Novelty and Its Ecological Implications to Dry Forest Functioning and Conservation
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Dry Forests in General
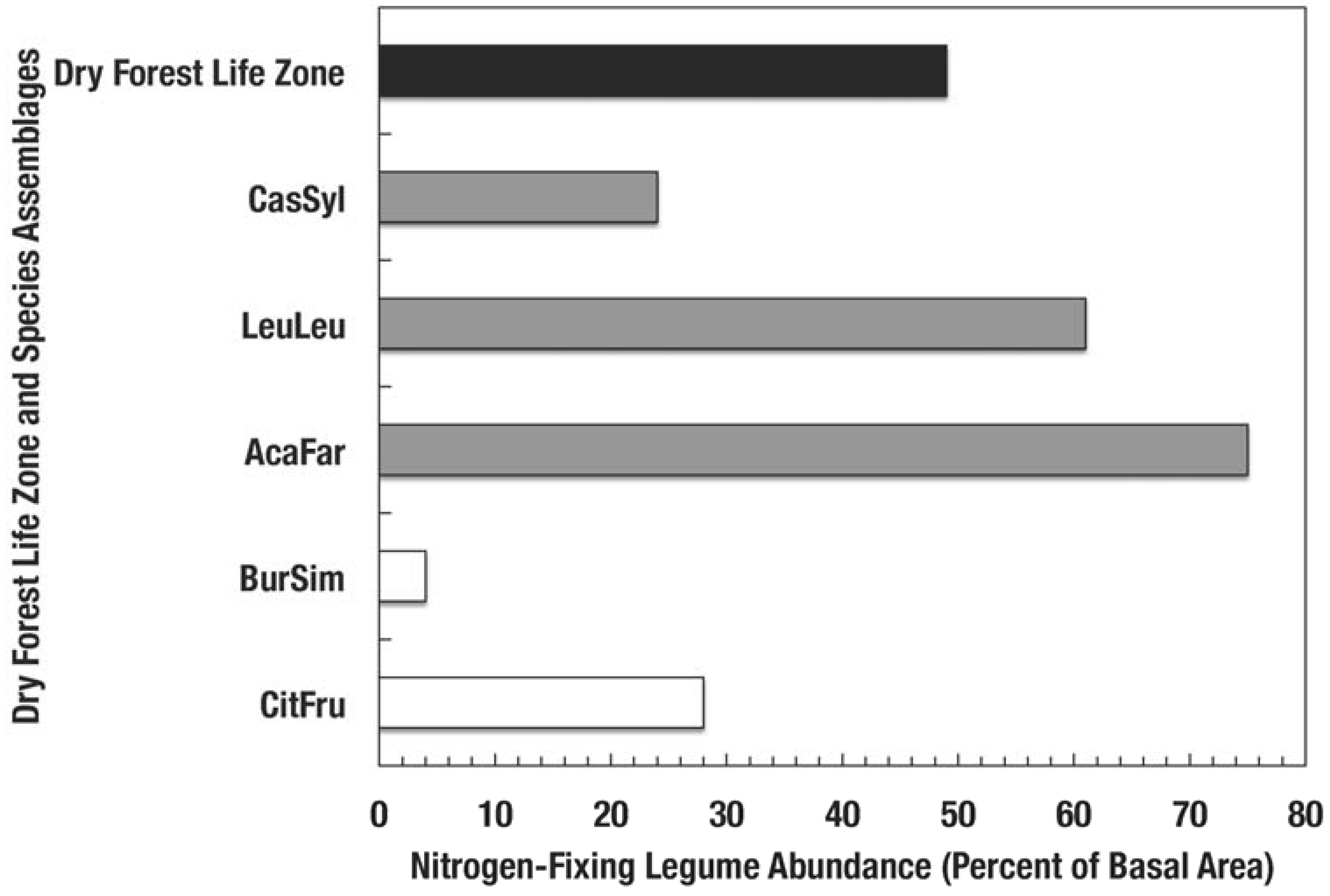
3.2. Naturalized Species and the Structure of Novel Dry Forests
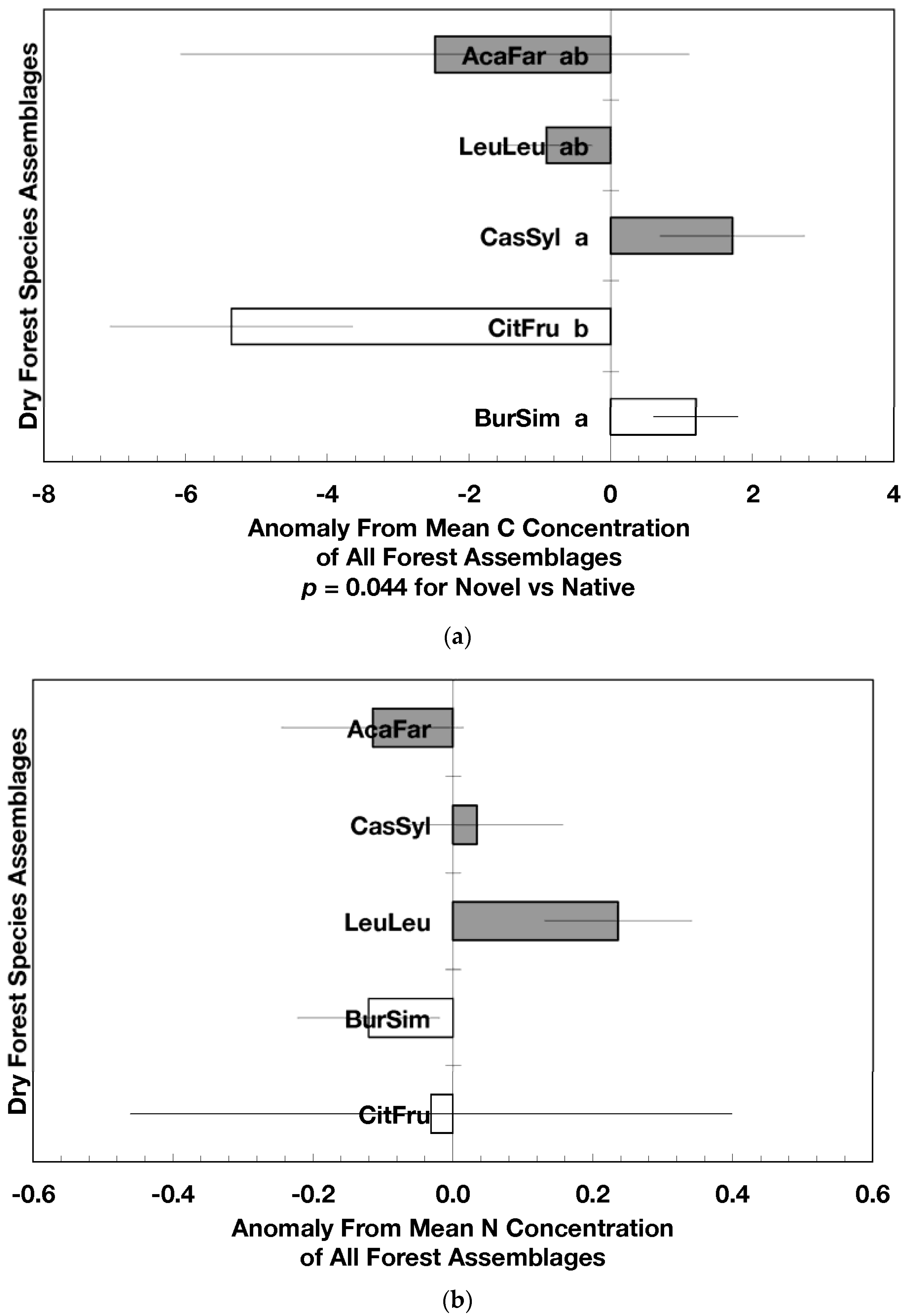
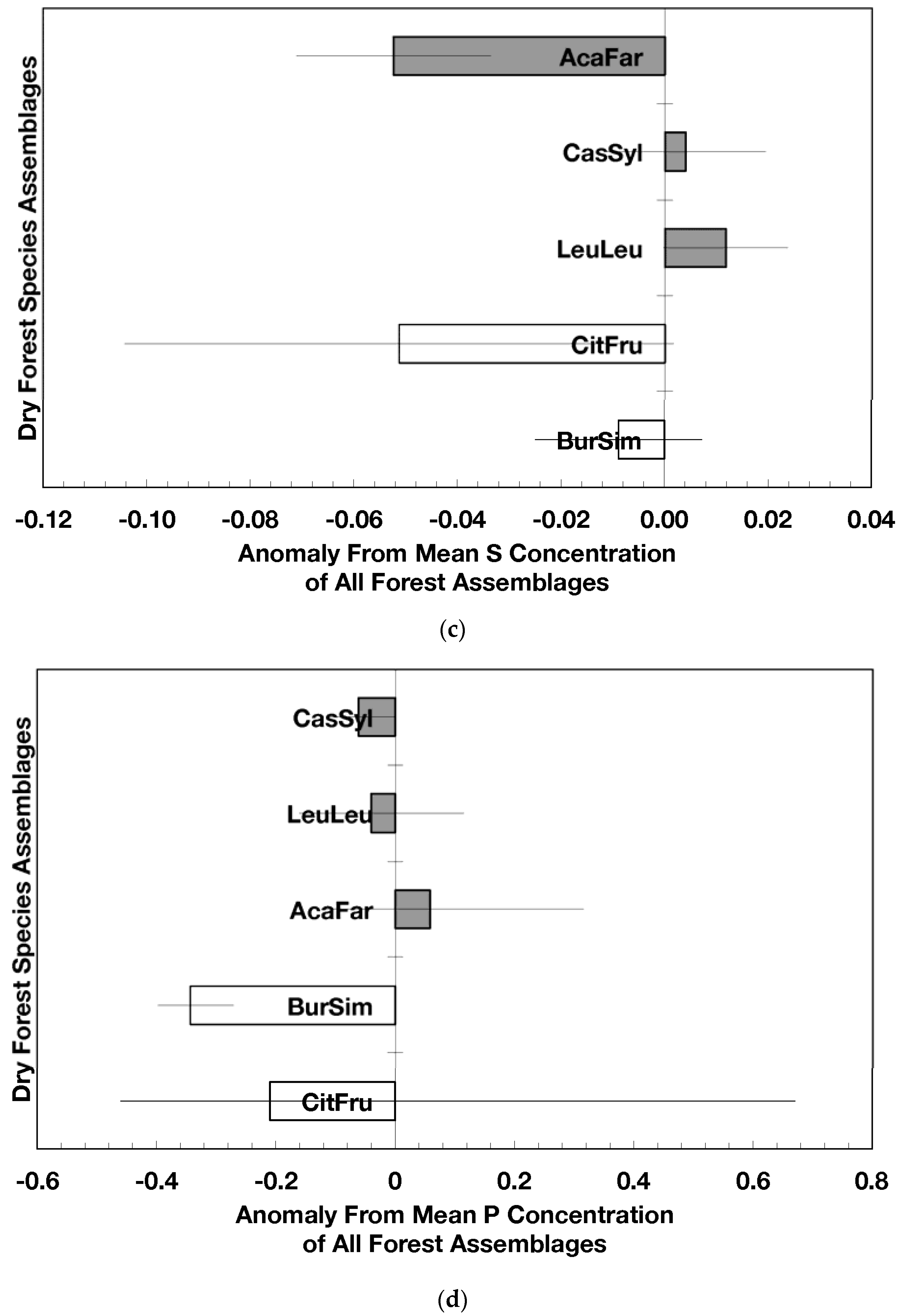
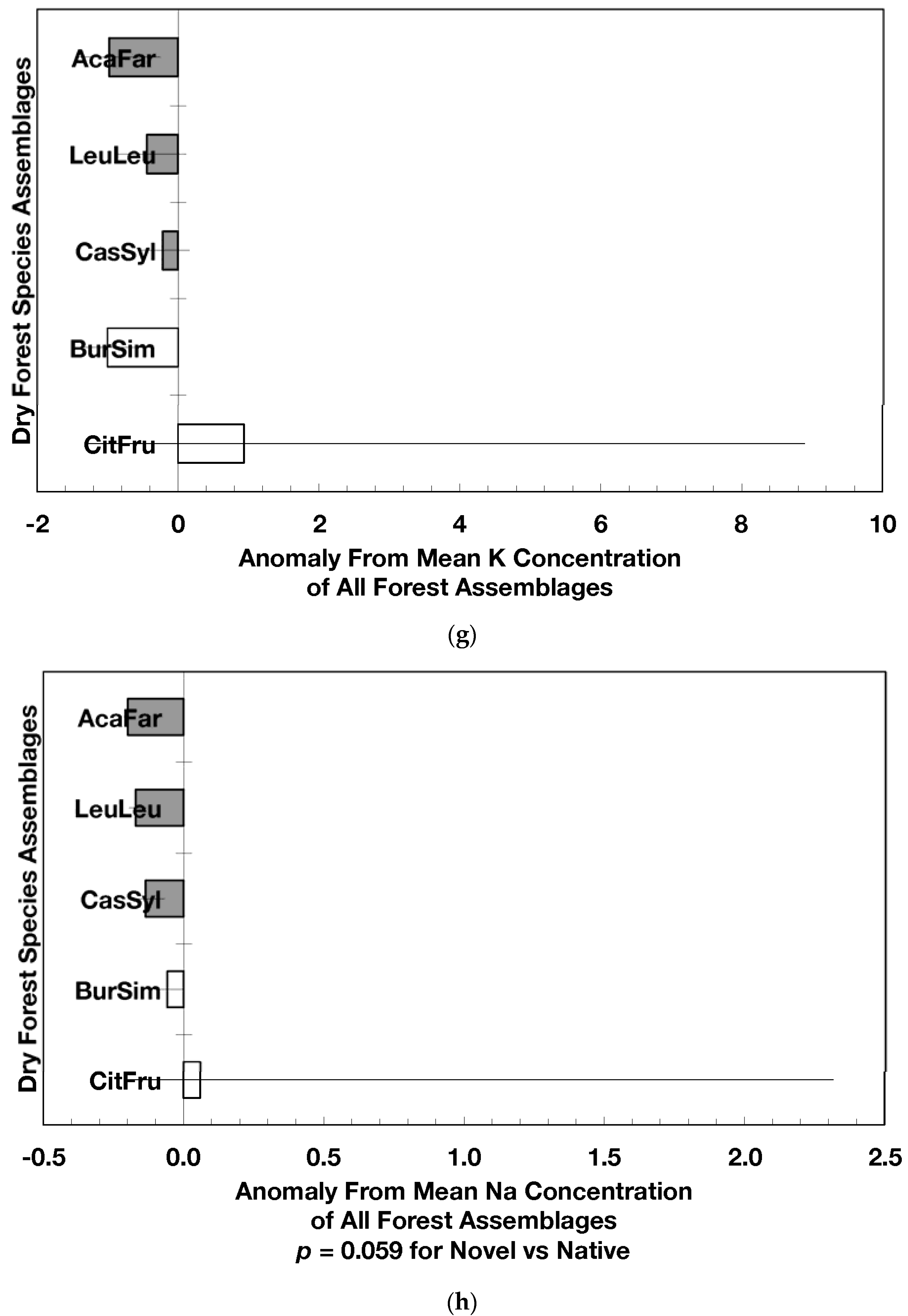
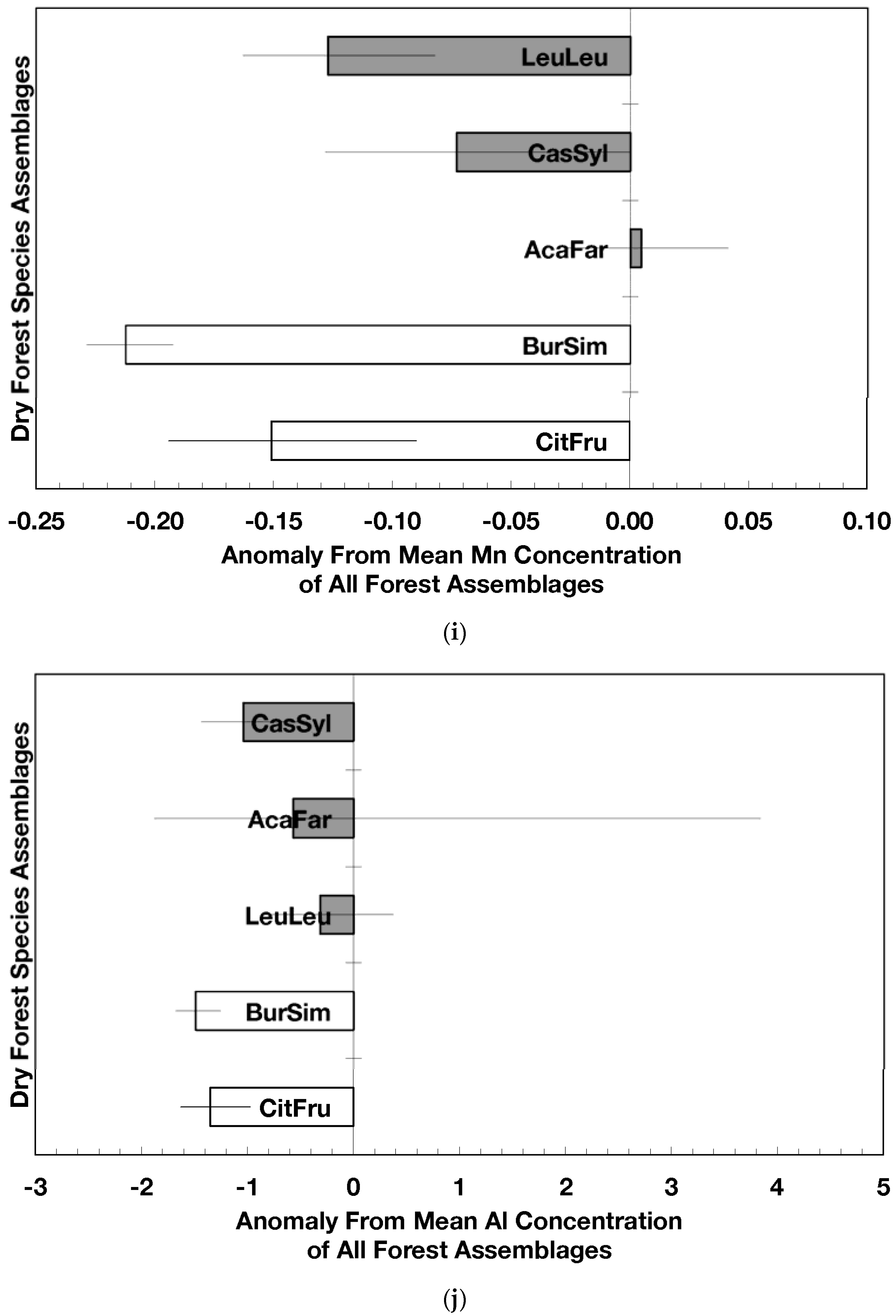
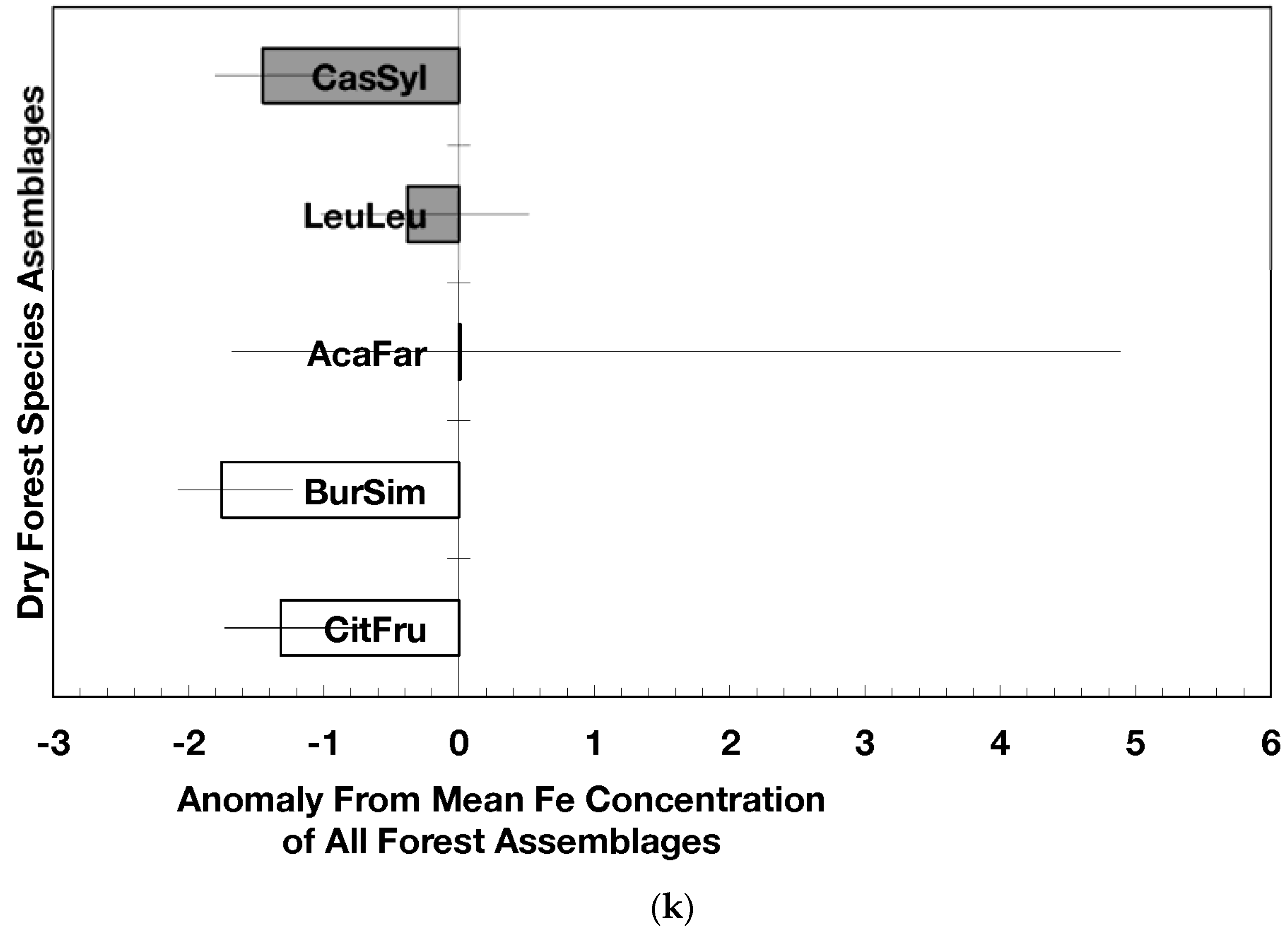
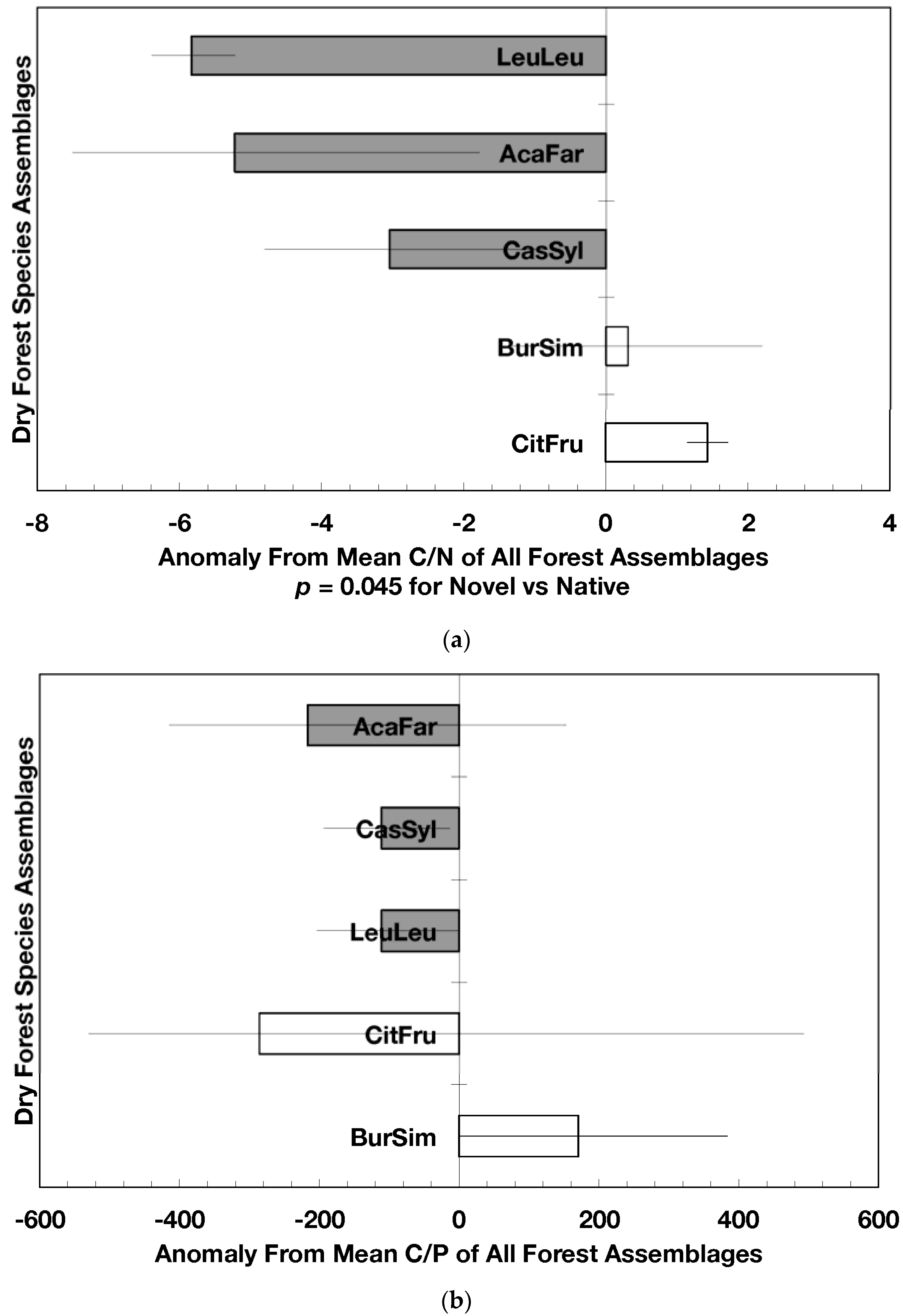
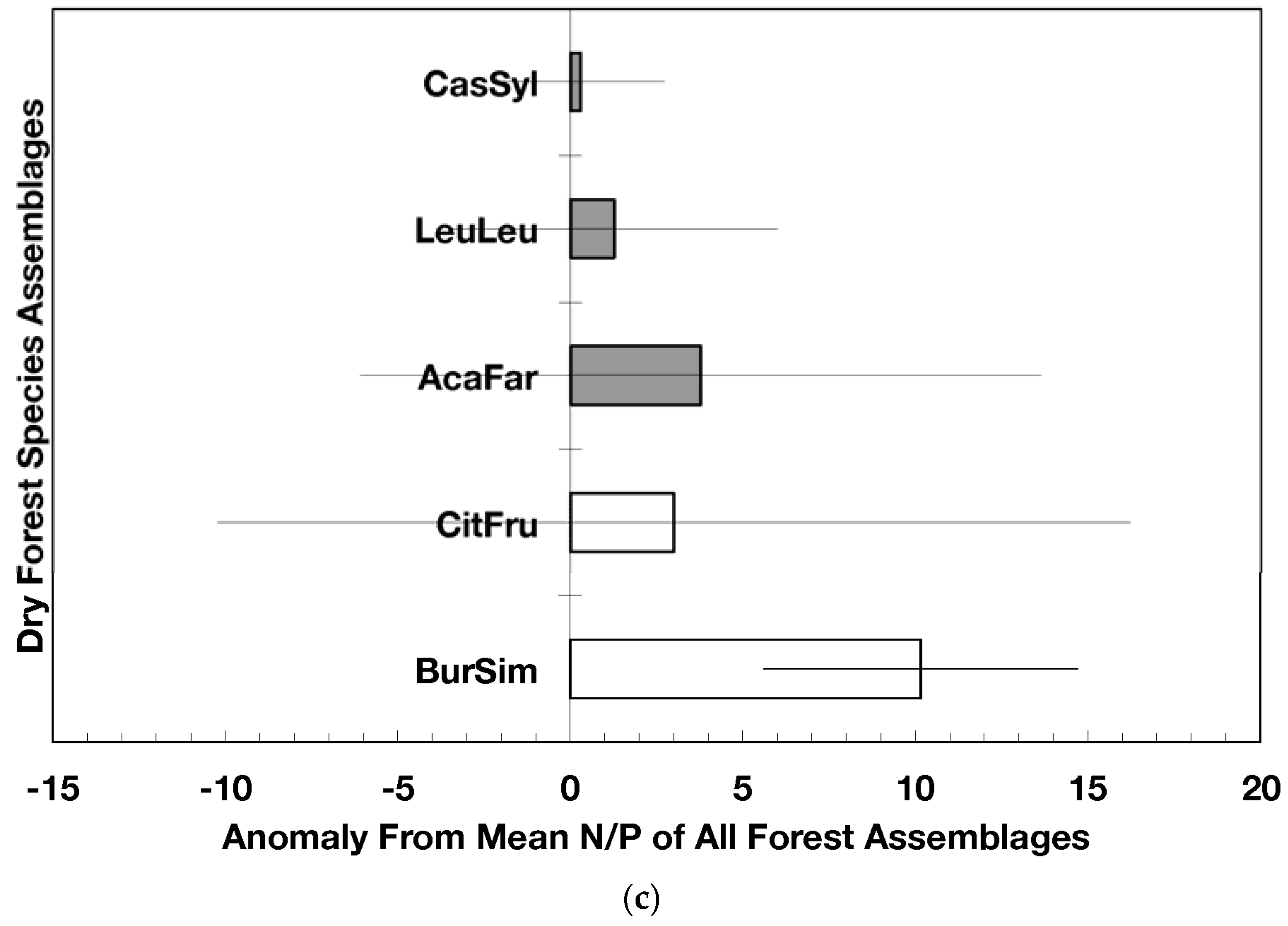
3.3. Stoichiometry of Leaf Litter
4. Discussion
4.1. Species Dominance, Density, and Novelty
4.2. The Importance of Species
4.3. Stoichiometry of Novel and Native Dry Forests
4.4. Implications of Novelty to Functioning and Conservation of Dry Forests
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holdridge, L.R. Life Zone Ecology; Tropical Science Center: San José, Costa Rica, 1967. [Google Scholar]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Dry forests of Central America and the Caribbean. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 9–34. [Google Scholar]
- Maass, J.M. Conversion of tropical dry forest to pasture and agriculture. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 399–422. [Google Scholar]
