Spatial Analysis of a Haloxylon Ammodendron Plantation in an Oasis-Desert Ecotone in the Hexi Corridor, Northwestern China
Abstract
:1. Introduction
2. Materials and Methods
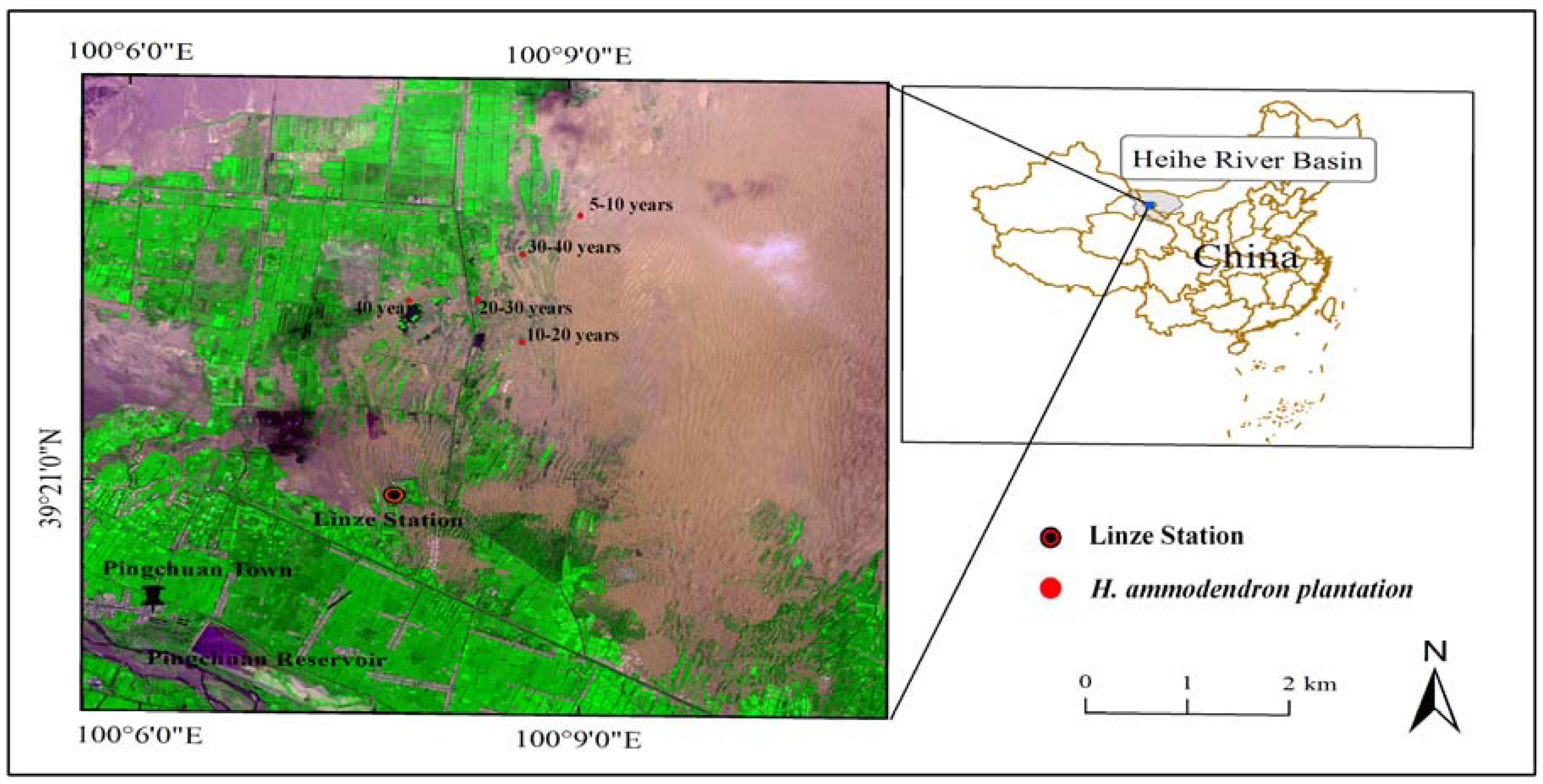
2.1. Study Area
2.2. Field Investigation
2.3. Data Analysis
3. Results
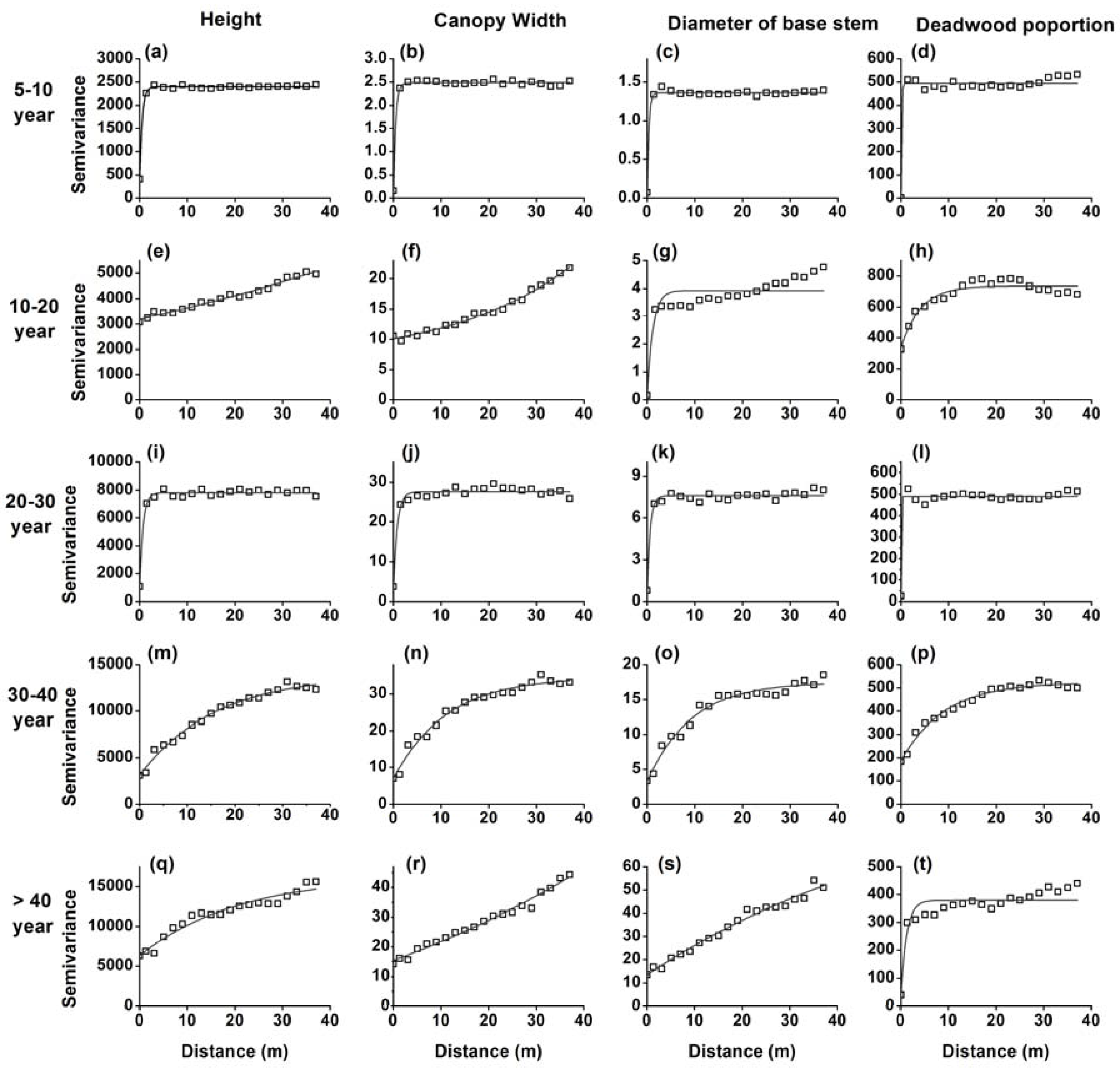
3.1. H. ammodendron Characteristics and Spatial Dependence Among Stages of Revegetation
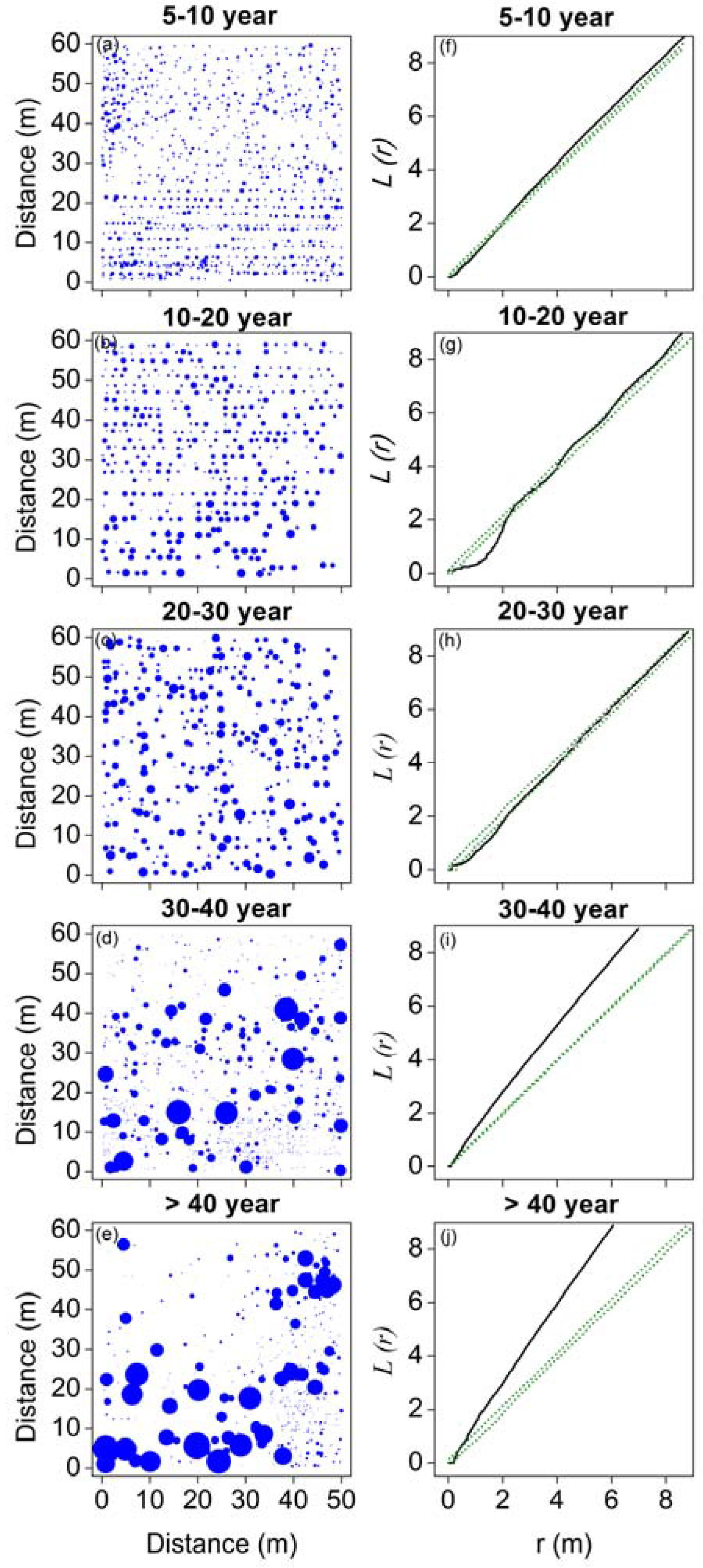
3.2. Spatial Patterns of H. ammodendron in Five Stages of Development
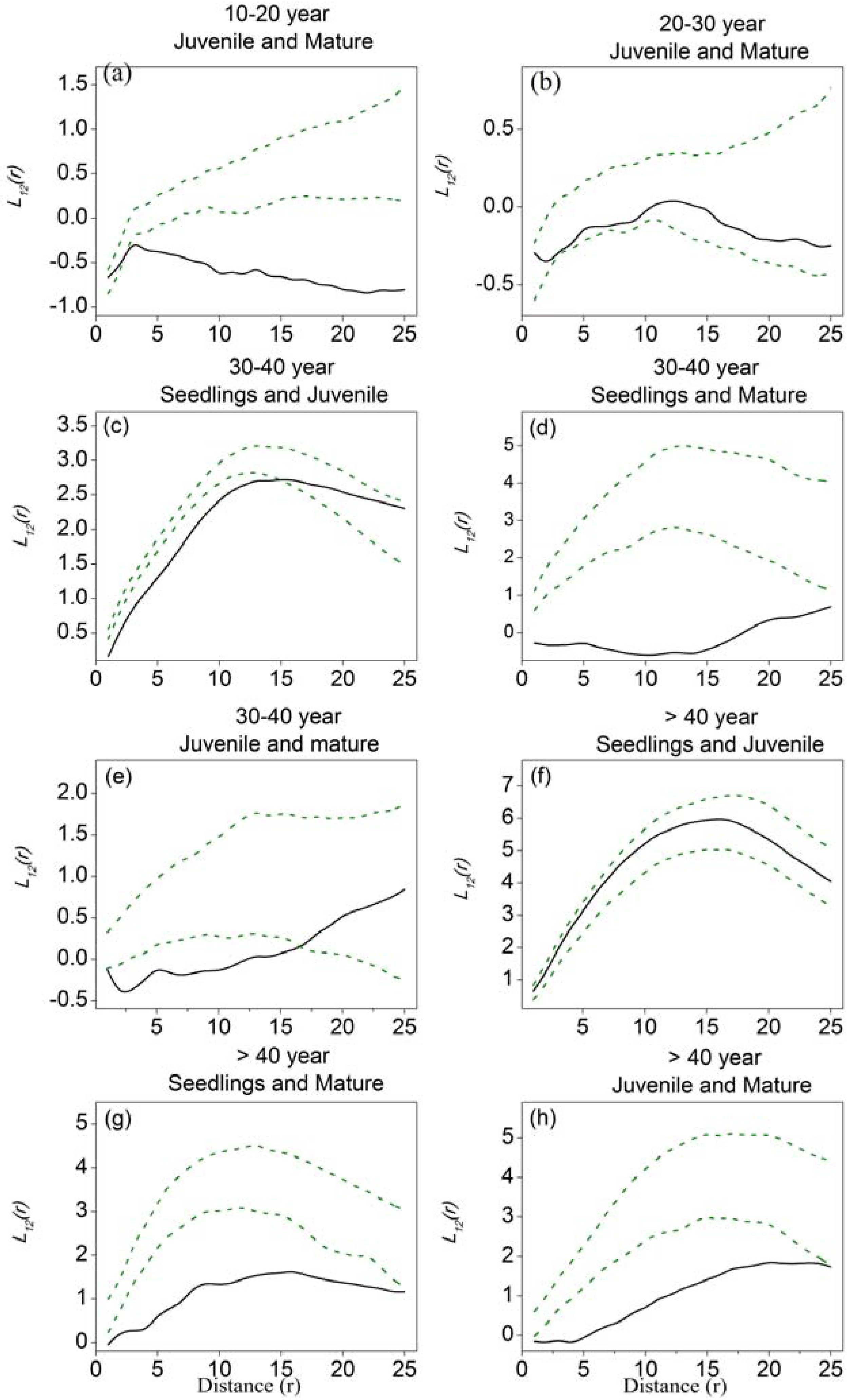
3.3. Spatial Associations among Different Tree-Size Classes
4. Discussion
4.1. Regeneration Dynamics across Stages of Revegetation
4.2. Changes in Spatial Patterns in the H. ammodendron Plantation
4.3. Spatial Associations and the Balance between Facilitation and Competition
4.4. Main Abiotic Factors Affected the Spatial Patterns and Associations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adeel, Z. Findings of the global desertification assessment by the Millennium Ecosystem Assessment—A perspective for better managing scientific knowledge. In The Future of Drylands; Lee, C., Schaaf, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 677–685. [Google Scholar]
- Hufkens, K.; Scheunders, P.; Ceulemans, R. Ecotones in vegetation ecology: Methodologies and definitions revisited. Ecol. Res. 2009, 24, 977–986. [Google Scholar] [CrossRef]
- Wang, Y.G.; Li, Y. Land exploitation resulting in soil salinization in a desert-oasis ecotone. Catena 2013, 100, 50–56. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Hu, G.L.; Zhang, Z.Z.; He, Z.B. Shielding effect of oasis-protection systems composed of various forms of wind break on sand fixation in an arid region: A case study in the Hexi Corridor, northwest China. Ecol. Eng. 2008, 33, 119–125. [Google Scholar] [CrossRef]
