Tree Diseases as a Cause and Consequence of Interacting Forest Disturbances
Abstract
:1. Introduction
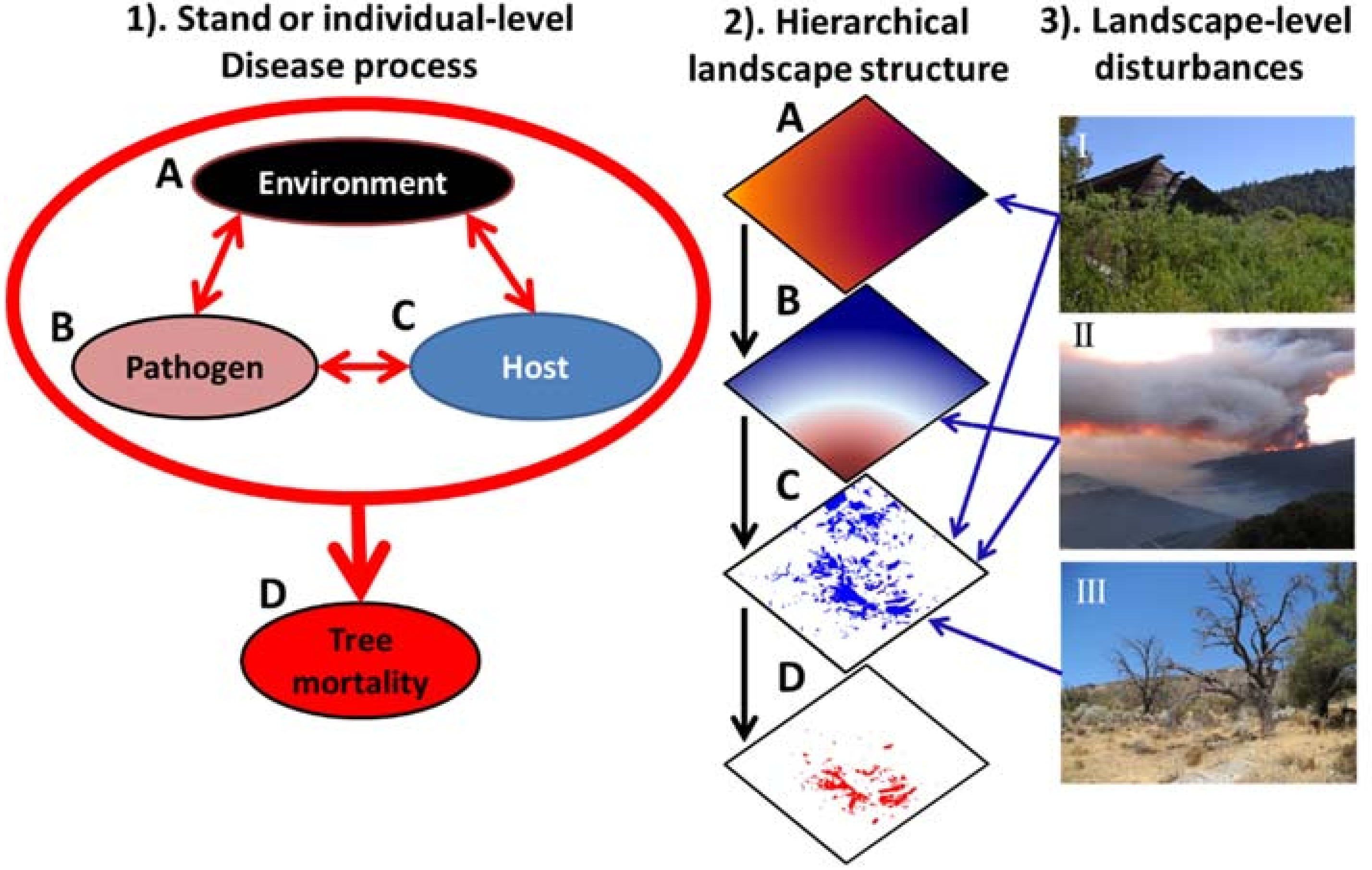
2. The Disease Triangle: A Primer for Landscape Ecologists
2.1. Pathogens
2.2. Tree Hosts
2.3. Environment
3. The Disease Triangle in the Context of Disturbance
3.1. Environmental vs. Biotic Stress on Tree Hosts
3.2. Example Disturbance Effects on Pathogens
3.3. N Deposition, an Example of Changing Environmental Conditions at Broad Spatial Extents
3.4. Disease as Disturbance
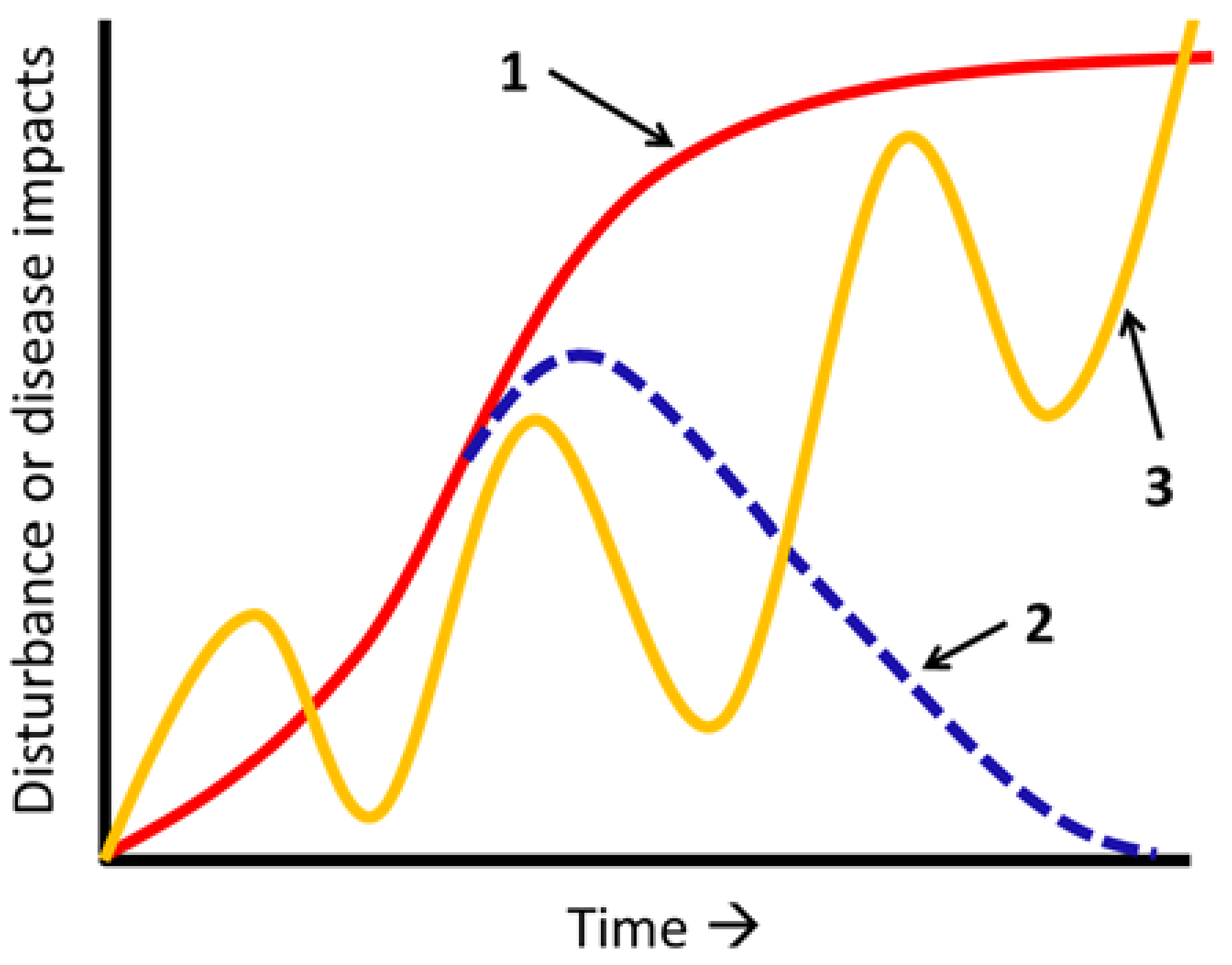
4. Disturbance Interactions: Perspectives from Landscape Ecology
4.1. Insect–Fire Interactions
4.2. Disease–Disturbance Interactions
5. Frontiers in the Study of Disease–Disturbance Interactions
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartig, R. Text-Book of the Diseases of Trees; Macmillan and Company: London, UK, 1894. [Google Scholar]
- Desprez-Loustau, M.-L.; Marçais, B.; Nageleisen, L.-M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Pautasso, M.; Schlegel, M.; Holdenrieder, O. Forest health in a changing world. Microb. Ecol. 2014, 69, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Bostock, R.M.; Pye, M.F.; Roubtsova, T.V. Predisposition in plant disease: Exploiting the nexus in abiotic and biotic stress perception and response. Annu. Rev. Phytopathol. 2014, 52, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J.; Oliva, J. Phenotypic interactions between tree hosts and invasive forest pathogens in the light of globalization and climate change. Philos. Trans. R. Soc. B 2016, 371, 20150455. [Google Scholar] [CrossRef] [PubMed]
- Paillet, F.L. Chestnut: History and ecology of a transformed species. J. Biogeogr. 2003, 29, 1517–1530. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Garbelotto, M.; Hansen, E.M. Phytophthora ramorum: Integrative research and management of an emerging pathogen in California and Oregon forests. Annu. Rev. Phytopathol. 2005, 43, 309–335. [Google Scholar] [CrossRef] [PubMed]
- Shearer, B.L.; Crane, C.E.; Barrett, S.; Cochrane, A. Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Aust. J. Bot. 2007, 55, 225–238. [Google Scholar] [CrossRef]
- Orwig, D.A.; Foster, D.R.; Mausel, D.L. Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. J. Biogeogr. 2002, 29, 1475–1487. [Google Scholar] [CrossRef]