- Ramjohn, I.A.; Murphy, P.G.; Burton, T.M.; Lugo, A.E. Survival and rebound of Antillean dry forests: Role of forest fragments. Forest Ecol. Manag. 2012, 284, 124–132. [Google Scholar] [CrossRef]
- Rudel, T.K.; Lugo, M.P.; Zichal, H. When fields revert to forest: Development and spontaneous reforestation in post-war Puerto Rico. Prof. Geogr. 2000, 52, 386–397. [Google Scholar] [CrossRef]
- Rudel, T.K. Tropical Forests: Regional Paths of Destruction and Regeneration in the Late Twentieth Century; Columbia University Press: New York, NY, USA, 2005. [Google Scholar]
- Lugo, A.E.; Helmer, E. Emerging forests on abandoned land: Puerto Rico’s new forests. For. Ecol. Manag. 2004, 190, 145–161. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.S.; Hall, C.M. (Eds.) Novel Ecosystems: Intervening in the New Ecological World Order; Willey-Blackwell: West Sussex, UK, 2013. [Google Scholar]
- Pearce, F. The New Wild: Why Invasive Species Will Be Nature's Salvation; Beacon Press: Boston, MA, USA, 2015. [Google Scholar]
- Martinuzzi, S.; Lugo, A.E.; Brandeis, T.J.; Helmer, E.H. Geographic distribution and level of novelty of Puerto Rican forests. In Novel Ecosystems: Intervening in the New Ecological World Order; Hobbs, R.J., Higgs, E.S., Hall, C., Eds.; Wiley: Oxford, UK, 2013; pp. 81–87. [Google Scholar]