- Cao, S.; Chen, L.; Shankman, D.; Wang, C.; Wang, X.; Zhang, H. Excessive reliance on afforestation in China’s arid and semi-arid regions: Lessons in ecological restoration. Earth Sci. Rev. 2011, 104, 240–245. [Google Scholar] [CrossRef]
- Li, X.R.; Zhao, Y.; Hui, R.; Su, J.Q.; Gao, Y.H. Progress and trend of development of restoration ecology research in the arid regions of China. Prog. Geo. 2014, 33, 1435–1443. [Google Scholar]
- Li, X.R.; Zhang, Z.S.; Huang, L.; Wang, X.P. Review of the ecohydrological processes and feedback mechanisms controlling sand-binding vegetation systems in sandy desert regions of China. Chin. Sci. Bull. 2013, 58, 1483–1496. [Google Scholar] [CrossRef]
- Hardenberg, J.V.; Meron, E.; Shachak, M.; Zarmi, Y. Diversity of vegetation patterns and desertification. Phys. Rev. Lett. 2001, 87, 198101. [Google Scholar] [CrossRef] [PubMed]
- Saco, P.M.; Willgoose, G.R.; Hancock, G.R. Eco-geomorphology of banded vegetation patterns in arid and semi-arid regions. Hydrol. Earth. Syst. Sci. 2007, 11, 1717–1730. [Google Scholar] [CrossRef]
- Du, B.M.; Kang, H.Z.; Zhu, Y.H.; Zhou, X.; Yin, S.; Burgess, P.J.; Liu, C.J. Variation of Oriental Oak (Quercus variabilis) leaf delta C-13 across temperate and subtropical China: Spatial patterns and sensitivity to precipitation. Forests 2015, 6, 2296–2306. [Google Scholar] [CrossRef]
- Perry, G.L.W.; Enright, N.J.; Miller, B.P.; Lamont, B.B. Nearest-neighbour interactions in species-rich shrublands: The roles of abundance, spatial patterns and resources. Oikos 2009, 118, 161–174. [Google Scholar] [CrossRef]
- Wang, X.T.; Liang, C.Z.; Wang, W. Balance between facilitation and competition determines spatial patterns in a plant population. Chin. Sci. Bull. 2014, 59, 1405–1415. [Google Scholar] [CrossRef]
- Couteron, P.; Kokou, K. Woody vegetation spatial patterns in a semi-arid savanna of Burkina Faso, West Africa. Plant. Ecol. 1997, 132, 211–227. [Google Scholar] [CrossRef]
