Influence of Cutting Type and Fertilization in Production of Containerized Poplar Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Cutting Preparation, Soil Substrates, Growth Containers and Planting
2.2. Experimental Design
2.3. Growth Conditions and Fertilizers
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Survival of Transplanted Cuttings
3.2. Growth of Fertilized and Un-Fertilized Plants, Interactions between Clone and Cutting Phenotype
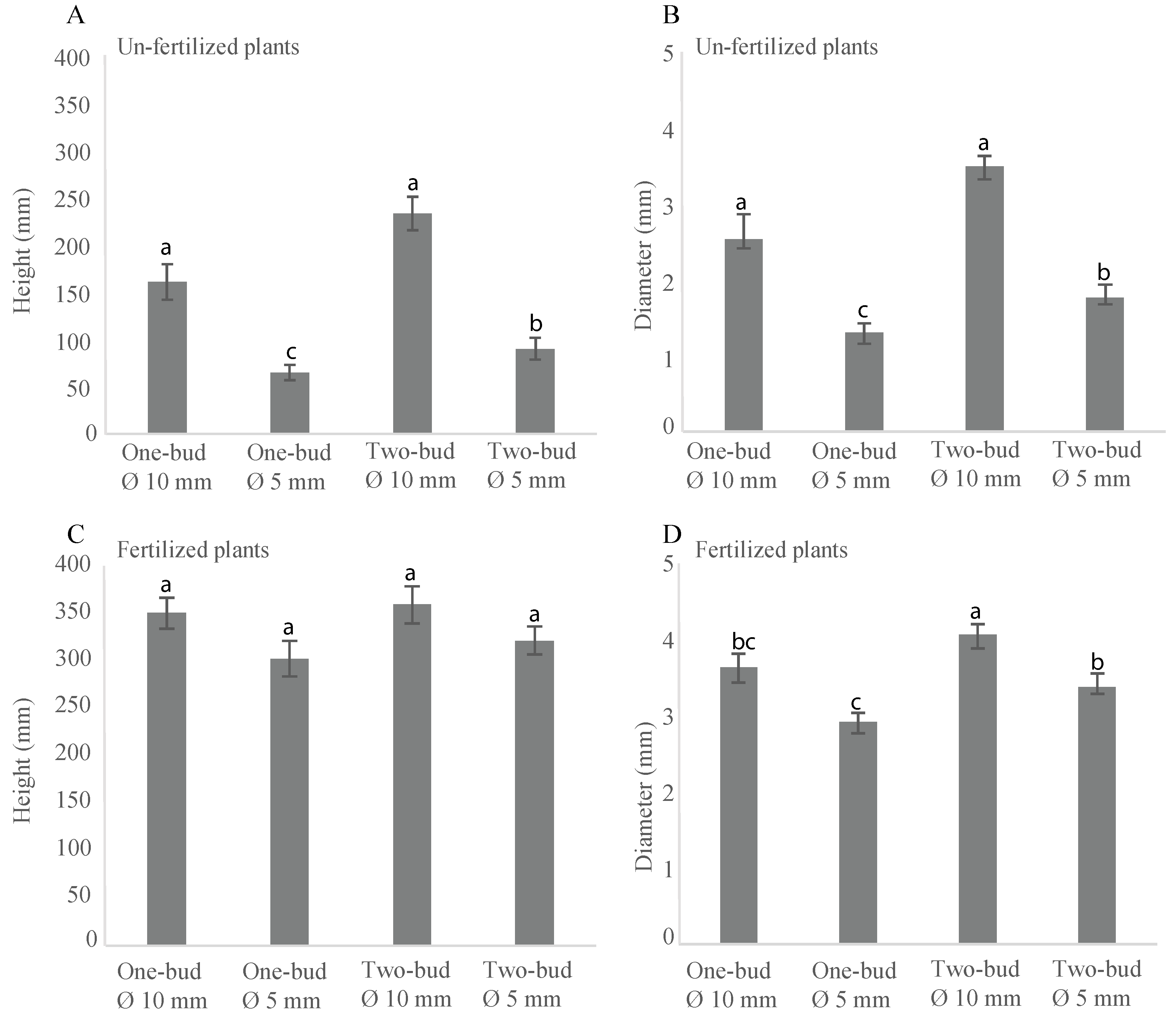
3.3. Height and Diameter Growth
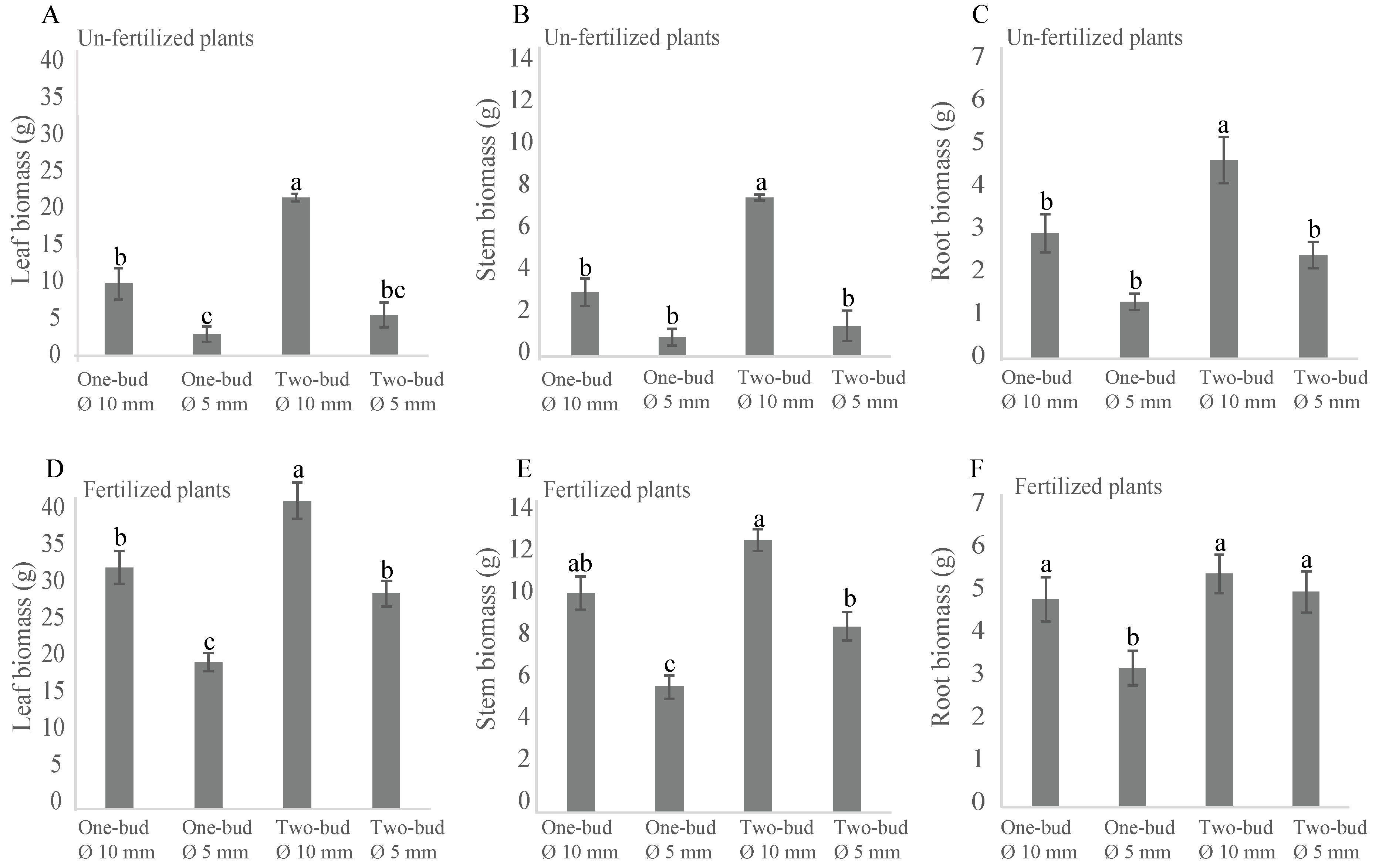
3.4. Analysis of Leaf Stem, and Root Biomasses
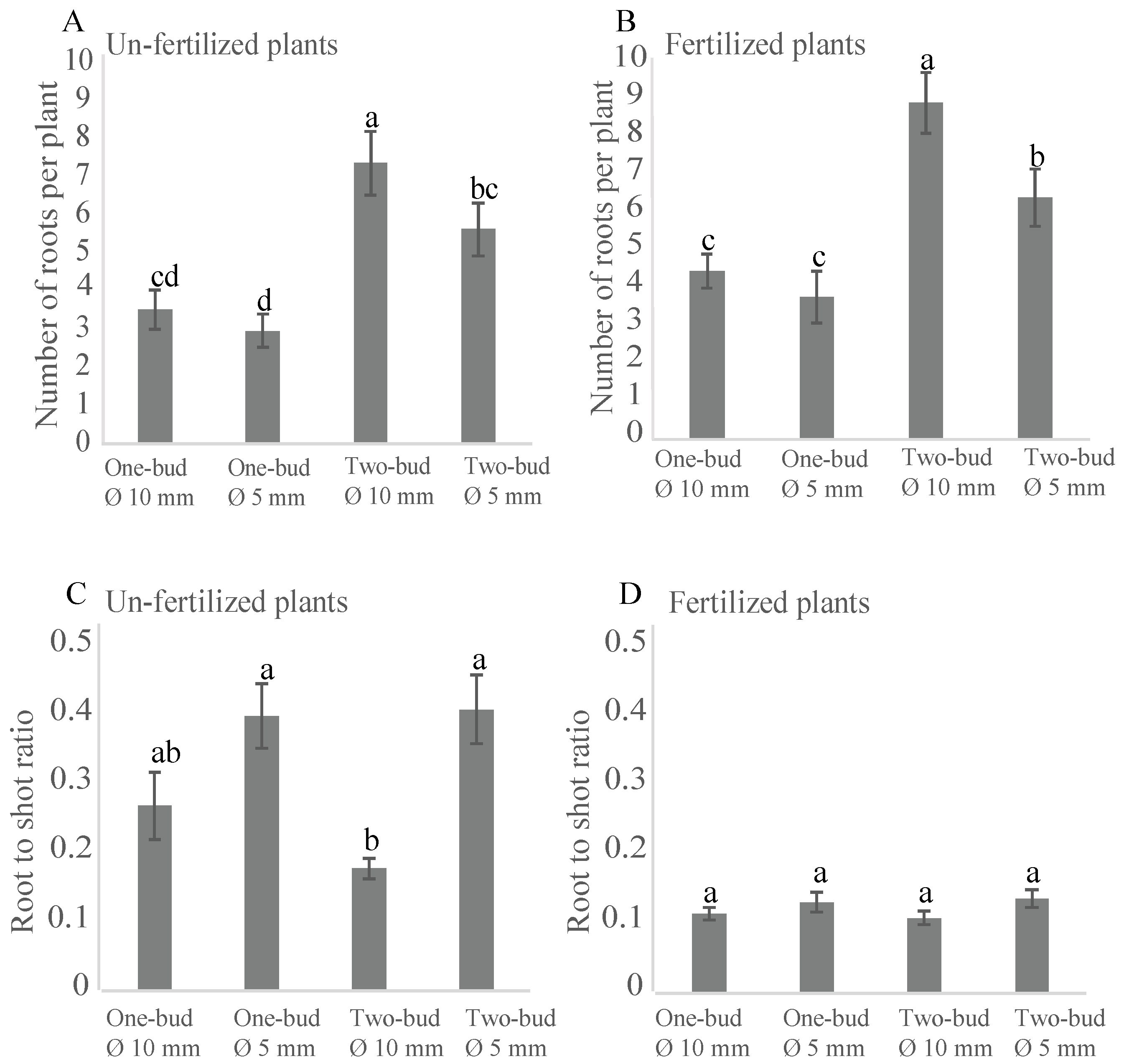
3.5. Root Number and Root-To-Shoot Ratios
4. Discussion
5. Conclusions and Practical Implications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christersson, L. Poplar plantations for paper and energy in the south of Sweden. Biomass Bioenergy 2008, 32, 997–1000. [Google Scholar] [CrossRef]
- Christersson, L. Wood production potential in poplar plantations in Sweden. Biomass Bioenergy 2010, 34, 1289–1299. [Google Scholar] [CrossRef]
- Simon, B.-G.; David, P.; Christian, M.; Bélanger, N. Juvenile growth of hybrid poplars on acidic boreal soil determined by environmental effects of soil preparation, vegetation control, and fertilization. For. Ecol. Manag. 2011, 261, 620–629. [Google Scholar]
- Stanturf, J.A.; von Oosten, C.; Netzer, D.A.; Colman, M.D.; Prtwood, C.J. Ecology and silviculture of poplar plantations. In Poplar Culture in North America; Dickman, D.I., Eckenwald, J.E., Richardson, J., Eds.; National Research Council of Canada