- Erickson, H.E.; Helmer, E.H.; Brandeis, T.J.; Lugo, A.E. Controls of fallen leaf chemistry and forest floor element masses in native and novel forests across a tropical island. Ecosphere 2014, 5, 48. [Google Scholar] [CrossRef]
- Brandeis, T.J.; Helmer, E.; Vega, H.M.; Lugo, A.E. Climate shapes the novel plant communities that form after deforestation in Puerto Rico and the U.S. Virgin Islands. For. Ecol. Manag. 2009, 258, 1704–1718. [Google Scholar] [CrossRef]
- Whittaker, R.H. Dominance and diversity in land plant communities: Numerical relations of species express the importance of competition in community function and evolution. Science 1965, 147, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H. Communities and Ecosystems; The Macmillan Company: Toronto, ON, Canada, 1970. [Google Scholar]
- Brown, S.; Lugo, A.E. The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica 1982, 14, 161–187. [Google Scholar] [CrossRef]
- Martínez, Y.A. Biomass distribution and primary productivity of tropical dry forests. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 326–345. [Google Scholar]
- Gentry, A.H. Diversity and floristic composition of neotropical dry forests. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 146–194. [Google Scholar]
- Acevedo-Rodríguez, P.; Strong, M.T. Floristic richness and affinities in the West Indies. Bot. Rev. 2008, 74, 5–36. [Google Scholar] [CrossRef]
- Kiehn, M. Invasive alien species and islands. In The Biology of Island Floras; Bramwell, D., Caujapé-Castells, J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 365–384. [Google Scholar]
- Francis, J.K.; Liogier, H.A. Naturalized Exotic Tree Species in Puerto Rico; General Technical Report SO-82; USDA, Forest Service, Southern Forest Experiment Station: New Orleans, LA, USA, 1991. [Google Scholar]
- Molina Colón, S.; Lugo, A.E.; Ramos, O. Novel dry forests in southwestern Puerto Rico. For. Ecol. Manag. 2011, 262, 170–177. [Google Scholar] [CrossRef]
- Molina-Colón, S.; Lugo, A.E. Recovery of a subtropical dry forest after abandonment of different land uses. Biotropica 2006, 38, 354–364. [Google Scholar] [CrossRef]
- Molina Colón, S. Long-Term Recovery of a Caribbean Dry Forest after Abandonment of Different Land Uses in Guánica, Puerto Rico. Dissertation, University of Puerto Rico, Río Piedras, Puerto Rico, 1998. [Google Scholar]