- Barot, S.; Gignoux, J.; Menaut, J.C. Demography of a savanna palm tree: Predictions from comprehensive spatial pattern analyses. Ecology 1999, 80, 1987–2005. [Google Scholar] [CrossRef]
- Stoll, P.; Bergius, E. Pattern and process: Competition causes regular spacing of individuals within plant populations. J. Ecol. 2005, 93, 395–403. [Google Scholar] [CrossRef]
- Malkinson, D.; Tielbörger, K. What does the stress-gradient hypothesis predict? Resolving the discrepancies. Oikos 2010, 119, 1546–1552. [Google Scholar] [CrossRef]
- Wiegand, T.; Gunatilleke, S.; Gunatilleke, N. Species associations in a heterogeneous Sri Lankan dipterocarp forest. Am. Nat. 2007, 170, E77–E95. [Google Scholar] [CrossRef] [PubMed]
- Gross, K. Positive interactions among competitors can produce species—Rich communities. Ecol. Lett. 2008, 11, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Han, G.D.; Zhao, M.L.; Chang, S.X. Spatial vegetation patterns as early signs of desertification: A case study of a desert steppe in Inner Mongolia, China. Landsc. Ecol. 2010, 25, 1519–1527. [Google Scholar] [CrossRef]
- Konings, A.G.; Dekker, S.C.; Rietkerk, M.; Katul, G.G. Drought sensitivity of patterned vegetation determined by rainfall—Land surface feedbacks. J. Geophys. Res. 2011, 116, G04008. [Google Scholar] [CrossRef]
- Bestelmeyer, B.T.; Duniway, M.C.; James, D.K.; Burkett, L.M.; Havstad, K.M. A test of critical thresholds and their indicators in a desertification-prone ecosystem: More resilience than we thought. Ecol. Lett. 2013, 16, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kulasova, A.; Blazkova, S.; Beven, K.; Rezacova, D.; Cajthaml, J. Vegetation pattern as an indicator of saturated areas in a Czech headwater catchment. Hydrol. Processes 2014, 28, 5297–5308. [Google Scholar] [CrossRef]
