R. Trunk reiteration promotes epiphytes and water storage in an old-growth redwood forest canopy. Ecol. Monogr. 2007, 77, 335–359. [Google Scholar] [CrossRef]
- Russell, W.; Sinclair, J.; Michels, K.H. Restoration of coast redwood (Sequoia sempervirens) forests through natural recovery. Open J. For. 2014, 4, 106–111. [Google Scholar]
- Lorimer, C.G.; Porter, D.J.; Madej, M.A.; Stuart, J.D.; Veirs, S.D., Jr.; Norman, S.P.; O’Hara, K.L.; Libby, W.J. Presettlement and modern disturbance regimes in coast redwood forests: Implications for the conservation of old-growth stands. For. Ecol. Manag. 2009, 258, 1038–1054. [Google Scholar] [CrossRef]
- Lewis, H.T. Patterns of Indian Burning in California: Ecology and Ethnohistory; Ballena Press: Ramona, CA, USA, 1973. [Google Scholar]
- Brown, P.M.; Baxter, W.T. Fire history in coast redwood forests of the Mendocino Coast, California. Northwest Sci. 2003, 77, 147–158. [Google Scholar]
- Stuart, J.D.; Stephens, S.L. North Coast Region. In Fire in California’s Ecosystems; Sugihara, N.G., van Wagtendonk, J.W., Shaffer, K.E., Fites-Kaufman, J., Thode, A.E., Eds.; University of California: Berkeley, CA, USA, 2006; pp. 147–169. [Google Scholar]
- Ramage, B.S.; O’Hara, K.L.; Caldwell, B.T. The role of fire in the competitive dynamics of coast redwood forests. Ecosphere 2010, 1, 1–18. [Google Scholar] [CrossRef]
- Berrill, J.-P.; O’Hara, K.L. How do biophysical factors contribute to height and basal area development in a mixed multiaged coast redwood stand? Forestry 2016, 89, 170–181. [Google Scholar] [CrossRef]
- Stone, E.C.; Vasey, R.B. Preservation of Coast Redwood on Alluvial Flats. Science 1968, 159, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Zinke, P.J. The redwood forest and associated north coast forests. In Terrestrial Vegetation of California, New Expanded, ed.; Barbour, M.G., Major, J., Eds.; California Native Plant Society: New York, NY, USA, 1977; pp. 679–698. [Google Scholar]
- Olson, D.F.J.; Roy, D.F.; Walters, G.A. Sequoia sempervirens (D. Don) Endl. Redwood (Agriculture Handbook 654). In Silvics of North America, Volume 1. Conifers; USDA Forest Service: Washington, DC, USA, 1990; pp. 541–551. [Google Scholar]
- Waring, K.M.; O’Hara, K.L. Estimating relative error in growth ring analyses of second-growth coast redwood (Sequoia sempervirens). Can. J. For. Res. 2006, 36, 2216–2222. [Google Scholar] [CrossRef]
- Berrill, J.P.; O’Hara, K.L. Estimating site productivity in irregular stand structures by indexing basal area or volume increment of the dominant species. Can. J. For. Res. 2014, 44, 92–100. [Google Scholar] [CrossRef]
- Mahony, T.M.; Stuart, J.D. Status of vegetation classification in redwood ecosystems. In Proceedings of the Redwood Science Symposium: What Does the Future Hold? PSW-GTR-194; USDA Forest Service: Albany, CA, USA, 2007; pp. 207–214. [Google Scholar]
- Lenihan, J.M.; Lennox, W.S.; Muldavin, E.H.; Veirs, S.D. A Handbook for Classifying Early Post-Logging Vegetation in the Lower Redwood Creek Basin; Technical Report Number 7; Redwood National Park, National Park Service: Arcata, CA, USA, 1982.