Research Press: Ottawa, ON, Canada, 2001; pp. 152–206. [Google Scholar]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Yield in 8 year-old hybrid poplar plantations on abandoned farmland along climatic and soil fertility gradients. For. Ecol. Manag. 2012, 267, 228–239. [Google Scholar] [CrossRef]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Biomass and volume yield in mature hybrid poplar plantations on temperate abandoned farmland. Forests 2014, 5, 3107–3130. [Google Scholar] [CrossRef]
- Tullus, A.; Rytter, L.; Tullus, T.; Weih, M.; Tullus, H. Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scand. J. For. Res. 2011, 27, 10–29. [Google Scholar] [CrossRef]
- Tullus, H. Short-Rotation Deciduous Plantations as an Alternative Land Use for Abandoned Agricultural Lands; Project Report No. 273 of the Estonian Agricultural Ministry; Department of Silviculture, Estonian University of Life Sciences: Tartu, Estonia, 2005. (In Estonian) [Google Scholar]
- Bouma, T.; Bryla, D. On the assessment of root and soil respiration for soils of different textures: Interactions with soil moisture contents and soil CO2 concentrations. Plant Soil 2000, 227, 215–221. [Google Scholar] [CrossRef]
- Coll, L.M.; Delagrange, C.; Berninger, S.; Berninger, F. Growth, allocation and leaf gas exchanges of hybrid poplar plants in their establishment phase on previously forested sites: Effect of different vegetation management techniques. Ann. For. Sci. 2007, 64, 275–285. [Google Scholar] [CrossRef]
- Stanturf, J.A.; van Oostem, C. Operational Poplar and Willow Culture. In Poplars and Willows, Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; CABI: Oxfordshire, UK, 2014; p. 231. [Google Scholar]