- Kulakowski, D.; Veblen, T.T. Effect of prior disturbances on the extent and severity of wildfire in Colorado subalpine forests. Ecology 2007, 88, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Garnas, J.R.; Ayres, M.P.; Liebhold, A.M.; Evans, C. Subcontinental impacts of an invasive tree disease on forest structure and dynamics. J. Ecol. 2011, 99, 532–541. [Google Scholar] [CrossRef]
- Holdenrieder, O.; Pautasso, M.; Weisberg, P.J.; Lonsdale, D. Tree diseases and landscape processes: The challenge of landscape pathology. Trends Ecol. Evol. 2004, 19, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Meentemeyer, R.K.; Haas, S.E.; Václavík, T. Landscape epidemiology of emerging infectious diseases in natural and human-altered ecosystems. Annu. Rev. Phytopathol. 2012, 50, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, N.J.; Gilligan, C.A. A theoretical framework for biological control of soil-borne plant pathogens: Identifying effective strategies. J. Theor. Biol. 2011, 278, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Buma, B. Disturbance interactions: Characterization, prediction, and the potential for cascading effects. Ecosphere 2015, 6, 70. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Metz, M.R.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Interacting disturbances: Wildfire severity affected by stage of forest disease invasion. Ecol. Appl. 2011, 21, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Desprez-Loustau, M.-L.; Aguayo, J.; Dutech, C.; Hayden, K.J.; Husson, C.; Jakushkin, B.; Marçais, B.; Piou, D.; Robin, C.; Vacher, C. An evolutionary ecology perspective to address forest pathology challenges of today and tomorrow. Ann. For. Sci. 2016, 73, 45–67. [Google Scholar] [CrossRef]
- Baumgartner, K.; Rizzo, D.M. Ecology of Armillaria spp. in Mixed-Hardwood Forests of California. Plant Dis. 2001, 85, 947–951. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; Gordon, T.R. Cryptic fungal infections: The hidden agenda of plant pathogens. Plant-Microbe Interact. 2014, 5, 506. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, G.; Rizzo, D. Past forest management promoted root disease in Yosemite Valley. Calif. Agric. 1999, 53, 17–24. [Google Scholar] [CrossRef]
- Woods, A.J.; Martín-García, J.; Bulman, L.; Vasconcelos, M.W.; Boberg, J.; La Porta, N.; Peredo, H.; Vergara, G.; Ahumada, R.; Brown, A.; et al. Dothistroma needle blight, weather and possible climatic triggers for the disease’s recent emergence. For. Pathol. 2016, 46, 443–452. [Google Scholar] [CrossRef]