- Powell, R.D. The role of spatial pattern in the population biology of Centaurea diffusa. J. Ecol. 1990, 78, 374–388. [Google Scholar] [CrossRef]
- Anderson, K.J. Temporal patterns in rates of community change during succession. Am. Nat. 2007, 169, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Lameire, S.; Hermy, M.; Honnay, O. Two decades of change in the ground vegetation of a mixed deciduous forest in an agricultural landscape. J. Veg. Sci. 2000, 11, 695–704. [Google Scholar] [CrossRef]
- Chesson, P.; Gebauer, R.L.E.; Schwinning, S.; Huntly, N.; Wiegand, K.; Ernest, M.S.K.; Sher, A.; Novoplansky, A.; Weltzin, J.F. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 2004, 141, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Sproull, G.J.; Quigley, M.F.; Sher, A.; González, E. Long-term changes in composition, diversity and distribution patterns in four herbaceous plant communities along an elevational gradient. J. Veg. Sci. 2015, 26, 552–563. [Google Scholar] [CrossRef]
- Allen, M.S.; Thapa, V.; Arévalo, J. R.; Palmer, M.W. Windstorm damage and forest recovery: Accelerated succession, stand structure, and spatial pattern over 25 years in two Minnesota forests. Plant. Ecol. 2012, 213, 1833–1842. [Google Scholar] [CrossRef]
- Larson, A.J.; Churchill, D. Tree spatial patterns in fire-frequent forests of western North America, including mechanisms of pattern formation and implications for designing fuel reduction and restoration treatments. For. Ecol. Manag. 2012, 267, 74–92. [Google Scholar] [CrossRef]
- Ross, L.C.; Woodin, S.J.; Hester, A.J.; Thompson, D.B.A.; Birks, H.J.B. Biotic homogenization of upland vegetation: Patterns and drivers at multiple spatial scales over five decades. J. Veg. Sci. 2012, 23, 755–770. [Google Scholar] [CrossRef]
- Ma, X.; Huete, A.; Yu, Q.; Coupe, N.R.; Davies, K.; Broich, M.; Ratana, P.; Beringer, J.; Hutley, L.B.; Cleverly, J.; et al. Spatial patterns and temporal dynamics in savanna vegetation phenology across the North Australian Tropical Transect. Remote Sens. Environ. 2013, 139, 97–115. [Google Scholar] [CrossRef]
