Separating Trends in Whitebark Pine Radial Growth Related to Climate and Mountain Pine Beetle Outbreaks in the Northern Rocky Mountains, USA
Abstract
:1. Introduction
Study Area
2. Materials and Methods
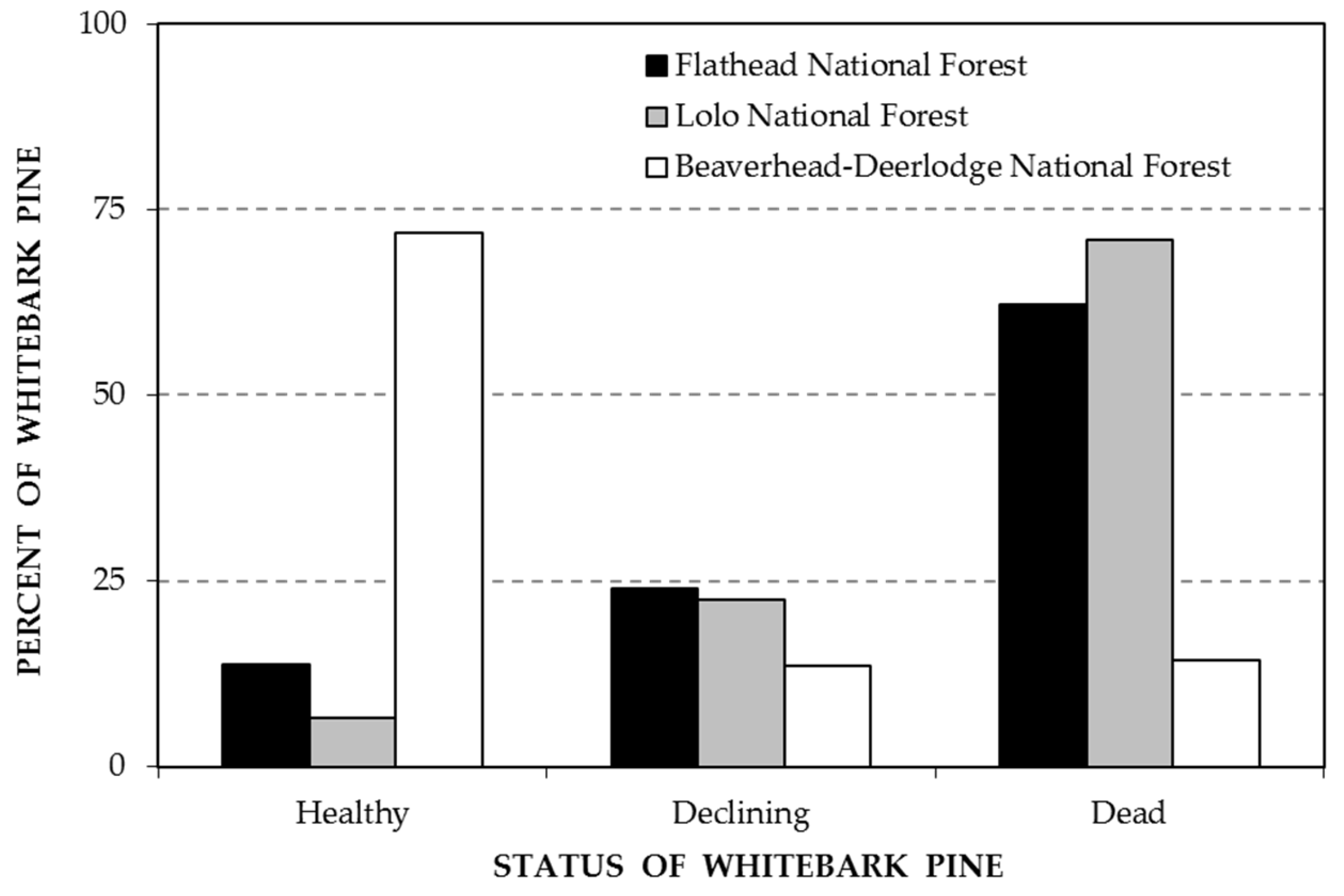
3. Results
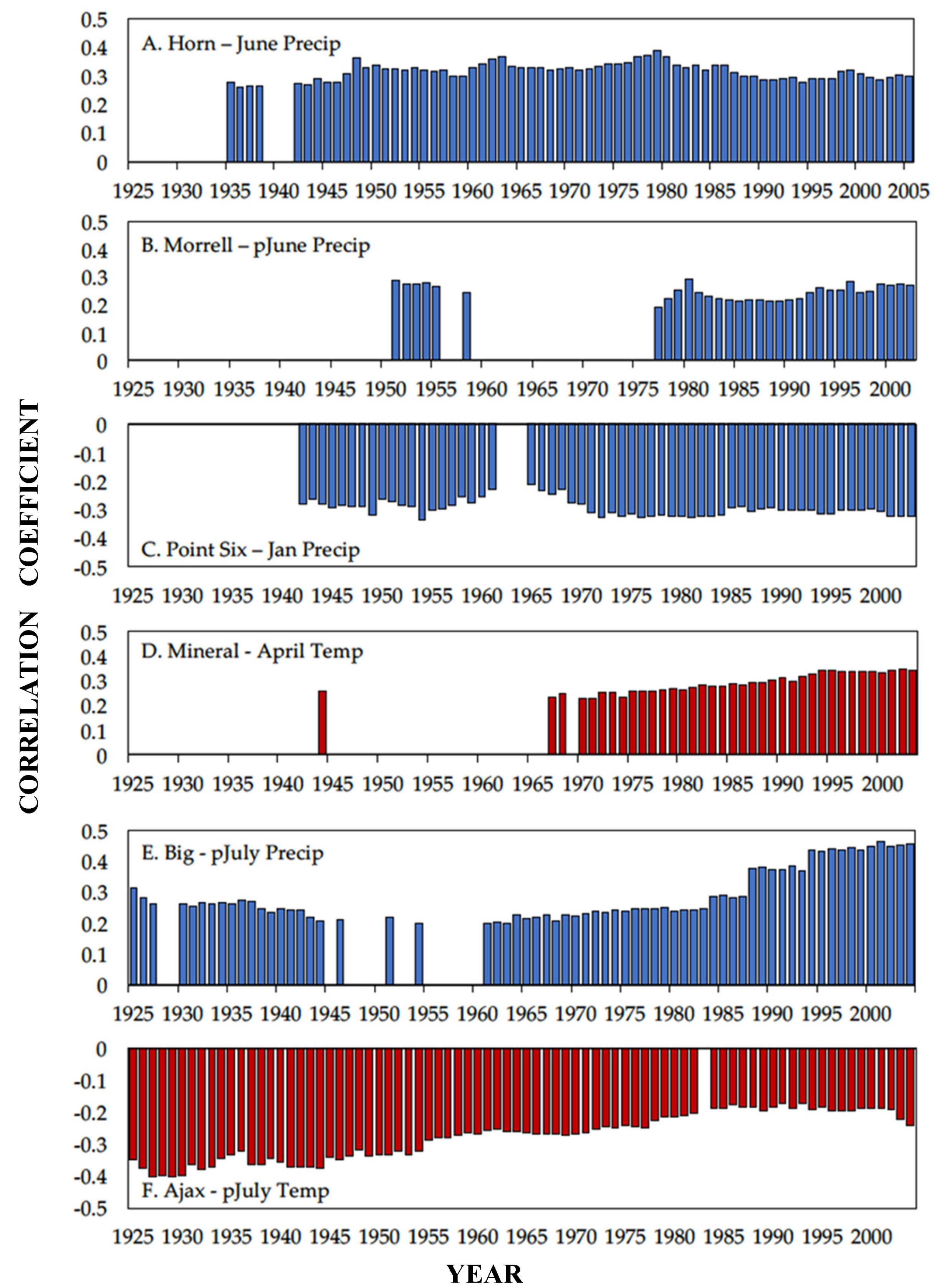
3.1. Climate Response
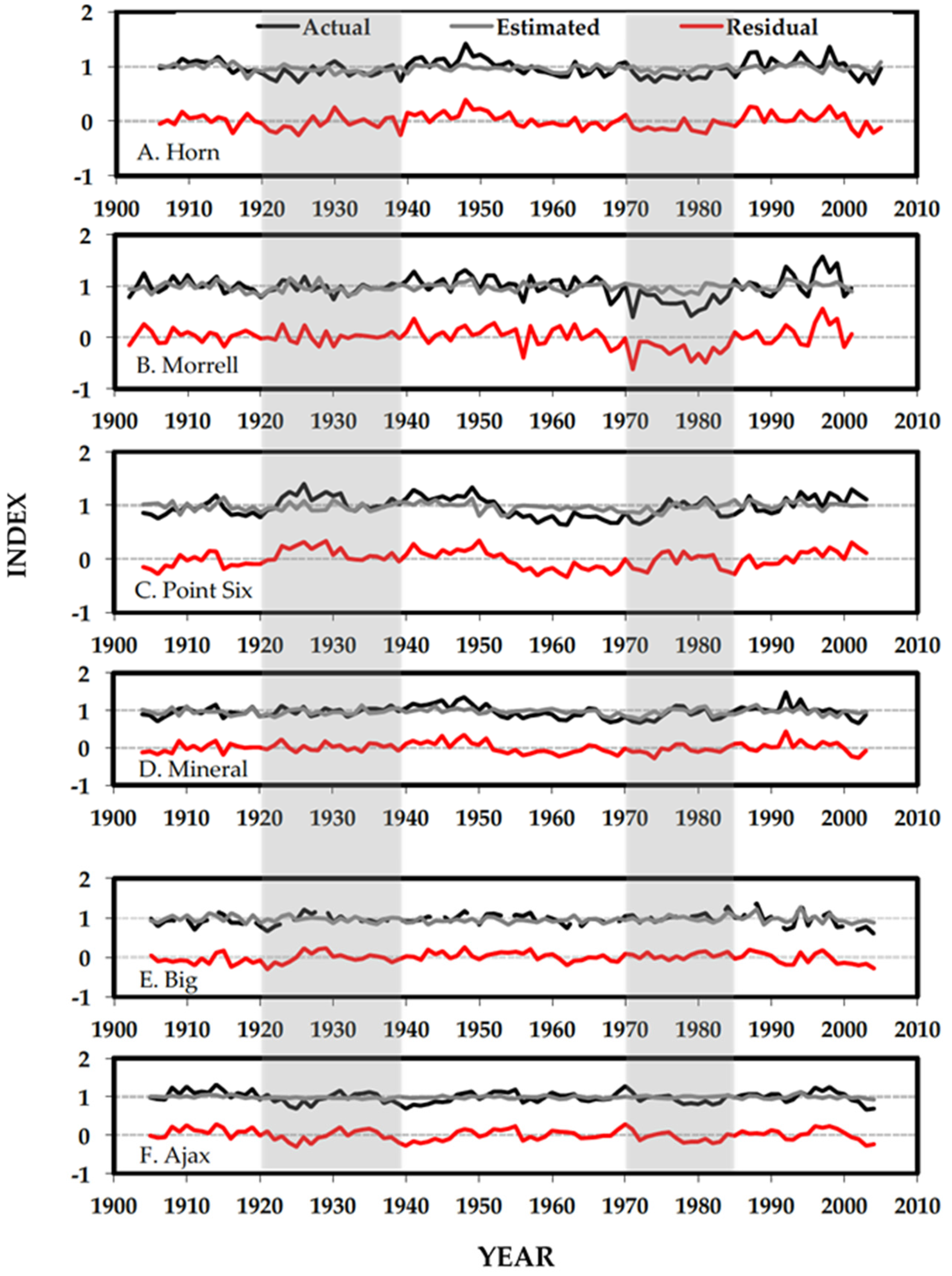
3.2. Separating Disturbance Events and Climate Response
4. Discussion
4.1. Climate Response
4.2. Separating Disturbance Events and Climate Response
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perkins, D.L.; Swetnam, T.W. A dendroecological assessment of whitebark pine in the Sawtooth-Salmon River region, Idaho. Can. J. For. Res. 1996, 26, 2123–2133. [Google Scholar] [CrossRef]
- Biondi, F.; Perkins, D.L.; Cayan, D.R.; Hughes, M.K. July temperature during the second millennium reconstructed from Idaho tree rings. Geophys. Res. Lett. 1999, 26, 1445–1448. [Google Scholar] [CrossRef]
- Luckman, B.H.; Villalba, R. Assessing the synchroneity of glacier fluctuations in the western cordillera of the Americas during the last millennium. In Interhemispheric Climate Linkages; Markgraf, V., Ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Tomback, D.F.; Arno, S.F.; Keane, R.E. The compelling case for management intervention. In Whitebark. Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 3–28. [Google Scholar]