- Cunniffe, N.J.; Cobb, R.C.; Meentemeyer, R.K.; Rizzo, D.M.; Gilligan, C.A. Modeling when, where, and how to manage a forest epidemic, motivated by sudden oak death in California. Proc. Natl. Acad. Sci. USA 2016, 113, 5640–5645. [Google Scholar] [CrossRef] [PubMed]
- Lieberei, R. South American Leaf Blight of the Rubber Tree (Hevea spp.): New Steps in Plant Domestication using Physiological Features and Molecular Markers. Ann. Bot. 2007, 100, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Bowcutt, F. Tanoak target: The rise and fall of herbicide use on a common native tree. Environ. Hist. 2011, 16, 197–225. [Google Scholar] [CrossRef]
- Cobb, R.C.; Rizzo, D.M.; Hayden, K.J.; Garbelotto, M.; Filipe, J.A.N.; Gilligan, C.A.; Dillon, W.W.; Meentemeyer, R.K.; Valachovic, Y.S.; Goheen, E.; et al. Biodiversity Conservation in the Face of Dramatic Forest Disease: An Integrated Conservation Strategy for Tanoak (Notholithocarpus densiflorus) Threatened by Sudden Oak Death. Madroño 2013, 60, 151–164. [Google Scholar] [CrossRef]
- Meentemeyer, R.K.; Cunniffe, N.J.; Cook, A.R.; Filipe, J.A.N.; Hunter, R.D.; Rizzo, D.M.; Gilligan, C.A. Epidemiological modeling of invasion in heterogeneous landscapes: Spread of sudden oak death in California (1990–2030). Ecosphere 2011, 2, 1–24. [Google Scholar] [CrossRef]
- Meentemeyer, R.K.; Rank, N.E.; Anacker, B.L.; Rizzo, D.M.; Cushman, J.H. Influence of land-cover change on the spread of an invasive forest pathogen. Ecol. Appl. 2008, 18, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Danneyrolles, V.; Arseneault, D.; Bergeron, Y. Pre-industrial landscape composition patterns and post-industrial changes at the temperate–boreal forest interface in western Quebec, Canada. J. Veg. Sci. 2016, 27, 470–481. [Google Scholar] [CrossRef]
- Nuñez, M.A.; Horton, T.R.; Simberloff, D. Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 2009, 90, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Dickie, I.A.; John, S.G.M.; Yeates, G.W.; Morse, C.W.; Bonner, K.I.; Orwin, K.; Peltzer, D.A. Belowground legacies of Pinus contorta invasion and removal result in multiple mechanisms of invasional meltdown. AoB Plants 2014, 6, plu056. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.J.; Thrall, P.H.; Ericson, L. The current and future dynamics of disease in plant communities. Annu. Rev. Phytopathol. 2006, 44, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Harrington, T.C. Root movement and root damage of red spruce and balsam fir on subalpine sites in the White Mountains, New Hampshire. Can. J. For. Res. 1988, 18, 991–1001. [Google Scholar] [CrossRef]