- Lugo, A.E.; Brandeis, T.A. New mix of alien and native species coexist in Puerto Rico’s landscapes. In Biotic Interactions in the Tropics. Their Role in the Maintenance of Species Diversity; Burslem, D., Pinard, M., Hartley, S., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 484–509. [Google Scholar]
- Lugo, A.E. Los bosques. In Biodiversidad de Puerto Rico. Vertebrados Terrestres y Ecosistemas; Joglar, R.L., Ed.; Editorial del Instituto de Cultura Puertorriqueña: San Juan, PR, USA, 2005; pp. 395–548. [Google Scholar]
- Jardim, F.C.S.; Hosokawa, R.T. Estrutura da floresta equa- torial úmida da Estação Experimental de Silvicultura Tropical do INPA. Acta Amazon. 1986, 16–17, 411–508. [Google Scholar]
- Beard, J.S. The Natural Vegetation of the Windward and Leeward Islands. Oxford Forestry Memoirs 21; The Clarendon Press: Oxford, UK, 1949. [Google Scholar]
- Whittaker, R.J. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Lugo, A.E. Visible and invisible effects of hurricanes on forest ecosystems: An international review. Austral Ecol. 2008, 33, 368–398. [Google Scholar] [CrossRef]
- Lugo, A.E. Dominancia y diversidad de plantas en Isla de Mona. Acta Cient. 1991, 5, 65–71. [Google Scholar]
- Kennaway, T.; Helmer, E.H. The forest types and ages cleared for land development in Puerto Rico. GISci. Remote Sens. 2007, 44, 356–382. [Google Scholar] [CrossRef]