- Kipmueller, K.F. Reconstructed summer temperature in the northern Rocky Mountains wilderness, USA. Quat. Res. 2008, 70, 173–187. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Jewett, J.T.; Lawrence, R.L.; Marshall, L.A.; Gessler, P.E.; Powell, S.L.; Savage, S.L. Spatiotemporal relationships between climate and whitebark pine mortality in the Greater Yellowstone Ecosystem. For. Sci. 2011, 57, 320–335. [Google Scholar]
- Larson, E.R.; van de Gevel, S.L.; Grissino-Mayer, H.D. Variability in fire regimes of high-elevation whitebark pine communities, western Montana, USA. Écoscience 2009, 16, 282–298. [Google Scholar] [CrossRef]
- Tomback, D.F.; Resler, L.M. Invasive pathogens at alpine treeline: Consequences for treeline dynamics. Phys. Geogr. 2008, 28, 397–418. [Google Scholar] [CrossRef]
- Millar, C.I.; Westfall, R.D.; Delany, D.L.; Bokach, M.J.; Flint, A.L.; Flint, L.E. Forest mortality in high-elevation whitebark pine (Pinus albicaulis) forests of eastern California, USA; influence of environmental context, bark beetles, climatic water deficit, and warming. Can. J. For. Res. 2012, 42, 749–765. [Google Scholar] [CrossRef]
- Kipfmueller, K.F. Fire-Climate-Vegetation Interactions in Subalpine Forests of the Selway-Bitterroot Wilderness Area, Idaho and Montana, USA. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 2003; p. 322. [Google Scholar]
- Bunn, A.G.; Lawrence, R.L.; Bellante, G.J.; Waggoner, L.A.; Graumlich, L.J. Spatial variation in distribution and growth patterns of old growth strip-bark pines. Arct. Antarct. Alp. Res. 2003, 35, 323–330. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Logan, J.A.; Bentz, B.J. Model analysis of mountain pine beetle (Coleoptera: Scolytidae) seasonality. Environ. Entomol. 1999, 28, 924–934. [Google Scholar] [CrossRef]