- Otto, S.L.; Loddo, D.; Zanin, G. Weed-poplar competition dynamics and yield loss in Italian short-rotation forestry. Weed Res. 2010, 50, 153–162. [Google Scholar] [CrossRef]
- Tuskan, G.A. Short-rotation woody crop supply systems in the United States: What do we know and what do we need to know? Biomass Bioenergy 1998, 14, 307–315. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Carlson, W.C. Root system considerations in the quality of loblolly pine seedlings. South. J. Appl. For. 1986, 10, 87–92. [Google Scholar]
- Burdett, A.N. Physiological processes in plantation establishment and the development of specifications for forest planting stock. Can. J. For. Res. 1990, 20, 415–427. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Davies, W.J. Control of water balance in transplanted trees. Arboriculture 1975, 1, 1–10. [Google Scholar]
- Rietveld, W.J. Transplanting stress in bareroot conifer seedlings: Its development and progression to establishment. North. J. Appl. For. 1989, 6, 99–107. [Google Scholar]
- Nilsson, U.; Luoranen, J.; Kolström, T.; Örlander, G.; Puttonen, P. Reforestation with planting in northern Europe. Scand. J. For. Res. 2010, 25, 283–294. [Google Scholar] [CrossRef]
- McDonald, P. Container seedlings outperform barefoot stock: Survival and growth after 10 years. New For. 1991, 5, 147–156. [Google Scholar] [CrossRef]
- Nilsson, U.; Örlander, G. Effects of regeneration methods on drought damage to newly planted Norway spruce seedlings. Can. J. For. Res. 1995, 25, 790–802. [Google Scholar] [CrossRef]