- Brandeis, T.J.; Turner, J.A. Puerto Rico’s Forests, 2009; USDA Forest Service Resource Bulletin SRS-191; Southern Research Station: Ashville, NC, USA, 2013. [Google Scholar]
- Hulshof, C.M.; Yrízar, A.M.; Burques, A.; Boyle, B.; Enquist, B.J. Plant functional trait variation in tropical dry forests: A review and synthesis. In Tropical Dry Forests in the Americas: Ecology, Conservation, and Management; Sánchez-Azofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 129–140. [Google Scholar]
- Lopezaraiza-Mikel, M.; Quesada, M.; Álvarez-Añorve, M.; Ávila-Cabadilla, L.; Martén-Rodriguez, S.; Calvo-Alvarado, J.; do-Santo, M.M.; Fernandes, G.W.; Sánchez-Azofeifa, A.; de J. Aguilar-Aguilar, M.; et al. Phenological patterns of tropical dry forests along latitudinal and successional gradients in the Neotropics. In Tropical Dry Forests in the Americas; Sánchez-Azofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 101–128. [Google Scholar]
- Abelleira Martínez, O.; Lugo, A.E. Post sugarcane succession in moist alluvial sites in Puerto Rico. In Post-Agricultural Succession in the Neotropics; Myster, R.W., Ed.; Springer: New York, NY, USA, 2008; pp. 73–92. [Google Scholar]
- Jaramillo, V.J.; Sanford, R.L. Nutrient cycling in tropical deciduous forests. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 346–361. [Google Scholar]