- Campbell, E.M.; Alfaro, R.I.; Hawkes, B. Spatial distribution of mountain pine beetle outbreaks in relation to climate and stand dynamics: A dendroecological analysis. J. Integr. Plant Biol. 2007, 49, 168–178. [Google Scholar] [CrossRef]
- Mitton, J.B.; Ferrenberg, S.M. Mountain pine beetle develops an unprecedented summer generation in response to climate warming. Am. Nat. 2012, 179, E163–E171. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.L.; Taylor, S.W.; Régnière, J.; Safranyik, L. Effects of climate change on range expansion by the mountain pine beetle in British Columbia. In Mountain Pine Beetle Symposium: Challenges and Solutions; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Information Report BC-X-399; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2003; pp. 223–232. [Google Scholar]
- Creeden, E.P.; Hicke, J.A.; Buotte, P.C. Climate, weather, and recent mountain pine beetle outbreaks in the western United States. For. Ecol. Manag. 2014, 312, 239–251. [Google Scholar] [CrossRef]
- Wong, C.M.; Daniels, L.D. Novel forest decline triggered by multiple interactions among climate, an introduced pathogen and bark beetles. Glob. Chang. Biol. 2017, 23, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.; Alfaro, R.I. Growth response in a Douglas-fir/lodgepole pine stand after thinning of lodgepole pine by the mountain pine beetle: A case study. J. Entomol. Soc. B. C. 1990, 87, 16–21. [Google Scholar]