- Lenihan, J.M. The Forest Associations of the Little Lost Man Creek Research Natural Area, Redwood National Park, CA; Humboldt State University: Arcata, CA, USA, 1986. [Google Scholar]
- Loya, D.T.; Jules, E.S. Use of species richness estimators improves evaluation of understory plant response to logging: a study of redwood forests. Plant Ecol. 2008, 194, 179–194. [Google Scholar] [CrossRef]
- Del Tredici, P. Lignotubers in Sequoia sempervirens: Development and ecological significance. Madrono 1998, 45, 255–260. [Google Scholar]
- Powers, R.F.; Wiant, H.V. Sprouting of old-growth coastal redwood stumps on slopes. For. Sci. 1970, 16, 339–341. [Google Scholar]
- Barrette, B.R. Redwood Sprouts on the Jackson State Forest; State Forestry Note No. 29; California Division of Forestry: Sacramento, CA, USA, 1966.
- Boe, K.N. Natural Regeneration in Old-Growth Cuttings; Research Note PSW-94; USDA Forest Service: Berkeley, CA, USA, 1965.
- Wiant, H.V., Jr.; Powers, R.F. Sprouting of old-growth redwood. In Proceedings Society of American Foresters Convention 1966; Society of American Foresters: Washington, DC, USA, 1967; pp. 88–90. [Google Scholar]
- Lindquist, J.L. Sprout Regeneration of Young-Growth Redwood: Sampling Methods Compared; Research Note PSW-337; USDA Forest Service: Berkeley, CA, USA, 1979.
- Neal, R.L., Jr. Sprouting of Old-Growth Redwood Stumps—First Year after Logging; Research Note PSW-137; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 1967.
- O’Hara, K.L.; Stancioiu, P.T.; Spencer, M.A. Understory stump sprout development under variable canopy density and leaf area in coast redwood. For. Ecol. Manag. 2007, 244, 76–85. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Berrill, J.-P. Dynamics of coast redwood sprout clump development in variable light environments. J. For. Res. 2010, 15, 131–139. [Google Scholar] [CrossRef]
- O’Hara, K.L. Multiaged Silviculture: Managing for Complex Forest Stand Structures; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Jameson, M.J.; Robards, T.A. Coast redwood regeneration survival and growth in Mendocino county, California. West. J. Appl. For. 2007, 22, 171–175. [Google Scholar]
- Dagley, C.M. Spatial pattern of coast redwood in three alluvial flat old-growth forests in northern California. For. Sci. 2008, 54, 294–302. [Google Scholar]
- Van Mantgem, P.J.; Stuart, J.D. Structure and dynamics of an upland old-growth forest at Redwood National Park, California. In Proceedings of Coast Redwood Forests in a Changing California: A Symposium for Scientists and Managers; Standiford, R.B., Weller, T.J., Piirto, D.D., Stuart, J.D., Eds.; PSW-GTR-238; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2012; pp. 333–343. [Google Scholar]
- Berrill, J.-P.; O’Hara, K.L. Influence of tree spatial pattern and sample plot type and size on inventory estimates for leaf area index, stocking, and tree size parameters. In Proceedings of Coast Redwood Forests in a Changing California: A Symposium for Scientists and Managers; Standiford, R.B., Weller, T.J., Piirto, D.D., Stuart, J.D., Eds.; PSW-GTR-238; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2012; pp. 485–497. [Google Scholar]
- Narayan, L. Clonal Diversity, Patterns, and Structure in Old Coast Redwood Forests; University of California: Berkeley, CA, USA, 2015. [Google Scholar]
- Rogers, D.L. Genotypic diversity and clone size in old-growth populations of coast redwood (Sequoia sempervirens). Can. J. Bot. 2000, 78, 1408–1419. [Google Scholar]
- Douhovnikoff, V.; Cheng, A.M.; Dodd, R.S. Incidences size and spatial structure of clones in second-growth stands of coast redwood Sequoia sempervirens (Cupressaceae). Am. J. Bot. 2004, 91, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; McGraw-Hill, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Reineke, L.H. Perfecting a stand-density index for even-aged forests. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Stein, W.I. Umbellularia californica (Hook and Arn.) Nutt. In Silvics of North America, Volume 2 Hardwoods. Agriculture Handbook 654; Barnes, R.H., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 826–834. [Google Scholar]
- Wensel, L.C.; Krumland, B.E. A site index system for redwood and Douglas-fir in California’s north coast forest. Hilgardia 1986, 54, 1–14. [Google Scholar] [CrossRef]
- Waring, K.M.; O’Hara, K.L. Redwood/tanoak stand development and response to tanoak mortality caused by Phytophthora ramorum. For. Ecol. Manag. 2008, 255, 2650–2658. [Google Scholar] [CrossRef]
