Predation on Early Recruitment in Mediterranean Forests after Prescribed Fires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Statistical Analyses
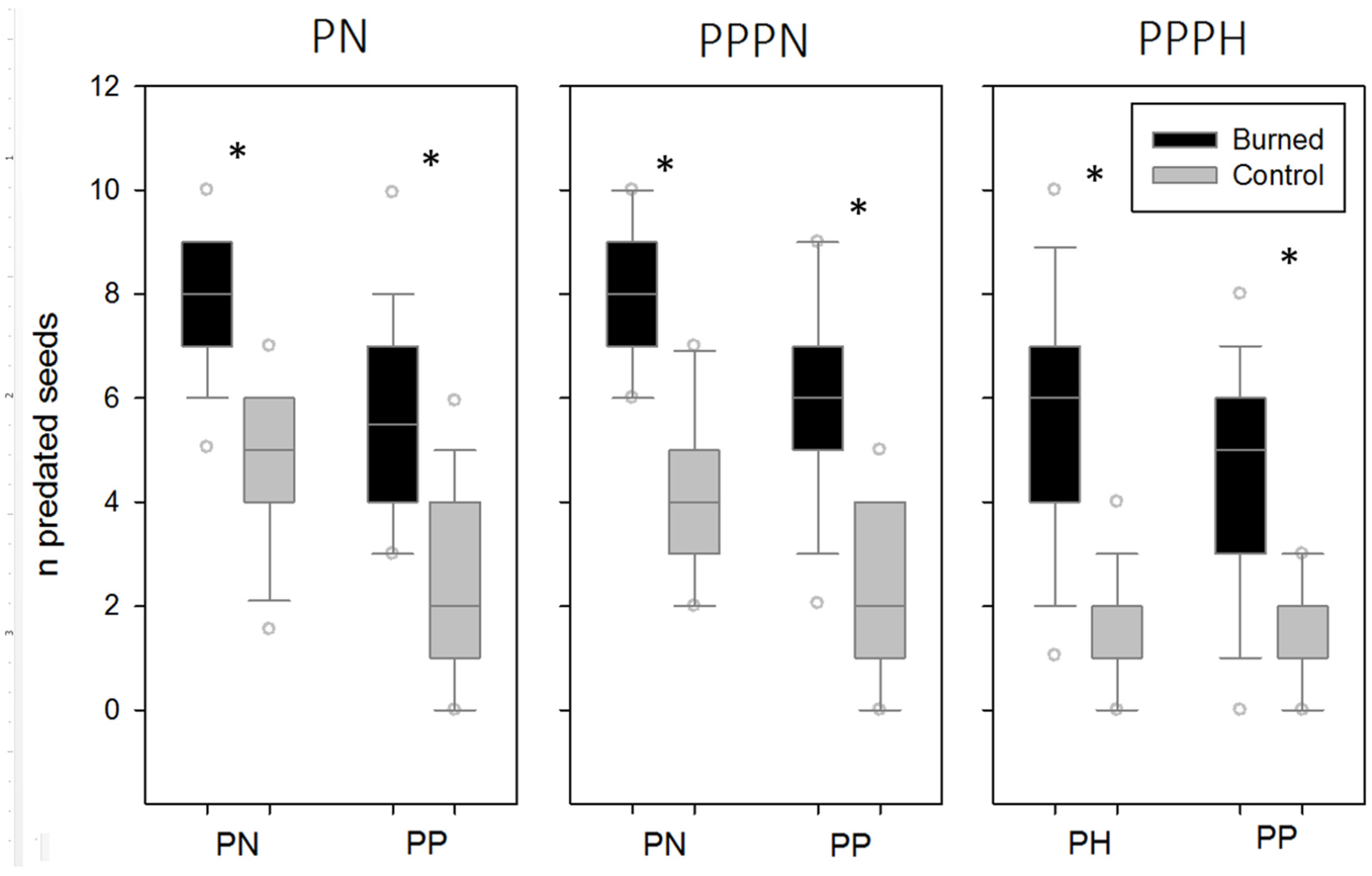
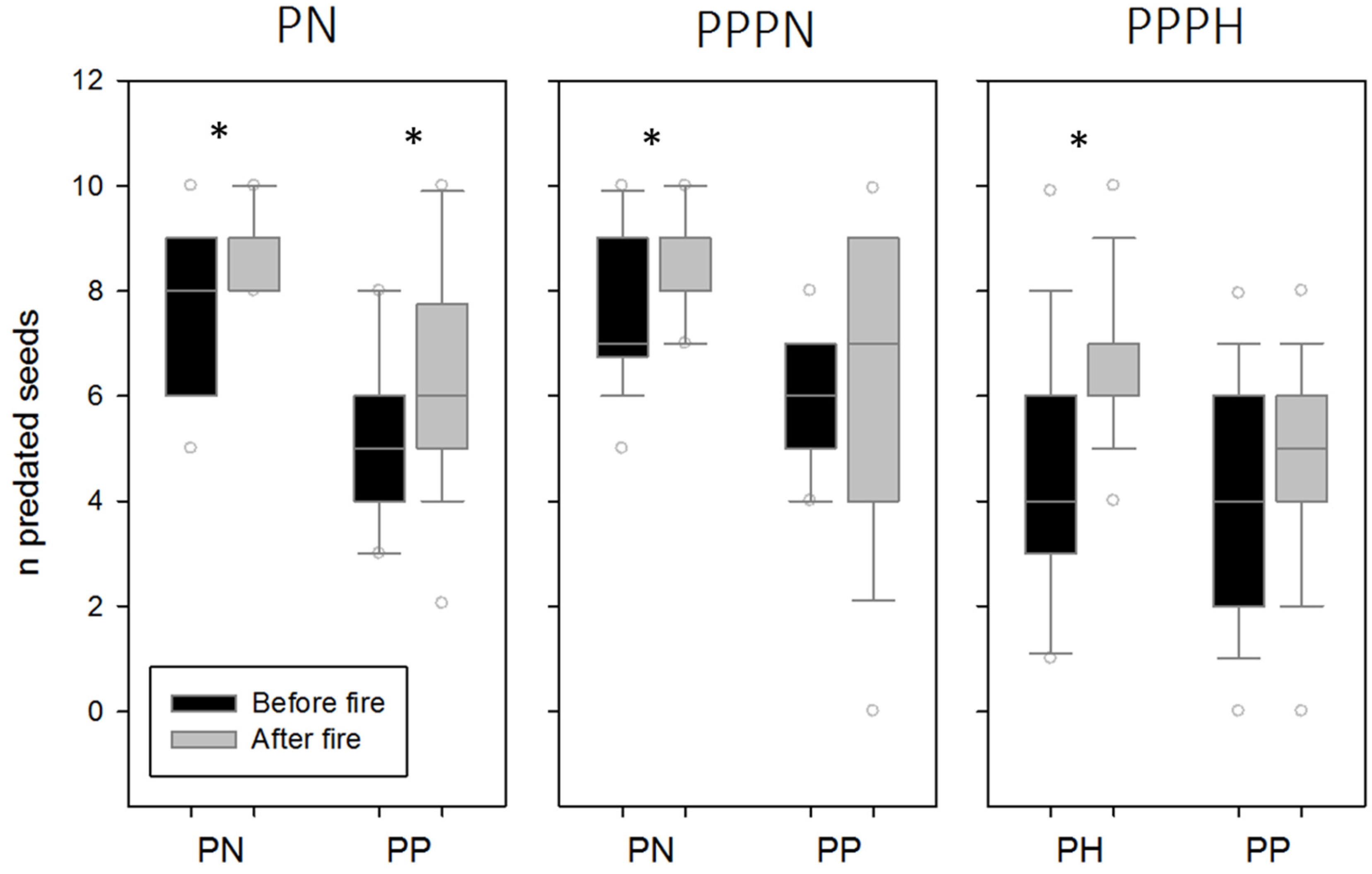
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| TOTAL | CONTROL | BURNED | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Before Fire | After Fire | |||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| PPPN | 5.98 | ± | 2.64 | 3.45 | ± | 1.82 | 7.25 | ± | 2.00 | 6.87 | ± | 1.57 | 7.64 | ± | 2.28 |
| P. nigra | 6.76 | ± | 2.37 | 4.09 | ± | 1.66 | 8.09 | ± | 1.32 | 7.56 | ± | 1.39 | 8.63 | ± | 0.97 |