- Lugo, A.E. Can we manage tropical landscapes? An answer from the Caribbean perspective. Landsc. Ecol. 2002, 17, 601–615. [Google Scholar] [CrossRef]
- Parrotta, J.A. Leucaena leucocephala (Lam.) de Wit. Leucaena; SO-ITF-SM-52; International Institute of Tropical Forestry, USDA Forest Service: Río Piedras, Puerto Rico, 1992. [Google Scholar]
- Santiago García, R.J.; Colón, S.M.; Sollins, P.; van Bloem, S.J. The role of nurse trees in mitigating fire effects on tropical dry forest restoration: A case study. Ambio 2008, 37, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin, Germany, 2003. [Google Scholar]
- Barberena-Arias, M.F. Single Tree Species Effects on Temperature, Nutrients and Arthropod Diversity in Litter and Humus in the Guánica Dry Forest. Ph.D. Thesis, University of Puerto Rico, Río Piedras, Puerto Rico, 2008. [Google Scholar]


| Forest Status | Stem Density (stems/ha) | Basal Area (m2/ha) | Aboveground Biomass (Mg/ha) | Tree Height (m) |
|---|---|---|---|---|
| Novel | 3103 | 9.71 | 36.43 | 5.83 |
| Native | 4348 | 16.4 | 70.33 | 7.3 |
| p value | 0.012 | 0.011 | 0.0002 | 0.002 |