- Badreldin, N.; Uria-Diez, J.; Mateu, J.; Youssef, A.; Stal, C.; El-Bana, M.; Magdy, A.; Goossens, R. A spatial pattern analysis of the halophytic species distribution in an arid coastal environment. Environ. Monit. Assess. 2015, 187, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Xiang, W.H.; Li, J.X.; Lei, P.F.; Deng, X.W.; Fang, X.; Peng, C.H. Effects of topographic and soil factors on woody species assembly in a Chinese subtropical evergreen broadleaved forest. Forests 2015, 6, 650–669. [Google Scholar] [CrossRef]
- Su, Y.Z.; Zhao, W.Z.; Su, P.X.; Zhang, Z.H.; Wang, T.; Ram, R. Ecological effects of desertification control and desertified land reclamation in an oasis-desert ecotone in an arid region: A case study in Hexi Corridor, northwest China. Ecol. Eng. 2007, 29, 117–124. [Google Scholar] [CrossRef]
- He, F.; Duncan, R.P. Density-dependent effects on tree survival in an old-growth Douglas fir forest. J. Ecol. 2000, 88, 676–688. [Google Scholar] [CrossRef]
- Dale, M.R.T.; Fortin, M.J. Spatial Analysis: A Guide for Ecologists, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; p. 382. [Google Scholar]
- Jia, Z.Q.; Lu, Q. Haloxylon Ammodendron; China Environmental Science Press: Beijing, China, 2004; p. 67. [Google Scholar]
- Diggle, P.J. Statistical Analysis of Spatial and Spatio-Temporal Point Patternss, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–5. [Google Scholar]
- Wiegand, T.; Kissling, W.D.; Cipriotti, P.A.; Aguiar, M.R. Extending point pattern analysis for objects of finite size and irregular shape. J. Ecol. 2006, 94, 825–837. [Google Scholar] [CrossRef]
- Besag, J. Contribution to the discussion of Dr. Ripley’s paper. Journal of the Royal Statistical. J. R. Stat. Soc. B 1977, 39, 193–195. [Google Scholar]