- Hunter, J.C.; Barbour, M.G. Through-growth by Pseudotsuga menziesii: A mechanism for change in forest composition without canopy gaps. J. Veg. Sci. 2001, 12, 445–452. [Google Scholar] [CrossRef]
- Sawyer, J.O.; Sillett, S.C.; Libby, W.J.; Dawson, T.E.; Popenoe, J.H.; Largent, D.L.; Van Pelt, R.; Viers, S.D.J.; Noss, R.F.; Thornburgh, D.A.; et al. Redwood trees, communities and ecosystems: A closer look. In The Redwood Forest: History, Ecology, and Conservation of the Coast Redwoods; Noss, R.F., Ed.; Island Press: Washington, DC, USA, 2000; pp. 81–118. [Google Scholar]
- Fritz, E. Some popular fallacies concerning California redwood. Madrono 1929, 1, 221–224. [Google Scholar]
- Viers, S.D.J. Coast redwood forest: Stand dynamics, successional status, and the role of fire. In Proceedings of the Symposium on Forest Succession and Stand Development in the Pacific Northwest; Means, J.E., Ed.; Oregon State University: Corvallis, OR, USA, 1982; pp. 119–141. [Google Scholar]
- Pelt, R.V.; Sillett, S.C. Crown development of coastal Pseudotsuga menziesii, including a conceptual model for tall conifers. Ecol. Monogr. 2008, 78, 283–311. [Google Scholar] [CrossRef]
- Dagley, C.M.; O’Hara, K.L. Potential for Old Forest Restoration and Development of Restoration Tools in Coast Redwood: A Literature Review and Synthesis; Save-the-Redwoods League: San Francisco, CA, USA, 2003. [Google Scholar]
- Westman, W.E.; Whittaker, R.H. Pygmy forest region of northern California—Studies on biomass and primary productivity. J. Ecol. 1975, 63, 493–520. [Google Scholar] [CrossRef]
- Fujimori, T. Stem biomass and structure of a mature Sequoia sempervirens stand on the Pacific Coast of northern California. J. Jpn. For. Soc. 1977, 59, 435–441. [Google Scholar]
- Busing, R.T.; Fujimori, T. Biomass, production and woody detritus in an old coast redwood (Sequoia sempervirens) forest. Plant Ecol. 2005, 177, 177–188. [Google Scholar] [CrossRef]
- Sugihara, N.G. The Role of Treefall Gaps and Fallen Trees in the Dynamics of Old Growth Coast Redwood (Sequoia sempervirens (D. Don) Endl.) Forests; University of California: Berkeley, CA, USA, 1992. [Google Scholar]
- Van Pelt, R.; Franklin, J.F. Influence of canopy structure on the understory environment in tall, old-growth, conifer forests. Can. J. For. Res. 2000, 30, 1231–1245. [Google Scholar] [CrossRef]
- Pillers, M.D.; Stuart, J.D. Leaf-litter accretion and decomposition in interior and coastal old-growth redwood stands. Can. J. For. Res. 1993, 23, 552–557. [Google Scholar] [CrossRef]
- Combs, W.E. Stand Structure and Composition on the Little Lost Man Creek Research Natural Area; Redwood National Park; Humboldt State University: Arcata, CA, USA, 1984. [Google Scholar]
- McBride, J.; Jacobs, D. Ecology of Redwood and the Impact of Man’s Use of the Redwood Forest as a Site for Recreational Activities: A Literature Review; Muir Woods Research Project Techinical Report No. 1; University of California for the Muir Wood National Monument, National Park Service: Mill Valley, CA, USA, 1977. [Google Scholar]
- Russell, W.; Michels, K.H. Stand development on a 127-yr chronosequence of naturally regenerating Sequoia sempervirens (Taxodiaceae) forests. Madrono 2010, 57, 229–241. [Google Scholar] [CrossRef]
- Schrepfer, S.R. The Fight to Save the Redwoods; A History of Environmental Reform, 1917–1978; University of Wisconsin Press: Madison, WI, USA, 1983. [Google Scholar]
- Harris, D. The Last Stand: The War Between Wall Street and Main Stree over California’s Ancient Redwoods; Times Books/Random House: New York, NY, USA, 1995. [Google Scholar]
- Rodrigues, K. The history of conflict over managing coast redwoods. In Coast Redwood Forest Ecology and Management, Proceedings of the Conference on Coast Redwood Ecology and Management, Humboldt State University, Arcata, CA, USA, 18–20 June 1996; LeBlanc, J., Ed.; University of California: Berkeley, CA, USA, 1996; pp. 52–54. [Google Scholar]
- Thornburgh, D.A. Managing redwoods. In The Redwood Forest: History, Ecology, and Conservation of the Coast Redwoods; Noss, R.F., Ed.; Island Press: Washington, DC, USA, 2000; pp. 229–262. [Google Scholar]
- California Department of Forestry and Fire Protection. California Forest Practice Rules; California Department of Forestry and Fire Protection: Sacramento, CA, USA, 2015.
