Modeled Shifts in Polylepis Species Ranges in the Andes from the Last Glacial Maximum to the Present
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Climate Data
2.3. Species Distribution Modeling
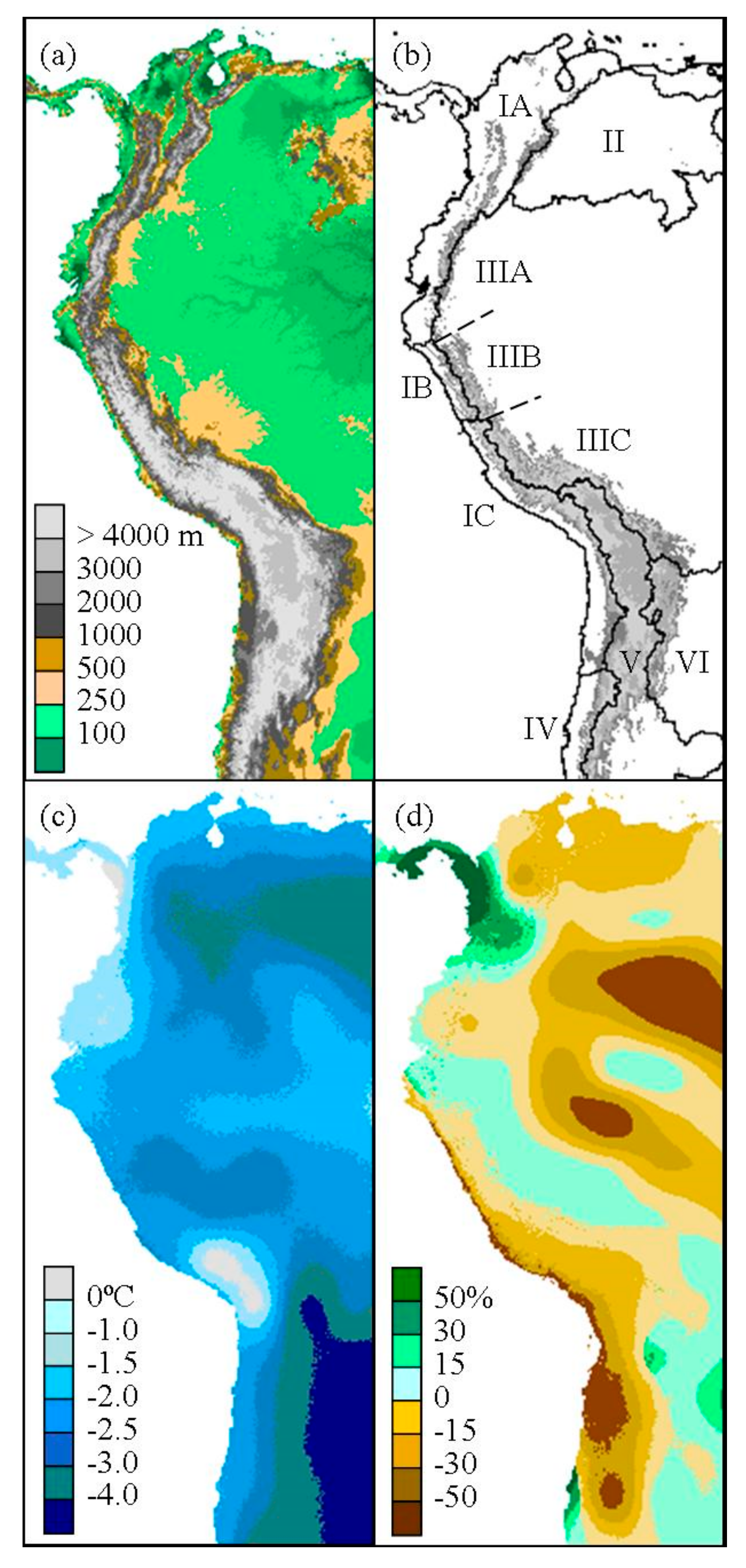
2.4. Biogeographic Zones
3. Results
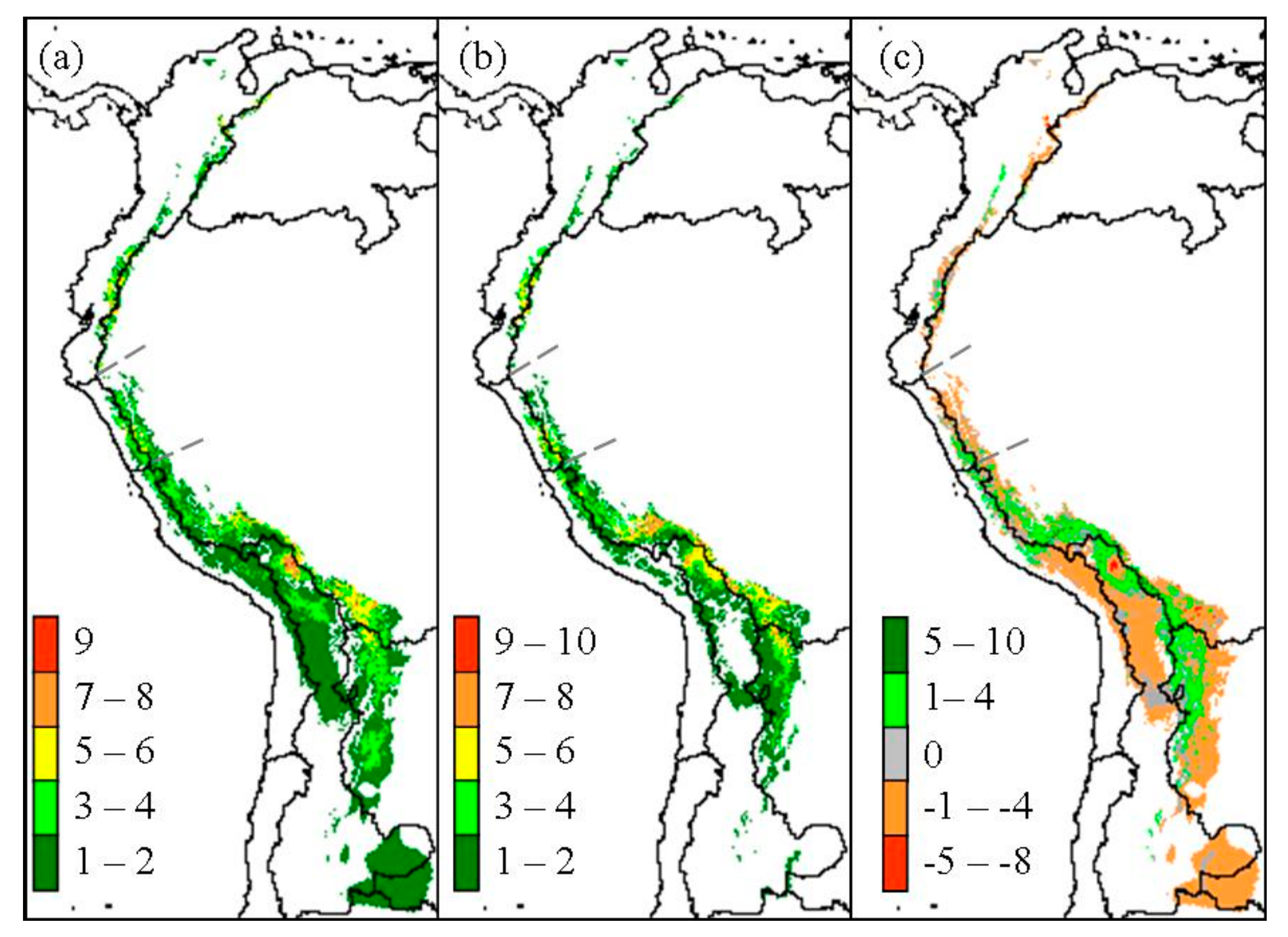
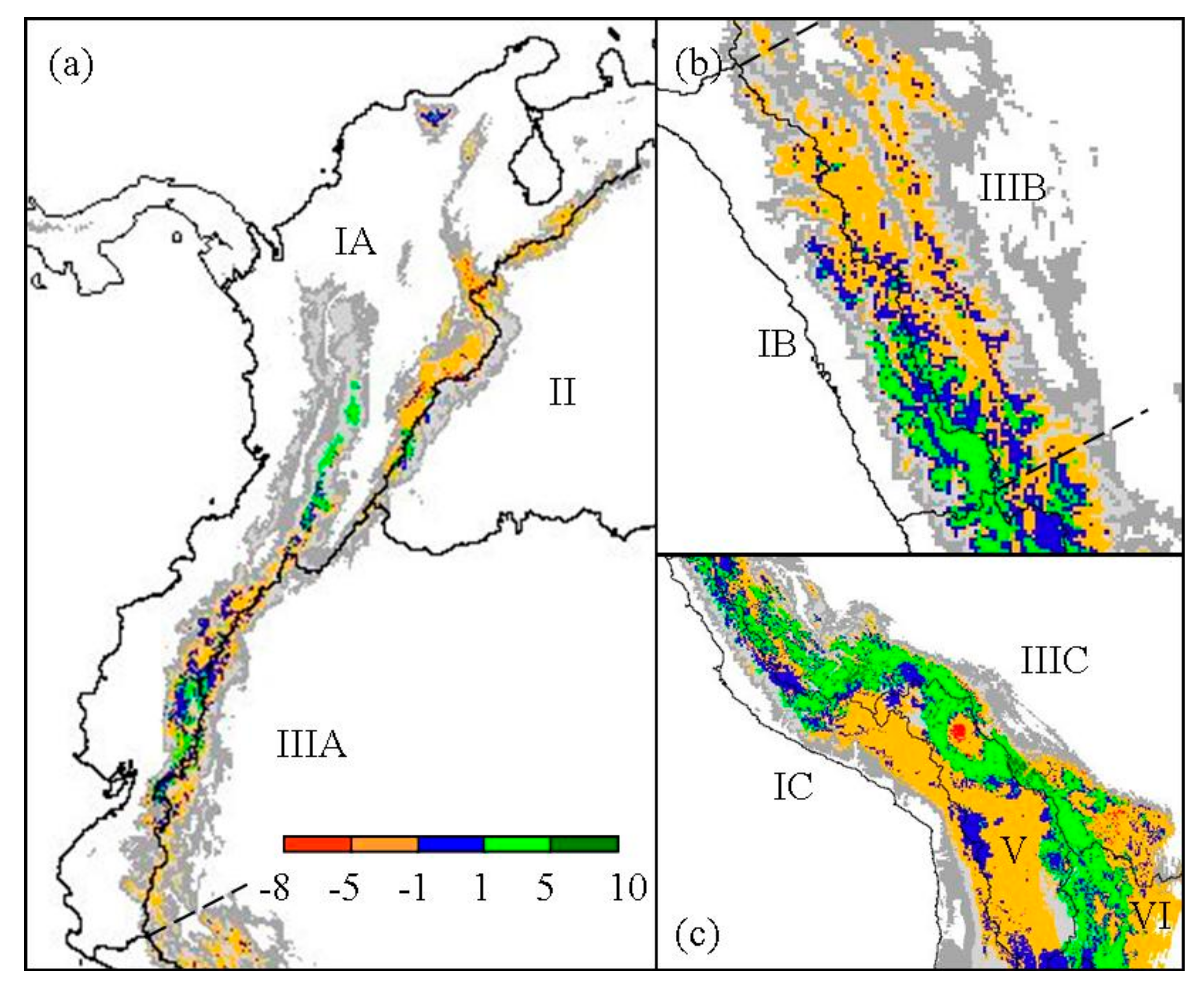
3.1. Polylepis Species Distribution
3.2. Biogeographic Zones
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simpson, B.B. A revision of the genus Polylepis (Rosaceae: Sanguisorbeae). Smithson. Contrib. Bot. 1979, 43, 1–62. [Google Scholar] [CrossRef]
- Kessler, M. The “Polylepis Problem”: Where do we stand? Ecotropica 2002, 8, 97–110. [Google Scholar]
- Purcell, J.; Brelsford, A.; Kessler, M. The world’s highest forest: A better understanding of the properties of Andean queñua woodlands has major implications for their conservation. Am. Sci. 2004, 92, 454–461. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Available online: www.iucnredlist.org (accessed on 7 March 2017).