- Alfaro, R.; Campbell, R.; Vera, P.; Hawkes, B.; Shore, T. Dendroecological reconstruction of mountain pine beetle outbreaks in the Chilcotin Plateau of British Columbia. In Mountain Pine Beetle Symposium: Challenges and Solutions; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Canadian Forest Service: Victoria, BC, Canada, 2004; pp. 258–266. [Google Scholar]
- Taylor, S.W.; Carroll, A.L.; Alfaro, R.I.; Safranyik, L. Forest, climate, and mountain pine beetle outbreak dynamics in western Canada. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, B., Eds.; Pacific Forestry Centre: Victoria, BC, Canada, 2006; pp. 67–94. [Google Scholar]
- Van de Gevel, S.L. Landscape-Level Dynamics in an Endangered Mountain Ecosystem in the Northern Rocky Mountains, USA. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2008; p. 253. [Google Scholar]
- Larson, E.R. Influences of the biophysical environment on blister rust and mountain pine beetle, and their interactions, in whitebark pine forests. J. Biogeogr. 2011, 38, 453–470. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976; p. 567. [Google Scholar]
- Arno, S.F.; Sneck, K.M. A Method for Determining Fire History in Coniferous Forests of the Mountain West; U.S. Department of Agriculture, Forest Service Research Paper INT-RP-301; Intermountain Research Station: Ogden, UT, USA, 1977.