- Boe, K.N. Thinning Promotes Growth of Sprouts on Old-Growth Redwood Stumps; Research Note PSW-290; USDA Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1974.
- Cole, D.W. Effects of Thinning on Redwood Sprout Growth; California Forestry Note No. 84; California Dept. Forestry: Sacramento, CA, USA, 1982. [Google Scholar]
- Cole, D.W. Redwood sprout growth 3 decades after thinning. J. For. 1983, 81, 148–150. [Google Scholar]
- Keyes, C.R.; Matzka, P.J.; Wright, K.C.; Glebocki, R.; Han, H.S. Early precommercial thinning of redwood sprout clumps: Evaluation of four techniques. Int. J. For. Eng. 2008, 19, 28–36. [Google Scholar]
- Lindquist, J.L. Precommercial Stocking Control of Coast Redwood: A Seventeen-Year Status Report (1981–1998); Report No. 2; California Department Forestry and Fire Protection: Sacramento, CA, USA, 2004.
- Lindquist, J. Precommercial stocking control of coast redwood at Caspar Creek, Jackson Demonstration State Forest. In Proceedings of the Redwood Region Forest Science Symposium: What Does the Future Hold? Standiford, R.B., Giusti, G.A., Valachovic, Y., Zielinski, W.J., Furniss, M.J., Eds.; PSW-GTR-194; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2007; pp. 295–304. [Google Scholar]
- O’Hara, K.L.; Narayan, L.; Cahill, K.G. Twelve-year response of coast redwood to precommercial thinning treatments. For. Sci. 2015, 61, 780–789. [Google Scholar] [CrossRef]
- Van Mantgem, P.; Das, A. An individual-based growth and competition model for coastal redwood forest restoration. Can. J. For. Res. 2014, 44, 1051–1057. [Google Scholar] [CrossRef]
- Berrill, J.-P.; Beal, C.B.; LaFever, D.H.; Dagley, C.M. Modeling young stand development towards the old-growth reference condition in evergreen mixed-conifer stands at Headwaters Forest Reserve, California. Forests 2013, 4, 455–470. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Narayan, L.; Leonard, L.P. Ten-year results from a variable-density thinning trial in coast redwood. 2017; In prep. [Google Scholar]
- O’Hara, K.L. Coast redwood responses to pruning. In Proceedings of Coast Redwood Forests in a Changing California: A Symposium for Scientists and Managers; Standiford, R.B., Weller, T.J., Piirto, D.D., Stuart, J.D., Eds.; PSW-GTR-238; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2012; pp. 529–538. [Google Scholar]
- O’Hara, K.L.; Berrill, J.-P. Epicormic sprout development in pruned coast redwood: Pruning severity, genotype, and sprouting characteristics. Ann. For. Sci. 2009, 66, 409. [Google Scholar] [CrossRef]
- Cown, D.; Marshall, H.; Silcock, P.; Meason, D. Sawn timber grade recovery from a planted coast redwood stand growing in New Zealand. N. Z. J. For. Sci. 2013, 43, 8. [Google Scholar] [CrossRef]
- Giusti, G.A. Black bear feeding on second growth redwoods: A critical asessment. In Proceedings of the 14th Vertebrate Pest Conference, Sacramento, CA, USA, 6–8 March 1990; Davis, L.R., Marsh, R.E., Eds.; University of California: Davis, CA, USA, 1990; pp. 214–217. [Google Scholar]
- Perry, D.W.; Breshears, L.W.; Gradillas, G.E.; Berrill, J.P. Thinning Intensity and Ease-of-Access Increase Probability of Bear Damage in a Young Coast Redwood Forest. J. Biodivers. Manag. For. 2016, 5, 3–9. [Google Scholar] [CrossRef]