- Fjeldså, J. Polylepis forests—Vestiges of a vanishing ecosystem in the Andes. Ecotropica 2002, 8, 111–123. [Google Scholar]
- Aucca, C.; Ramsay, P.M. Management of biodiversity and land use in southern Peru: ECOAN’s activities to help conserve Polylepis woodlands. Mt. Res. Dev. 2005, 25, 287–289. [Google Scholar] [CrossRef]
- Ellenberg, H. Wald oder Steppe? Die natürliche Pflanzendecke der Anden Perus (I, II). Umschau Wissen. Technol. 1958, 21, 645–648, 22, 679–681. [Google Scholar]
- Simpson, B.B. Speciation and Specialization of Polylepis in the Andes. High Altitude Tropical Biogeography; Vulllemeire, F., Monasterio, M., Eds.; Oxford University Press: Oxford, UK, 1986; pp. 304–316. [Google Scholar]
- Gosling, W.D.; Hanselman, J.A.; Knox, C.; Valencia, B.G.; Bush, M.B. Long-term drivers of change in Polylepis woodland distribution in the central Andes. J. Veg. Sci. 2009, 20, 1041–1052. [Google Scholar] [CrossRef]
- Toivonen, J.M.; Horna, V.; Kessler, M.; Ruokolainen, K.; Hertel, D. Interspecific variation in functional traits in relation to species climatic niche optima in Andean Polylepis (Rosaceae) tree species: Evidence for climatic adaptations. Funct. Plant Biol. 2014, 41, 301–312. [Google Scholar] [CrossRef]
- Gosling, W.D.; Bunting, M.J. A role of palaeoecology in anticipating future change in mountain regions? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 259, 1–5. [Google Scholar] [CrossRef]
- MacDonald, G.M.; Bennett, K.D.; Jackson, S.T.; Parducci, L.; Smith, F.A.; Smol, J.P.; Willis, K.J. Impacts of climate change on species, populations and communities: Palaeobiogeographical insights and frontiers. Prog. Phys Geogr. 2008, 32, 139–172. [Google Scholar] [CrossRef]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The last glacial maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Seltzer, G.O.; Farber, D.L.; Rodell, D.T.; Finkel, R.C. Early local last glacial maximum in the tropical Andes. Science 2005, 308, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.B.; Silman, M.R.; Urrego, D.H. 48,000 years of climate and forest change in a biodiversity hot spot. Science 2004, 303, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.H.; Bush, M.B.; Silamn, M.R.; Correa-Metrio, A.; Ledru, M.-P.; Mayle, F.E.; Paduano, G.; Valencia, B.G. Millennial-scale ecological changes in tropical South America since the last glacial maximum. In Past Climate Variability in South America and Surrounding, Developments in Paleoenvironmental Research 14; Vimeux, F., Ed.; Springer: New York, NY, USA, 2009; pp. 283–300. [Google Scholar]
- Carnaval, A.C.; Moritz, C. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J. Biogeogr. 2008, 35, 1187–1201. [Google Scholar] [CrossRef]
- Nogués-Bravo, D. Predicting the past distribution of species climatic niches. Glob. Ecol. Biogeogr. 2009, 18, 521–531. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, F.; Arroyo, J. Reconstructing the demise of Tethyan plants: Climate-driven range dynamics of Laurus since the Pliocene. Glob. Ecol. Biogeogr. 2008, 17, 685–695. [Google Scholar] [CrossRef]