- Ripley, B.D. Modelling spatial patterns. J. R. Stat. Soc. B 1977, 39, 172–212. [Google Scholar]
- Goreaud, F.; Pe´lissier, R. On explicit formulas of edge effect correction for Ripley’s K-function. J. Veg. Sci. 1999, 10, 433–438. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Baddeley, A.; Turner, R. Spatstat: An R package for analyzing spatial point patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar] [CrossRef]
- Baddeley, A.; Turner, R.; Møller, J.; Hazelton, M. Residual analysis for spatial point processes (with discussion). J. R. Stat. Soc. B 2005, 67, 617–666. [Google Scholar] [CrossRef]
- Pelissier, R.; Goreaud, F.; Pelissier, M.R. Package ‘ads’. Science 2015, 14, 681–692. [Google Scholar]
- Carrer, M.; Soraruf, L.; Lingua, E. Convergent space-time tree regeneration patterns along an elevation gradient at high altitude in the Alps. For. Ecol. Manag. 2013, 304, 1–9. [Google Scholar] [CrossRef]
- Cipriotti, P.A.; Aguiar, M.R. Is the balance between competition and facilitation a driver of the patch dynamics in arid vegetation mosaics? Oikos 2015, 124, 139–149. [Google Scholar] [CrossRef]
- Nishimura, N.; Kato, K.; Sumida, A.; Ono, K.; Tanouchi, H.; Iida, S.; Hoshino, D.; Yamamoto, S.; Hara, T. Effects of life history strategies and tree competition on species coexistence in a sub-boreal coniferous forest of Japan. Plant. Ecol. 2010, 206, 29–40. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity influences spatial patterns and demographics in forest stands. J. Ecol. 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Seiwa, K.; Miwa, Y.; Sahashi, N.; Kanno, H.; Tomita, M.; Ueno, N.; Yamazaki, M. Pathogen attack and spatial patterns of juvenile mortality and growth in a temperate tree, Prunus grayana. Can. J. For. Res. 2008, 38, 2445–2454. [Google Scholar] [CrossRef]