- Kimball, B.A.; Nolte, D.L.; Engeman, R.M.; Johnston, J.J.; Frank, R. Chemically mediated foraging preference of black bears (Ursus americanus). J. Mammal. 2008, 79, 448–456. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Nesmith, J.C.B.; Leonard, L.; Porter, D.J. Restoration of old forest features in coast redwood forests using early-stage variable-density thinning. Restor. Ecol. 2010, 18, 125–135. [Google Scholar] [CrossRef]
- Oliver, W.W.; Lindquist, J.L.; Strothmann, R.O. Young-growth redwood stands respond well to various thinning intensities. West. J. Appl. For. 1994, 9, 106–112. [Google Scholar]
- Webb, L.A.; Lindquist, J.L.; Wahl, E.; Hubbs, A. Whiskey Springs long-term coast redwood density management; Final grown, sprout, and yield results. In Proceedings of Coast Redwood Forests in a Changing California: A Symposium for Scientists and Managers; Standiford, R.B., Weller, T.J., Piirto, D.D., Stuart, J.D., Eds.; PSW-GTR-238; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2012; pp. 571–581. [Google Scholar]
- Berrill, J.P.; O’Hara, K. Modeling coast redwood variable retention management regimes. In Proceedings of the Redwood Region Forest Science Symposium: What Does the Future Hold? Standiford, R.B., Giusti, G.A., Valachovic, Y., Zielinski, W.J., Furniss, M.J., Eds.; PSW-GTR-194; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2007; pp. 261–269. [Google Scholar]
- Berrill, J.-P.; O’Hara, K.L. Simulating multiaged coast redwood stand development: Interactions between regeneration, structure, and productivity. West. J. Appl. For. 2009, 24, 24–32. [Google Scholar]
- Carey, A.B. Biocomplexity and restoration of biodiversity in temperate coniferous forest: Inducing spatial heterogeneity with variable-density thinning. Forestry 2003, 76, 127–136. [Google Scholar] [CrossRef]
- Comfort, E.J.; Roberts, S.D.; Harrington, C.A. Midcanopy growth following thinning in young-growth conifer forests on the Olympic Peninsula western Washington. For. Ecol. Manag. 2010, 259, 1606–1614. [Google Scholar] [CrossRef]
- Pukkala, T.; Lähde, E.; Laiho, O. Variable-density thinning in uneven-aged forest management—A case for Norway spruce in Finland. Forestry 2011, 84, 557–565. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Leonard, L.P.; Keyes, C.R. Variable-density thinning and a marking paradox: Comparing prescription protocols to attain stand variability in coast redwood. West. J. Appl. For. 2012, 27, 143–149. [Google Scholar] [CrossRef]
- Dodson, E.K.; Ares, A.; Puettmann, K.J. Early responses to thinning treatments designed to accelerate late successional forest structure in young coniferous stands of western Oregon, USA. Can. J. For. Res. 2012, 355, 345–355. [Google Scholar] [CrossRef]
- Kuehne, C.; Weiskittel, A.R.; Fraver, S.; Puettmann, K.J. Effects of thinning induced changes in structural heterogeneity on growth, ingrowth, and mortality in secondary coastal Douglas-fir forests. Can. J. For. Res. 2015, 45, 1448–1461. [Google Scholar] [CrossRef]
- Burns, R.M. Silvicultural systems for the major forest types of the United States. In Agricultural Handbook No. 445; U.S. Department of Agriculture: Quilcene, WA, USA, 1983. [Google Scholar]