- Hooghiemstra, H.; van der Hammen, T. Quaternary Ice-Age dynamics in the Colombian Andes: Developing an understanding of our legacy. Philos. Trans. R. Soc. B 2004, 359, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Chepstow-Lusty, A.; Bush, M.B.; Frogley, M.R.; Baker, P.A.; Fritz, S.C.; Aronson, J. Vegetation and climate change on the Bolivian Altiplano between 108,000 and 18,000 yr ago. Quat. Res. 2005, 63, 90–98. [Google Scholar] [CrossRef]
- Gosling, W.D.; Bush, M.B.; Hanselman, J.A.; Chepstow-Lusty, A. Glacial-interglacial changes in moisture balance and the impact on vegetation in the southern hemisphere tropical Andes (Bolivia/Peru). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 259, 35–50. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Otto-Bliesner, B.L.; Brady, E.C.; Clauzet, G.; Tomas, R.; Levis, S.; Kothavala, Z. Last Glacial Maximum and Holocene climate in CCSM3. J. Clim. 2006, 19, 2526–2544. [Google Scholar] [CrossRef]
- Braconnot, P.; Otto-Bliesner, B.; Harrison, S.; Joussaume, S.; Peterchmitt, J.-Y.; Abe-Ouchi, A.; Crucifix, M.; Driesschaert, E.; Fichefet, T.; Hewitt, C.D.; et al. Results of PMIP2 coupled simulations of the Mid-Holocene and Last Glacial Maximum—Part 1: Experiments and large-scale features. Clim. Past 2007, 3, 261–277. [Google Scholar] [CrossRef]
- Phillips, S.; Anderson, R.P.; Schapire, R.E. Maximum entropy modelling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Phillips, S.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Csuti, B. Mapping animal distribution areas for gap analysis. In Gap Analysis: A Landscape Approach to Biodiversity Planning; Scott, J.M., Tear, T.H., Davis, F.W., Eds.; American Society for Photogrammetry and Remote Sensing: Bethesda, MD, USA, 1996; pp. 135–145. [Google Scholar]
- Hurlbert, A.P.; Jetz, W. Species richness, hotspots, and the scale-dependence of range maps in ecology and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
- Loarie, S.R.; Carter, B.E.; Hayhoe, K.; McMahon, S.; Moe, R.; Knight, C.A.; Ackerly, D.D. Climate change and the future of California’s endemic flora. PLoS ONE 2008, 3, e2502. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S. A brief Tutorial on Maxent; ATandT Research: Florham Park, NJ, USA, 2005; Available online: http://www.cs.princeton.edu/_schapire/maxent/tutorial/tutorial.doc (accessed on 7 March 2017).
- Buermann, W.; Saatchi, S.; Smith, T.B.; Zutta, B.R.; Chaves, J.A.; Milá, B.; Graham, C.H. Predicting species distribution across the Amazonian and Andean regions using remote sensing data. J. Biogeogr. 2008, 35, 1160–1176. [Google Scholar] [CrossRef]
- Riordan, E.C.; Rundel, P.W. Modelling the distribution of a threatened habitat: The California sage scrub. J. Biogeogr. 2009, 36, 2176–2188. [Google Scholar] [CrossRef]
- US Geological Survey (USGS). HYDRO1K Elevation Derivative Database; Earth Resources Observation and Science (EROS) Data Center (EDC): Sioux Falls, SD, USA, 1996. Available online: http://edc.usgs.gov/products/elevation/gtopo30/hydro/index.html (accessed on 7 March 2017).