- Murrell, D.J. On the emergent spatial structure of size-structured populations: When does self-thinning lead to a reduction in clustering? J. Ecol. 2009, 97, 256–266. [Google Scholar] [CrossRef]
- Jia, X.; Dai, X.F.; Shen, Z.X.; Zhang, J.Y.; Wang, G.X. Facilitation can maintain clustered spatial pattern of plant populations during density-dependent mortality: Insights from a zone-of-influence model. Oikos 2011, 120, 472–480. [Google Scholar] [CrossRef]
- Tirado, R.; Pugnaire, F.I. Shrub spatial aggregation and consequences for reproductive success. Oecologia 2003, 136, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Schurr, F.M.; Bossdorf, O.; Milton, S.J.; Schumacheret, J. Spatial pattern formation in semi-arid shrubland: A priori predicted versus observed pattern characteristics. Plant. Ecol. 2004, 173, 271–282. [Google Scholar] [CrossRef]
- Wang, G.H.; Zhao, W.Z.; Liu, H.; Zhang, G.F.; Li, F. Changes in soil and vegetation with stabilization of dunes in a desert-oasis ecotone. Ecol. Res. 2015, 30, 639–650. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, W.Z.; Zhang, G.F. Varying water utilization of Haloxylon ammodendron plantations in a desert-oasis ecotone. Hydrol. Processes 2016, 31, 825–835. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, Z.S.; Tan, H. J.; Gao, Y.H.; Liu, L.C.; Wang, X.P. Ecological restoration and recovery in the wind-blown sand hazard areas of northern China: Relationship between soil water and carrying capacity for vegetation in the Tengger Desert. Sci. China Life Sci. 2014, 57, 539–548. [Google Scholar] [CrossRef] [PubMed]
| Traits | Stages of Development | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5–10 Years | 10–20 Years | 20–30 Years | 30–40 Years | >40 Years | |||||||||||
| Seedling | Juvenile | Mature | Seedling | Juvenile | Mature | Seedling | Juvenile | Mature | Seedling | Juvenile | Mature | Seedling | Juvenile | Mature | |