- Etheridge, D.E.; Craig, H.M. Factors influencing infection and initiation of decay by the Indian paint fungus (Echinodontiumtinctorium) in western hemlock. Can. J. For. Res. 1976, 6, 299–318. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Plavcová, L.; Anderegg, L.D.L.; Hacke, U.G.; Berry, J.A.; Field, C.B. Drought’s legacy: Multiyear hydraulic deterioration underlies widespread aspen forest die-off and portends increased future risk. Glob. Chang. Biol. 2013, 19, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Dillon, W.W.; Meentemeyer, R.K.; Vogler, J.B.; Cobb, R.C.; Metz, M.R.; Rizzo, D.M. Range-wide threats to a foundation tree species from disturbance interactions. Madroño 2013, 60, 139–150. [Google Scholar] [CrossRef]
- Thompson, J.R.; Carpenter, D.N.; Cogbill, C.V.; Foster, D.R. Four centuries of change in Northeastern United States forests. PLoS ONE 2013, 8, e72540. [Google Scholar] [CrossRef] [PubMed]
- Mehl, H.K.; Mori, S.R.; Frankel, S.J.; Rizzo, D.M. Mortality and growth of dwarf mistletoe-infected red and white fir and the efficacy of thinning for reducing associated losses. For. Pathol. 2013, 43, 193–203. [Google Scholar] [CrossRef]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.M.; Whiting, E.C.; Elkins, R.B. Spatial distribution of armillaria mellea in pear orchards. Plant Dis. 1998, 82, 1226–1231. [Google Scholar] [CrossRef]
- Beh, M.M.; Metz, M.R.; Frangioso, K.M.; Rizzo, D.M. The key host for an invasive forest pathogen also facilitates the pathogen’s survival of wildfire in California forests. New Phytol. 2012, 196, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.E.; Henkel, T.W. Native forest pathogens facilitate persistence of Douglas-fir in old-growth forests of Northwestern California. Can. J. For. Res. 2011, 41, 1256–1266. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem Consequences of Biological Invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Cobb, R.C.; Meentemeyer, R.K.; Rizzo, D.M. Apparent competition in canopy trees determined by pathogen transmission rather than susceptibility. Ecology 2010, 91, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.D.; Dobson, A.P.; Begon, M.; Bowers, R.G.; Schauber, E.M. Parasite establishment in host communities. Ecol. Lett. 2003, 6, 837–842. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Reich, P.B.; Tilman, D.; Groth, J.V. Effects of elevated CO2, nitrogen deposition, and decreased species diversity on foliar fungal plant disease. Glob. Chang. Biol. 2003, 9, 438–451. [Google Scholar] [CrossRef]
- Strengbom, J.; Englund, G.; Ericson, L. Experimental scale and precipitation modify effects of nitrogen addition on a plant pathogen. J. Ecol. 2006, 94, 227–233. [Google Scholar] [CrossRef]
- Eviner, V.T.; Likens, G.E. Effects of pathogens on terrestrial ecosystem function. In Infectious Disease Ecology. Effects of Ecosystems on Disease and Disease on Ecosystems; Ostfeld, R.S., Keesing, F., Eviner, V.T., Eds.; Princeton University Press: Princeton, NJ, USA, 2008; pp. 260–283. [Google Scholar]
- Matson, P.A.; Boone, R.D. Natural Disturbance and Nitrogen Mineralization: Wave-Form Dieback of Mountain Hemlock in the Oregon Cascades. Ecology 1984, 65, 1511–1516. [Google Scholar] [CrossRef]
- Hobara, S.; Tokuchi, N.; Ohte, N.; Koba, K.; Katsuyama, M.; Kim, S.J.; Nakanishi, A. Mechanism of nitrate loss from a forested catchment following a small-scale, natural disturbance. Can. J. For. Res. 2001, 31, 1326–1335. [Google Scholar] [CrossRef]
- Cobb, R.C.; Eviner, V.T.; Rizzo, D.M. Mortality and community changes drive sudden oak death impacts on litterfall and soil nitrogen cycling. New Phytol. 2013, 200, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.M.; Goheen, E.M. Phellinus weirii and other native root pathogens as determinants of forest structure and process in western North America. Annu. Rev. Phytopathol. 2000, 38, 515–539. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.R.; Frangioso, K.M.; Wickland, A.C.; Meentemeyer, R.K.; Rizzo, D.M. An emergent disease causes directional changes in forest species composition in coastal California. Ecosphere 2012, 3, 86. [Google Scholar] [CrossRef]
- Paine, R.T.; Tegner, M.J.; Johnson, E.A. Compounded perturbations yield ecological surprises. Ecosystems 1998, 1, 535–545. [Google Scholar] [CrossRef]
- Simard, M.; Romme, W.H.; Griffin, J.M.; Turner, M.G. Do mountain pine beetle outbreaks change the probability of active crown fire in lodgepole pine forests? Ecol. Monogr. 2010, 81, 3–24. [Google Scholar] [CrossRef]