- Tappeiner, J.C.; McDonald, P.M.; Hughes, T.F. Survival of tanoak (Lithocarpus densiflorus) and Pacific madrone (Arbutus menziesii) seedlings in forests of southwestern Oregon. New For. 1986, 1, 43–55. [Google Scholar] [CrossRef]
- Piirto, D.D.; Smith, B.; Huff, E.K.; Robinson, S.T. Efficacy of herbicide application methods used to control tanoak (Lithocarpus densiflorus) in an uneven-aged coast redwood management context. In Coast Redwood Forest Ecology and Management; LeBlanc, J., Ed.; University of California: Berkeley, CA, USA, 1996. [Google Scholar]
- Ireland, K.B.; Hardy, G.E.S.J.; Kriticos, D.J. Combining inferential and deductive approaches to estimate the potential geographical range of the invasive plant pathogen, Phytophthora ramorum. PLoS ONE 2013, 8, e63508. [Google Scholar] [CrossRef] [PubMed]
- Beh, M.M.; Metz, M.R.; Frangioso, K.M.; Rizzo, D.M. The key host for an invasive forest pathogen also facilitates the pathogen’s survival of wildfire in California forests. New Phytol. 2012, 196, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Ramage, B.S.; O’Hara, K.L. Sudden oak death-induced tanoak mortality in coast redwood forests: Current and predicted impacts to stand structure. Forests 2010, 1, 114–130. [Google Scholar] [CrossRef]
- Cobb, R.C.; Filipe, J.A.N.; Meentemeyer, R.K.; Gilligan, C.A.; Rizzo, D.M. Ecosystem transformation by emerging infectious disease: Loss of large tanoak from California forests. J. Ecol. 2012, 100, 712–722. [Google Scholar] [CrossRef]
- Metz, M.R.; Varner, J.M.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Unexpected redwood mortality from synergies between wildfire and an emerging infectious disease. Ecology 2013, 94, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.C.; Letourneau, D.K.; Bothwell, S.G. Disturbance, resources, and exotic plant invasion: Gap size effects in a redwood forest. Madrono 2010, 57, 11–19. [Google Scholar] [CrossRef]
- DiTomaso, J.M.; Drewitz, J.J.; Kyser, G.B. Jubatagrass (Cortaderia jubata) control using chemical and mechanical methods. Invasive Plant Sci. Manag. 2008, 1, 82–90. [Google Scholar] [CrossRef]
- Johnson, E.A.; Miyanishi, K. Testing the assumptions of chronosequences in succession. Ecol. Lett. 2008, 11, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E. Fog in the Calfornia redwood forest: Ecosystem inputs and use by plants. Oecologia 1998, 117, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Limm, E.B.; Simonin, K.A.; Bothman, A.G.; Dawson, T.E. Foliar water uptake: A common water acquisition strategy for plants of the redwood forest. Oecologia 2009, 161, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Templer, P.H.; Weathers, K.C.; Ewing, H.A.; Dawson, T.E.; Mambelli, S.; Lindsey, A.M.; Webb, J.; Boukili, V.K.; Firestone, M.K. Fog as a source of nitrogen for redwood trees: Evidence from fluxes and stable isotopes. J. Ecol. 2015, 103, 1397–1407. [Google Scholar] [CrossRef]
- Johnstone, J.A.; Dawson, T.E. Climatic context and ecological implications of summer fog decline in the coast redwood region. Proc. Natl. Acad. Sci. USA 2010, 107, 4533–4538. [Google Scholar] [CrossRef] [PubMed]