- Luteyn, J.L. Páramos: A Checklist of Plant Diversity, Geographical Distribution, and Botanical Literature; New York Botanical Garden Press: New York, NY, USA, 1999. [Google Scholar]
- Weigend, M. Observations on the biogeography of the Amotape-Huancabamba zone in northern Peru. Bot. Rev. 2002, 68, 38–54. [Google Scholar] [CrossRef]
- Weng, C.; Hooghiemstra, H.; Duivenvoorden, J.F. Response of pollen diversity to the climate-driven altitudinal shift of vegetation in the Colombian Andes. Philos. Trans. R. Soc. B 2007, 362, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Jomelli, V.; Favier, V.; Rabatel, A.; Brunstein, D.; Hoffman, G.; Francou, B. Fluctuations of glaciers in the tropical Andes over the last millennium and paleoclimatic implications: A review. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 281, 269–282. [Google Scholar] [CrossRef]
- Rodríguez, F.; Behling, H. Late Quaternary vegetation, climate and fire dynamics, and evidence of early to mid-Holocene Polylepis forests in the Jimbura region of the southernmost Ecuadorian Andes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 350, 247–257. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 10, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Tingley, M.W. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Cierjacks, A.; Wesche, K.; Hensen, I. Potential lateral expansion of Polylepis forest fragments in central Ecuador. For. Ecol. Manag. 2007, 242, 477–486. [Google Scholar] [CrossRef]
- Garzione, C.N.; Hoke, G.D.; Libarkin, J.C.; Withers, S.; MacFadden, B.; Eiler, J.; Ghosh, P.; Mulch, A. Rise of the Andes. Science 2008, 6, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Cuatrecasas, J. Speciation and radiation of the Espeletiinae in the Andes. In High. Altitude Tropical Biogeography; Vuilleumier, F., Monasterio, M., Eds.; Oxford University Press: New York, NY, USA, 1986; pp. 267–303. [Google Scholar]
- Carnaval, A.C.; Hickerson, M.J.; Haddad, C.F.B.; Rodrigues, M.T.; Moritz, C. Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science 2009, 303, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Cossios, D.; Lucherini, M.; Ruiz-García, M.; Angers, B. Influence of ancientglacial periods on the Andean fauna: the case of the pampas cat (Leopardus colocolo). BMC Evol. Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Hughes, L.; Poulsen, M. Predicting species distributions: Use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol. Model. 2005, 186, 250–269. [Google Scholar] [CrossRef]
- Hampe, A. Bioclimatic envelope models: What they detect and what they hide. Glob. Ecol. Biogeogr. 2004, 13, 469–471. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Bioclimatic envelope models: What they detect and what they hide—Response to Hampe. Glob. Ecol. Biogeogr. 2004, 13, 471–473. [Google Scholar] [CrossRef]
- Kessler, M.; Schmidt-Lebuhn, A.N. Taxonomical and distributional notes on Polylepis (Rosaceae). Org. Divers. Evol. 2006, 6, 67–69. [Google Scholar] [CrossRef]
| Species | Site | Range | Hydrologic Basin | Predicted Area (km2) | Middle Elevation (m) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Past | Present | Stable | Past | Present | Change of Middle | ||||