- James, P.M.A.; Robert, L.-E.; Wotton, B.M.; Martell, D.L.; Fleming, R.A. Lagged cumulative spruce budworm defoliation affects the risk of fire ignition in Ontario, Canada. Ecol. Appl. 2016, 27, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.R.; Varner, J.M.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Unexpected redwood mortality from synergies between wildfire and an emerging infectious disease. Ecology 2013, 94, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Cobb, R.C.; Meentemeyer, R.K.; Rizzo, D.M. Wildfire and forest disease interaction lead to greater loss of soil nutrients and carbon. Oecologia 2016, 182, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Worrall, J.J.; Harrington, T.C. Etiology of canopy gaps in spruce–fir forests at Crawford Notch, New Hampshire. Can. J. For. Res. 1988, 18, 1463–1469. [Google Scholar] [CrossRef]
- Negrón, J.F.; McMillin, J.D.; Anhold, J.A.; Coulson, D. Bark beetle-caused mortality in a drought-affected ponderosa pine landscape in Arizona, USA. For. Ecol. Manag. 2009, 257, 1353–1362. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
| Biological Agent | Interacting Disturbance | Comments | Examples | |
|---|---|---|---|---|
| Landscape-level examples | ||||
| Native insects | Fire, wind, salvage harvest | Tested for interactive effects | [11,57,58] | |
| Invasive pathogen | Fire | Tested for interactive effects | [18,43,59,60] | |
| Root pathogens | Wind | Focused on environmental drivers of disease | [35,61] | |
| Root pathogens | Management, fire suppression | Etiological investigation of landscape-level disease drivers | [22] | |
| Individual-level examples | ||||
| Insects or pathogens | Drought, salt stress, and heat | Synthesis, laboratory experiments | [4] | |
| Pathogens | Drought | Synthesis | [5] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobb, R.C.; Metz, M.R. Tree Diseases as a Cause and Consequence of Interacting Forest Disturbances. Forests 2017, 8, 147. https://doi.org/10.3390/f8050147
Cobb RC, Metz MR. Tree Diseases as a Cause and Consequence of Interacting Forest Disturbances. Forests. 2017; 8(5):147. https://doi.org/10.3390/f8050147
Chicago/Turabian StyleCobb, Richard C., and Margaret R. Metz. 2017. "Tree Diseases as a Cause and Consequence of Interacting Forest Disturbances" Forests 8, no. 5: 147. https://doi.org/10.3390/f8050147