- Douhovnikoff, V.; Dodd, R.S. Lineage divergence in coast redwood (Sequoia sempervirens), detected by a new set of nuclear microsatellite loci. Am. Midl. Nat. 2011, 165, 22–37. [Google Scholar] [CrossRef]
- Chin, A.R.O.; Sillett, S.C. Phenotypic plasticity of leaves enhances water-stress tolerance and promotes hydraulic conductivity in a tall conifer. Am. J. Bot. 2016, 103, 796–807. [Google Scholar] [CrossRef] [PubMed]




| Location | Latitude° N | Upper Canopy Redwood | Total Density | Source |
|---|---|---|---|---|
| Trees ha−1 | ||||
| Mendocino County—slopes | 39.33 | n/a | 913 | [55] 1 |
| Mendocino County—alluvial flats | 39.33 | n/a | 337 | |
| Humboldt Redwoods St. Pk—(Bull Creek) | 40.35 | 66 | 167 | [5,56,57] |
| Humboldt Redwoods St. Pk—(Bull Creek) | 40.35 | 88 | 225 | [58] 2 |
| Humboldt Redwoods St. Pk—(Bull Creek) | 40.35 | 48 | 163 | [59] |
| Redwood N. Pk. (Prairie Creek) flat | 41.38 | 163 | 180 | [60] 3 |
| Redwood N. Pk. (Prairie Creek) slope | 41.38 | 177 | 276 | |
| Humboldt Redwood St. Pk. flat | 40.35 | 160 | 239 | |
| Humboldt Redwoods St. Pk. Slope | 40.35 | 59 | 1046 | |
| Redwood N. Pk. | n/a | 46 | 70 | [52] 4 |
| Redwood N. Pk. (xeric) | 41.33 | 128 | 311 | [61] 5 |
| Redwood N. Pk. (mesic) | 41.33 | 180 | 272 | |
| Muir Woods | 37.89 | n/a | 462 | [62] |
| Prairie Creek St. Pk. | 107 | 137 | [50] | |
| Humboldt Redwoods St. Pk. (Bull Creek) | 143 | 146 | ||
| Armstrong St. Pk. | 38.88 | 74 | 192 | [38] 6 |
| Humboldt Redwoods St. Pk. | 40.31 | 59 | 122 | |
| Various—Mendocino County | Approx. 38 | n/a | 763 | [63] 7 |
| Various—Santa Cruz and San Mateo Co. | Approx. 37 | n/a | 1308 | [11] |
| Big Basin St. Pk. | 37.18 | n/a | 272 | [41] 8 |
| Humboldt Redwoods St. Pk. | 40.34 | n/a | 183 | |
| Redwood N. Pk. | 41.34 | n/a | 170 | |
| Jedediah Smith St. Pk. upper | 41.78 | 39 | 266 | [6] 9 |
| Jedediah Smith St. Pk. lower | 41.77 | 48 | 246 | |
| Prairie Creek St. Pk. upper | 41.37 | 58 | 247 | |
| Prairie Creek Sk. Pk. lower | 41.36 | 41 | 145 | |
| Redwood N. Pk. upper | 41.26 | 49 | 471 | |
| Redwood N. Pk. lower | 41.20 | 64 | 426 | |
| Humboldt Redwoods St. Pk. | 40.34 | 64 | 375 | |
| Montgomery Woods St. Res. | 39.23 | 68 | 336 | |
| Samuel P. Taylor St. Pk. | 38.02 | 53 | 475 | |
| Big Basin St. Pk. | 37.19 | 48 | 552 | |
| Landeis-Hill Big Creek Res. | 36.09 | 76 | 550 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Hara, K.L.; Cox, L.E.; Nikolaeva, S.; Bauer, J.J.; Hedges, R. Regeneration Dynamics of Coast Redwood, a Sprouting Conifer Species: A Review with Implications for Management and Restoration. Forests 2017, 8, 144. https://doi.org/10.3390/f8050144
O’Hara KL, Cox LE, Nikolaeva S, Bauer JJ, Hedges R. Regeneration Dynamics of Coast Redwood, a Sprouting Conifer Species: A Review with Implications for Management and Restoration. Forests. 2017; 8(5):144. https://doi.org/10.3390/f8050144
Chicago/Turabian StyleO’Hara, Kevin L., Lauren E. Cox, Sasha Nikolaeva, Julian J. Bauer, and Rachelle Hedges. 2017. "Regeneration Dynamics of Coast Redwood, a Sprouting Conifer Species: A Review with Implications for Management and Restoration" Forests 8, no. 5: 144. https://doi.org/10.3390/f8050144