| P. australis | 61 | Central and north-western Argentina (e) | Mostly VI and some neighboring areas of the V and IV basins | 63,460 | 12,450 | 2290 | 809 (52–3732) | 2263 (805–3951) | +1454 |
| P. besseri | 80 | Southern Peru to Bolivia | VI, eastern V, southern Amazon and San Juan basins | 17,185 | 31,445 | 3130 | 3142 (1276–4334) | 3757 (2216–4892) | +615 |
| P. crista-galli | 33 | Southern Bolivia to northernmost Argentina | Mostly VI and some neighboring areas of IIIC | 18,225 | 16,520 | 1590 | 2048 (337–3883) | 2963 (1264–4024) | +915 |
| P. hieronymi | 41 | Southern Bolivia to northern Argentina | VI and sIIIC | 23,850 | 12,645 | 1490 | 1299 (292–3026) | 2153 (926–3563) | +854 |
| P. incana | 115 | Ecuador to southern Peru | Western and southern Amazon, northern and central San Juan basins | 25,885 | 22,485 | 8160 | 3736 (559–4908) | 4001 (1588–5035) | +265 |
| P. incarum | 57 | Southern Peru to Bolivia | Southern Amazon and V basins | 18,075 | 28,310 | 3235 | 3586 (1162–5037) | 4076 (1591–5243) | +490 |
| P. lanata | 40 | Southern Peru to Bolivia | IIIC | 19,690 | 15,525 | 4160 | 3443 (1771–4525) | 3628 (1693–4775) | +185 |
| P. lanuginosa | 20 | Ecuador (e) | Northwest San Juan and northwest Amazon basins | 6165 | 6125 | 600 | 3112 (1673–4728) | 3342 (2234–5280) | +230 |
| P. neglecta | 32 | Central and southern Bolivia (e) | Mostly IIIC and some neighboring areas of VI basin | 13,315 | 15,035 | 2465 | 2327 (1056–3634) | 2883 (1273–4049) | +556 |
| P. pacensis | 20 | Western Bolivia (e) | Mostly IIIC and some neighboring areas of V | 17,085 | 20,385 | 3680 | 3564 (1492–5057) | 3847 (1425–5407) | +283 |
| P. pauta | 42 | Ecuador and isolated populations in southeastern Peru and northern Bolivian Andes | Northern San Juan, neighboring areas of the northwestern Amazon Basin and isolated areas of the IIIC | 23,375 | 16,690 | 5590 | 3141 (1366–4908) | 3449 (1569–5280) | +308 |
| P. pepei | 30 | Central Peru to northeastern Bolivia | IIIC | 12,890 | 16,215 | 3990 | 3843 (1614–5130) | 3984 (1631–5585) | +141 |
| P. racemosa | 56 | Northern Peru to northwestern Bolivia, isolated population in Ecuador | Northern San Juan to IIIC | 30,300 | 29,945 | 7745 | 3577 (1495–4600) | 3873 (1759–5236) | +296 |
| P. reticulata | 34 | Ecuador and isolated populations in northern and central Peru | Northern to central San Juan basin | 7300 | 5890 | 3555 | 3516 (2564–5280) | 3613 (2927–5280) | +97 |
| P. rugulosa | 24 | Southwestern Peru to northernmost Chile | Central San Juan to western V | 38,495 | 8840 | 745 | 4034 (1340–5804) | 4063 (1811–5498) | +29 |
| P. sericea | 90 | Venezuela to Bolivia | Northern San Juan to IIIC | 36,455 | 35,740 | 15,010 | 3708 (1202–5280) | 3957 (1613–5280) | +249 |
| P. subtusalbida | 92 | Central Bolivia (e) | IIIC | 15,485 | 19,415 | 2120 | 3155 (1927–4305) | 3645 (1932–4608) | +490 |
| P. tarapacana | 62 | Border regions of Argentina, Bolivia, Chile, and Peru | V and VI | 52,145 | 13,515 | 85 | 4168 (2224–5804) | 4517 (2749–5498) | +349 |