- Thiffault, N.; Jobidon, R.; Munson, A.D. Performance and physiology of large containerized and blare-root spruce seedlings in relation to scarification and competition in Quebec (Canada). Ann. For. Sci. 2003, 60, 645–655. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Ecophysiology of Northern Spruce Species, the Performance of the Planted Seedling; NRC Research Press: Vancouver, BC, Canada, 2000. [Google Scholar]
- Böhlenius, H.; Övergaard, R. Impact of seedling type on early growth of poplar plantations on forest and agricultural land. Scand. J. For. Res. 2016, 31, 733–741. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E. Plant Propagation: Principles and Practices; Prentice-Hall: Englewood Cliffs, NJ, USA, 1975. [Google Scholar]
- DeBell, D.S.; Harrington, C.A. Productivity of Populus in monoclonal and polyclonal blocks at three spacings. Can. J. For. Res. 1997, 27, 978–985. [Google Scholar] [CrossRef]
- Rossi, P. Length of cuttings in juvenile development of a hybrid poplar clone. New For. 1991, 5, 211–218. [Google Scholar] [CrossRef]
- Ferm, A.; Hytönen, J.; Vuori, J. Effect of spacing and nitrogen fertilization on the establishment and biomass production of short rotation poplar in Finland. Biomass 1989, 18, 95–108. [Google Scholar] [CrossRef]
- DesRochers, A.; Thomas, B.R. A comparison of pre-planting treatments on hardwood cuttings of four hybrid poplar clones. New For. 2003, 26, 17–32. [Google Scholar] [CrossRef]
- R CoreTeam. A language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.Org/ (accessed on 8 October 2014).