| Species Assemblage and Replicates | Total Mass | Leaf Mass | C | N | S | P | Ca | Mg | K | Na | Mn | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CitFru (3) | 285 | 94 | 131 | 4.2 | 1.2 | 0.18 | 7.9 | 0.79 | 0.55 | 0.07 | 0.04 | 0.49 | 0.65 |
| BurSim (9) | 666 | 434 | 333 | 10.1 | 1.9 | 0.26 | 19.2 | 1.39 | 0.9 | 0.23 | 0.09 | 0.87 | 0.63 |
| CasSyl (14) | 465 | 234 | 224 | 7.5 | 1.4 | 0.29 | 8.1 | 1.29 | 1.06 | 0.09 | 0.10 | 0.98 | 0.83 |
| LeuLeu (10) | 691 | 225 | 325 | 11.7 | 1.9 | 0.44 | 16.7 | 2.05 | 1.33 | 0.08 | 0.11 | 0.69 | 0.46 |
| AcaFar (5) | 325 | 89 | 154 | 4.8 | 1.2 | 0.15 | 7.6 | 0.66 | 0.44 | 0.03 | 0.03 | 0.68 | 0.70 |
| Life Zone (12) | 543 | 145 | 253 | 6.7 | 1.4 | 0.21 | 14.1 | 1.22 | 0.69 | 0.04 | 0.05 | 0.76 | 0.82 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugo, A.E.; Erickson, H.E. Novelty and Its Ecological Implications to Dry Forest Functioning and Conservation. Forests 2017, 8, 161. https://doi.org/10.3390/f8050161
Lugo AE, Erickson HE. Novelty and Its Ecological Implications to Dry Forest Functioning and Conservation. Forests. 2017; 8(5):161. https://doi.org/10.3390/f8050161
Chicago/Turabian StyleLugo, Ariel E., and Heather E. Erickson. 2017. "Novelty and Its Ecological Implications to Dry Forest Functioning and Conservation" Forests 8, no. 5: 161. https://doi.org/10.3390/f8050161