| Frequency/(%) | 2.9 | 96 | 1 | 0 | 63.4 | 36.6 | 1.7 | 56.4 | 41.9 | 45.6 | 45.8 | 8.6 | 24.0 | 61.3 | 14.7 |
| Diameter of base stem/(cm) | 0.96 ± 0.15 | 3.06 ± 1.06 | 7.11 ± 0.42 | / | 3.82 ± 1.32 | 7.40 ± 1.03 | 0.95 ± 0.20 | 4.53 ± 1.46 | 8.91 ± 1.80 | 0.92 ± 0.18 | 2.56 ± 1.44 | 12.87 ± 6.11 | 0.98 ± 0.18 | 2.35 ± 1.16 | 17.69 ± 7.53 |
| Height/(cm) | 102.12 ± 24.79 | 193.14 ± 46.60 | 213.33 ± 78.10 | / | 220.11 ± 48.89 | 314.11 ± 57.41 | 121.67 ± 16.93 | 262.24 ± 77.47 | 355.26 ± 61.86 | 62.44 ± 21.22 | 136.2 ± 70.96 | 373.46 ± 97.14 | 74.55 ± 25.53 | 127.45 ± 57.62 | 372.94 ± 108.76 |
| Crown width/(m2) | 0.84 ± 0.57 | 3.15 ± 1.54 | 3.40 ± 1.94 | / | 4.66 ± 2.48 | 9.86 ± 4.80 | 1.35 ± 0.57 | 6.67 ± 4.18 | 12.54 ± 4.36 | 0.27 ± 0.23 | 2.29 ± 2.76 | 16.63 ± 7.49 | 0.45 ± 0.37 | 1.65 ± 2.07 | 14.95 ± 6.73 |
| Deadwood proportion/(%) | 5.96 ± 10.77 | 13.41 ± 23.39 | 10.00 ± 20.00 | / | 39.47 ± 27.93 | 43.08 ± 25.05 | 53.33 ± 36.15 | 58.30 ± 22.36 | 53.81 ± 21.34 | 15.21 ± 18.61 | 20.79 ± 22.21 | 38.96 ± 22.81 | 17.18 ± 20.07 | 19.88 ± 19.05 | 35.84 ± 17.42 |
| Density/individual/(m−2) | 0.009 | 0.284 | 0.003 | / | 0.091 | 0.053 | 0.002 | 0.068 | 0.050 | 0.128 | 0.128 | 0.024 | 0.034 | 0.086 | 0.021 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhao, W.; Zhang, G. Spatial Analysis of a Haloxylon Ammodendron Plantation in an Oasis-Desert Ecotone in the Hexi Corridor, Northwestern China. Forests 2017, 8, 200. https://doi.org/10.3390/f8060200
Zheng Y, Zhao W, Zhang G. Spatial Analysis of a Haloxylon Ammodendron Plantation in an Oasis-Desert Ecotone in the Hexi Corridor, Northwestern China. Forests. 2017; 8(6):200. https://doi.org/10.3390/f8060200
Chicago/Turabian StyleZheng, Ying, Wenzhi Zhao, and Gefei Zhang. 2017. "Spatial Analysis of a Haloxylon Ammodendron Plantation in an Oasis-Desert Ecotone in the Hexi Corridor, Northwestern China" Forests 8, no. 6: 200. https://doi.org/10.3390/f8060200