- Robison, D.J.; Raffa, K.F. Importance of cutting diameter and method of production on early growth hybrid poplar. Tree Plant. Notes 1996, 47, 76–80. [Google Scholar]
- Dickmann, D.I.; Isebrands, J.G.; Eckenwalder, J.E.; Richardson, J. (Eds.) Poplar Culture in North America; National Research Council of Canada Research Press: Ottawa, ON, Canada, 2001. [Google Scholar]
- Thompson, B.E. Seedling morphological evaluation: What you can tell by looking. In Evaluating Seedling Quality: Principles, Procedures and Predictive Abilities of Major Tests; Duryea, M.L., Ed.; Oregon State University Forest Research Laboratory: Corvallis, OR, USA, 1985. [Google Scholar]
- Johnson, J.D.; Cline, M.L. Seedling quality of southern pines. In Forest Regeneration Manual; Duryea, M.L., Dougherty, P.M., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 143–159. [Google Scholar]
- Mexal, J.G.; Landis, T.D. Target seedling concepts: Height and diameter. In Target Seedling Symposium; Rose, R., Cambell, S.J., Landis, T.D., Eds.; UDSA Forest Service: Roseburg, OR, USA, 1990; pp. 17–35. [Google Scholar]
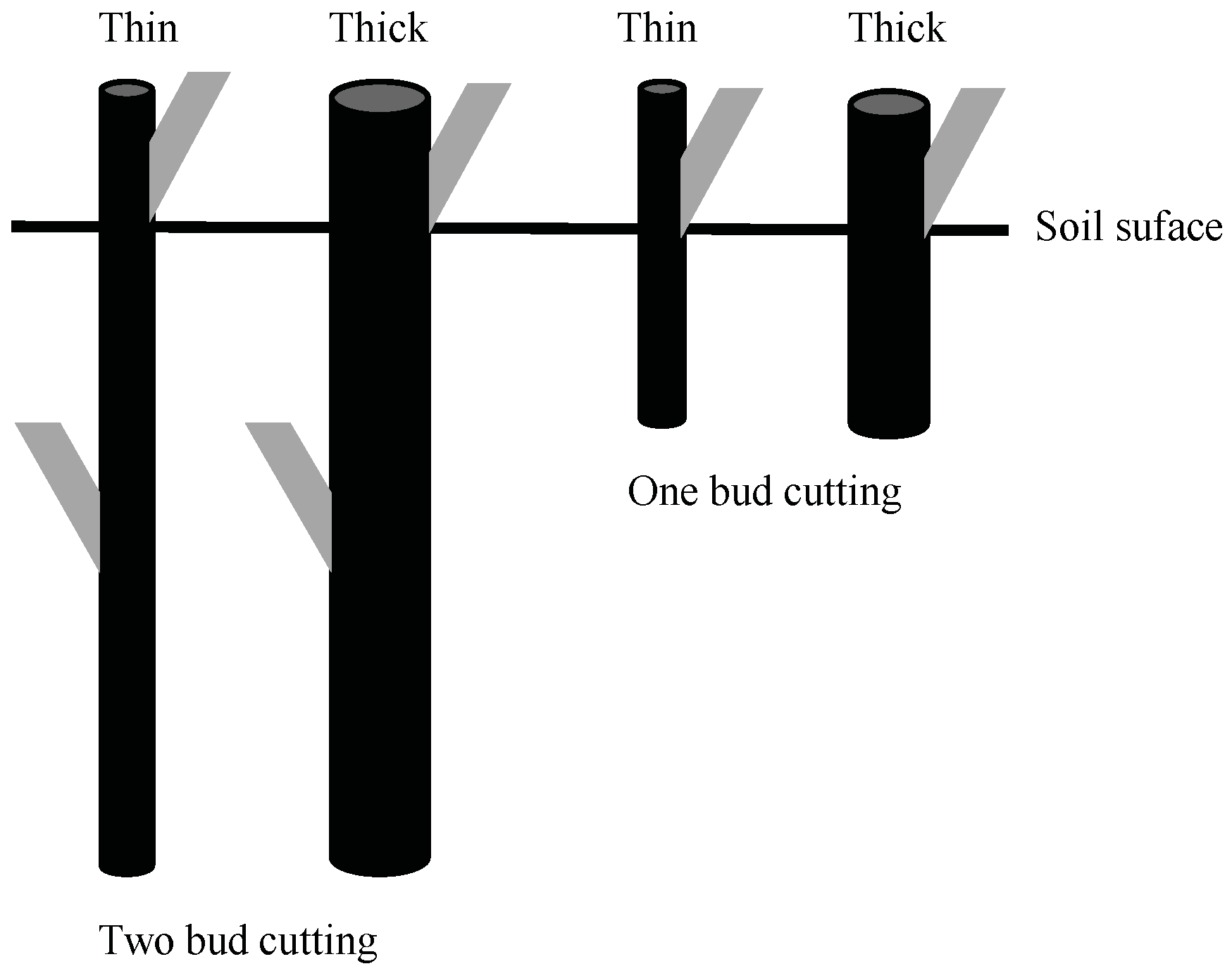
| Poplar Clone | Cutting Type | Diamenter | Length | ||||
|---|---|---|---|---|---|---|---|
| Mean | SE | mean | SE | ||||
| Rochester | Two-bud Ø 5 mm | 4.1 | ± | 0.8 | 44 | ± | 5 |
| Two-bud Ø 10 mm | 9.8 | ± | 1.3 | 53 | ± | 6 | |
| One-bud Ø 5 mm | 4.2 | ± | 0.7 | 96 | ± | 15 | |
| One-bud Ø 10 mm | 9.4 | ± | 1.4 | 106 | ± | 11 | |
| Clone 15 | Two-bud Ø 5 mm | 3.9 | ± | 0.7 | 47 | ± | 5 |