| P. tomentella | 122 | Southern Bolivia to northwestern Argentina with isolated populations in central Peru | Central San Juan, V, IIIC and VI | 40,515 | 35,630 | 4940 | 3315 (930–4568) | 3635 (1720–4670) | +320 |
| P. triacontandra | 61 | Southern Peru to Bolivia, with few isolated populations in central Peru | Mostly IIIC and some neighboring areas of the V. Isolated areas in the central San Juan basin | 22,740 | 21,190 | 11,690 | 4177 (2835–5563) | 4222 (1955–5585) | +45 |
| P. weberbaueri | 82 | Ecuador to southern Peru | Northen San Juan to IIIC | 26,745 | 13,535 | 4130 | 3924 (1437–5280) | 3990 (1776–5280) | +66 |
| Basin | Biogeo. Zone | Species Richness Response | Area (km2) | Mean Elevation (m) | Temp from Present (°C) | Prec. from Present (%) |
|---|---|---|---|---|---|---|
| San Juan | IA | Decrease | 9960 | 2458 (559–4335) | −4.1 | |
| Stable | 3760 | 3450 (1997–4834) | −1.5 | −1.1 | ||
| Increase | 2320 | 3499 (2251–5280) | −1.8 | 15.0 | ||
| IB | Decrease | 1975 | 3258 (2154–5563) | −2.0 | −5.6 | |
| Stable | 1710 | 3733 (2752–5229) | −2.2 | −10.2 | ||
| Increase | 1840 | 4341 (2294–5243) | −2.3 | −10.1 | ||
| IC | Decrease | 16,870 | 3869 (1340–5804) | −1.2 | −29.3 | |
| Stable | 6760 | 4094 (2233–5347) | −1.9 | −24.1 | ||
| Increase | 4060 | 4154 (1972–4921) | −2.1 | −19.4 | ||
| Orinoco | II | Decrease | 1815 | 2797 (1673–4024) | −2.6 | −12.1 |
| Stable | 285 | 3352 (2838–3923) | −2.8 | 12.4 | ||
| Increase | 270 | 3604 (2698–4110) | −2.8 | 24.3 | ||
| Amazon | IIIA | Decrease | 2975 | 2996 (2085–4183) | −1.5 | −1.8 |
| Stable | 1335 | 3481 (2613–4908) | −1.3 | −5.5 | ||
| Increase | 450 | 3663 (2640–5035) | −1.3 | −4.7 | ||
| IIIB | Decrease | 7030 | 3275 (2132–4348) | −2.1 | −2.3 | |
| Stable | 2580 | 3787 (2669–5119) | −2.3 | −4.0 | ||
| Increase | 850 | 4233 (3336–5219) | −2.4 | −5.2 | ||
| IIIC | Decrease | 20,745 | 2939 (1056–5053) | −2.9 | −8.5 | |
| Stable | 10,595 | 3795 (1605–5374) | −2.4 | −10.8 | ||
| Increase | 20,775 | 4026 (1758–5585) | −2.4 | −11.7 | ||
| Argentina | IV | Decrease | 3420 | 602 (121–1148) | −3.9 | 8.7 |
| Stable | 325 | 1401 (1053–1978) | −3.6 | 6.7 | ||
| Increase | - | - | - | - | ||
| Altiplano | V | Decrease | 47,680 | 2761 (152–5476) | −2.2 | −16.2 |
| Stable | 15,755 | 3854 (805–5498) | −2.7 | −16.5 | ||
| Increase | 11,640 | 4000 (1867–5195) | −2.8 | −12.7 | ||
| Paraná | VI | Decrease | 39,900 | 924 (92–4901) | −4.3 | 5.3 |
| Stable | 10,010 | 3006 (106–5930) | −4.0 | 1.7 | ||
| Increase | 15,865 | 3539 (1380–5161) | −4.0 | 5.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zutta, B.R.; Rundel, P.W. Modeled Shifts in Polylepis Species Ranges in the Andes from the Last Glacial Maximum to the Present. Forests 2017, 8, 232. https://doi.org/10.3390/f8070232
Zutta BR, Rundel PW. Modeled Shifts in Polylepis Species Ranges in the Andes from the Last Glacial Maximum to the Present. Forests. 2017; 8(7):232. https://doi.org/10.3390/f8070232
Chicago/Turabian StyleZutta, Brian R., and Philip W. Rundel. 2017. "Modeled Shifts in Polylepis Species Ranges in the Andes from the Last Glacial Maximum to the Present" Forests 8, no. 7: 232. https://doi.org/10.3390/f8070232