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1968; p. 73. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, Arizona, 2010. [Google Scholar]
- Holmes, R.L. Computer assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree-Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985; p. 171. [Google Scholar]
- NCEI. National Centers for Environmental Information (Formerly National Climatic Data Center). 2015. Available online: http://www.ncdc.noaa.gov (accessed on 10 December 2015).
- Daly, C.; Kittel, T.G.F.; McNab, A.; Gibson, W.P.; Royle, J.A.; Nychk, A.D.; Parzybok, T.; Rosenbloom, N.; Taylor, G.H. Development of a 103-year high-resolution climate data set for the conterminous United States. In Proceedings of the 12th AMS Conference of Applied Climatology, Asheville, NC, USA, 8–11 May 2000; pp. 249–252. [Google Scholar]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- Fritts, H.C. PRECON Version 5.17: A Statistical Model for Analyzing the Tree-Ring Response to Variations in Climate. Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1999. Available online: http://www.ltrr.arizona.edu (accessed on 15 March 2008).
- Safranyik, L.; Carroll, A.L. The biology and epidemiology of the mountain pine beetle in lodgepole pine forests. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, B., Eds.; Pacific Forestry Centre: Victoria, BC, Canada, 2006; pp. 3–67. [Google Scholar]
- Evenden, J.C. History of the Mountain Pine Beetle Infestation in the Lodgepole Stands of Montana; United States Department of Agriculture Bureau of Entomology Forest Insect Field Station: Coeur d’Alene, ID, USA, 1934.
- Evenden, J.C. Montana’s Thirty-Year Mountain Pine Beetle Infestation; United States Department of Agriculture Bureau of Entomology and Plant Quarantine, Forest Insect Laboratory: Coeur d’Alene, ID, USA, 1944.
- Bartos, D.L.; Gibson, K.E. Insects of whitebark pine with emphasis on mountain pine beetle. In Proceedings—Symposium on Whitebark Pine Ecosystems: Ecology and Management of a High Mountain Resource; Schmidt, J., Wyman, C., McDonald, K., Eds.; USDA Forest Servive General Technical Report INT-270; U.S. Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1990; pp. 171–178. [Google Scholar]
- Frelich, L.E. Forest Dynamics and Disturbance Regimes: Studies from Temperate Evergreen-Deciduous Forests; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Kipfmueller, K.F.; Salzer, M.W. Linear trend and climate response of five-needle pines in the western United States related to treeline proximity. Can. J. For. Res. 2010, 40, 134–142. [Google Scholar] [CrossRef]