| Two-bud Ø 10 mm | 9.2 | ± | 0.8 | 55 | ± | 6 | |
| One-bud Ø 5 mm | 4.3 | ± | 0.8 | 97 | ± | 18 | |
| One-bud Ø 10 mm | 10.2 | ± | 1.8 | 105 | ± | 11 | |
| OP42 | Two-bud Ø 5 mm | 4.2 | ± | 0.6 | 49 | ± | 6 |
| Two-bud Ø 10 mm | 10.5 | ± | 1.6 | 59 | ± | 5 | |
| One-bud Ø 5 mm | 4.3 | ± | 0.7 | 93 | ± | 13 | |
| One-bud Ø 10 mm | 10.6 | ± | 1.5 | 117 | ± | 12 | |
| Interactions | Fertilization | Height | Diameter | Biomasses | Root to Shot Ratio | Number of Roots | ||
|---|---|---|---|---|---|---|---|---|
| Stem | Leaf | Root | ||||||
| Clone: cutting length | Yes | 0.5 | 0.94 | 0.02 | 0.06 | 0.21 | 0.8 | 0.83 |
| Clone: cutting diameter | Yes | 0.82 | 0.19 | 0.18 | 0.09 | 0.26 | 0.06 | 0.21 |
| Clone: cutting length | No | 0.07 | 0.34 | 0.003 | 0.002 | 0.51 | 0.81 | 0.6 |
| Clone: cutting diameter | No | 0.35 | 0.0001 | 0.21 | 0.36 | 0.09 | 0.64 | 0.11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhlenius, H.; Fransson, T.; Holmström, E.; Salk, C. Influence of Cutting Type and Fertilization in Production of Containerized Poplar Plants. Forests 2017, 8, 164. https://doi.org/10.3390/f8050164
Böhlenius H, Fransson T, Holmström E, Salk C. Influence of Cutting Type and Fertilization in Production of Containerized Poplar Plants. Forests. 2017; 8(5):164. https://doi.org/10.3390/f8050164
Chicago/Turabian StyleBöhlenius, Henrik, Thomas Fransson, Emma Holmström, and Carl Salk. 2017. "Influence of Cutting Type and Fertilization in Production of Containerized Poplar Plants" Forests 8, no. 5: 164. https://doi.org/10.3390/f8050164