- Gray, S.T.; Fastie, C.L.; Jackson, S.T.; Betancourt, J.L. Tree-ring-based reconstruction of precipitation in the Bighorn Basin, Wyoming, since 1260 AD. J. Clim. 2004, 17, 3855–3865. [Google Scholar] [CrossRef]
- Peterson, D.W.; Peterson, D.L. Mountain hemlock growth responds to climatic variability at annual and decadal time scales. Ecology 2001, 82, 3330–3345. [Google Scholar] [CrossRef]
- Watson, E.; Luckman, B.H. Dendroclimatic reconstruction of precipitation for sites in the southern Canadian Rockies. Holocene 2001, 11, 203–213. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Kirdyanov, A.V.; Schweingruber, F.H.; Silkin, P.P. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 1999, 400, 149–151. [Google Scholar]
- Peterson, D.L. Climate, limiting factors, and environmental change in high-altitude forests of western North America. In The Impacts of Climate Variability on Forests; Beniston, M., Innes, J.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 191–208. [Google Scholar]
- Marshall, J.D.; Blair, J.M.; Peters, D.P.C.; Okin, G.; Rango, A.; Williams, M. Predicting and understanding ecosystem responses to climate change at continental scales. Front. Ecol. Environ. 2008, 6, 273–280. [Google Scholar] [CrossRef]
- Pederson, G.T.; Betancourt, J.L.; McCabe, G.L. Regional patterns and proximal causes of the recent snowpack decline in the Rocky Mountains, US. Geophys. Res. Lett. 2013, 40, 1811–1816. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate change 2013: The physical science basis. In Intergovernmental Panel on Climate Change, Working Group I Contribution to the IPCC Fifth Assessment Report (AR5); Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.; Kane, J.M.; Anderegg, L.D. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Cook, E.R.; Woodhouse, C.A.; Eakin, C.M.; Meko, D.M.; Stahle, D.W. Long-term aridity changes in the western United States. Science 2004, 306, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.B.; Veblen, T.T.; Schoennagel, T. Spatiotemporal patterns of mountain pine beetle activity in the southern Rocky Mountains. Ecology 2012, 93, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Preisler, H.K.; Hicke, J.A.; Ager, A.A.; Hayes, J.L. Climate and weather influences on spatial temporal patterns of mountain pine beetle populations in Washington and Oregon. Ecology 2012, 93, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
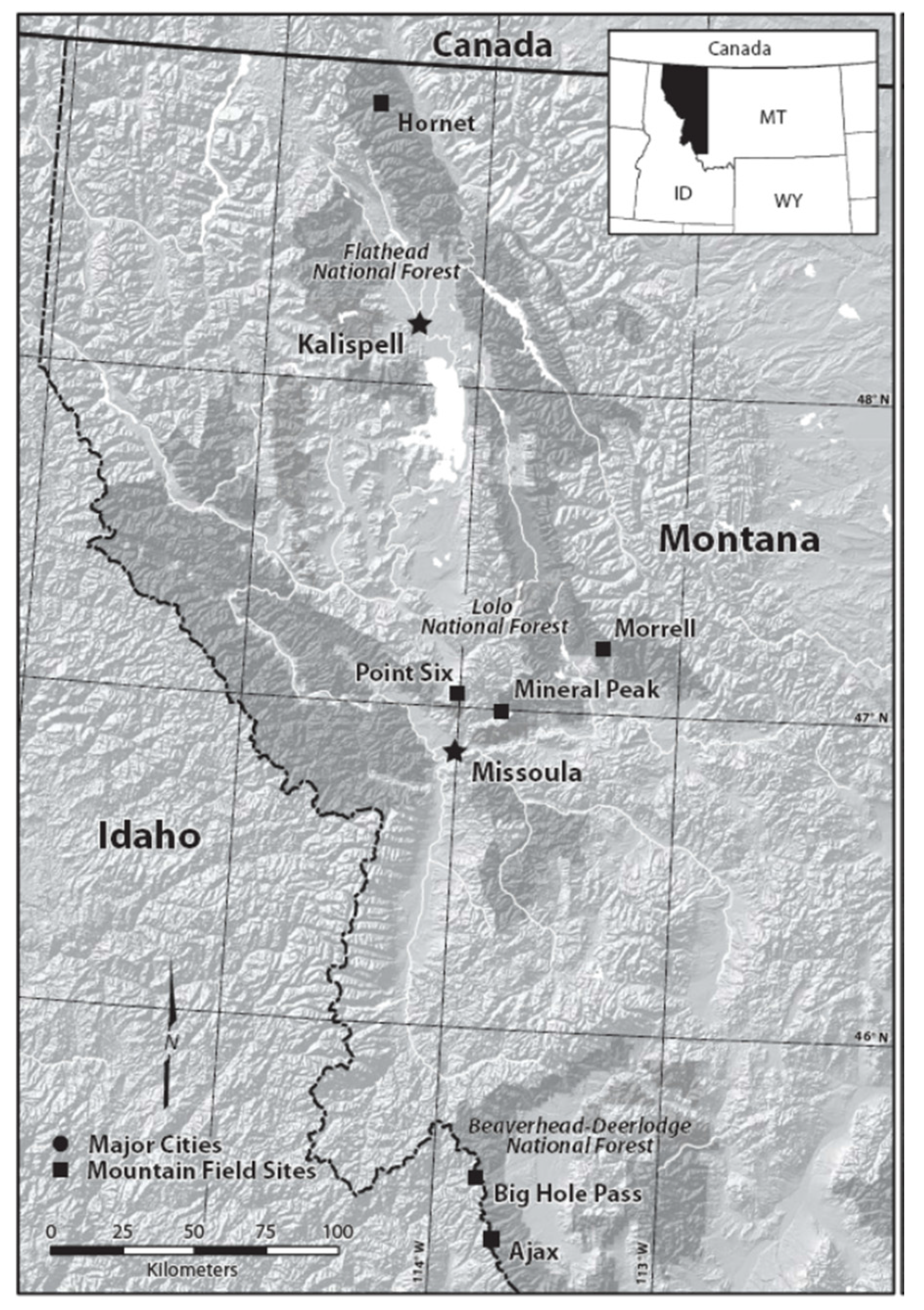
| Study Site | National Forest | Elevation (m) | Latitude (Degrees N) | Longitude (Degrees W) |
|---|---|---|---|---|
| Hornet Peak | Flathead | 2040 | 48.52.44 | 114.31.33 |
| Mineral Peak | Lolo | 2250 | 47.00.13 | 113.48.51 |
| Morrell Mountain | Lolo | 2370 | 47.11.53 | 113.21.25 |
| Point Six | Lolo | 2350 | 47.02.34 | 113.59.14 |
| Ajax Peak | Beaverhead-Deerlodge | 2535 | 45.20.25 | 113.42.57 |
| Big Hole Pass | Beaverhead-Deerlodge | 2255 | 45.31.14 | 113.48.16 |
| Study Site | National Forest | Period of Record | Number of Samples | Interseries Correlation | Mean Sensitivity |
|---|---|---|---|---|---|
| Hornet Peak | Flathead | 1682–2005 | 64 | 0.48 | 0.23 |
| Morrell Mountain | Lolo | 1489–2003 | 60 | 0.51 | 0.24 |
| Point Six | Lolo | 1581–2003 | 62 | 0.47 | 0.22 |
| Mineral Peak | Lolo | 1171–2003 | 76 | 0.47 | 0.21 |
| Big Hole Pass | Beaverhead-Deerlodge | 1778–2004 | 27 | 0.50 | 0.22 |
| Ajax Peak | Beaverhead-Deerlodge | 1832–2004 | 33 | 0.52 | 0.21 |
| Study Site | Period | Climate Variable | Month | Correlation Coefficient |
|---|---|---|---|---|
| Hornet Peak | 1895–2005 | Precipitation | June | 0.29 * |
| Morrell Mountain | 1895–2003 | Precipitation | Previous June | 0.27 * |
| Point Six | 1895–2003 | Precipitation | January | −0.32 ** |
| Mineral Peak | 1895–2003 | Temperature | April | 0.34 ** |
| Big Hole Pass | 1895–2004 | Precipitation | Previous July | 0.45 *** |
| Ajax Peak | 1895–2004 | Temperature | Previous July | −0.24 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van de Gevel, S.L.; Larson, E.R.; Grissino-Mayer, H.D. Separating Trends in Whitebark Pine Radial Growth Related to Climate and Mountain Pine Beetle Outbreaks in the Northern Rocky Mountains, USA. Forests 2017, 8, 195. https://doi.org/10.3390/f8060195
Van de Gevel SL, Larson ER, Grissino-Mayer HD. Separating Trends in Whitebark Pine Radial Growth Related to Climate and Mountain Pine Beetle Outbreaks in the Northern Rocky Mountains, USA. Forests. 2017; 8(6):195. https://doi.org/10.3390/f8060195
Chicago/Turabian StyleVan de Gevel, Saskia L., Evan R. Larson, and Henri D. Grissino-Mayer. 2017. "Separating Trends in Whitebark Pine Radial Growth Related to Climate and Mountain Pine Beetle Outbreaks in the Northern Rocky Mountains, USA" Forests 8, no. 6: 195. https://doi.org/10.3390/f8060195