| Similar | 6.13 | ± | 2.64 | 3.60 | ± | 1.50 | 8.03 | ± | 1.46 | 7.15 | ± | 1.46 | 8.90 | ± | 0.77 |
| More Humid | 6.78 | ± | 2.34 | 4.00 | ± | 1.15 | 8.17 | ± | 1.32 | 7.83 | ± | 1.44 | 8.50 | ± | 1.09 |
| Drier | 7.15 | ± | 2.13 | 4.67 | ± | 2.02 | 8.08 | ± | 1.23 | 7.55 | ± | 1.26 | 8.60 | ± | 0.94 |
| P. Pinaster | 4.82 | ± | 2.60 | 2.48 | ± | 1.62 | 5.99 | ± | 2.18 | 5.83 | ± | 1.23 | 6.15 | ± | 2.82 |
| More Humid | 4.73 | ± | 2.66 | 2.60 | ± | 1.47 | 5.80 | ± | 2.47 | 5.80 | ± | 1.14 | 5.80 | ± | 3.30 |
| Drier | 4.91 | ± | 2.54 | 2.37 | ± | 1.74 | 6.18 | ± | 1.82 | 5.87 | ± | 1.31 | 6.50 | ± | 2.17 |
| PN | 6.02 | ± | 2.59 | 3.69 | ± | 1.90 | 7.19 | ± | 2.03 | 6.59 | ± | 1.92 | 7.79 | ± | 1.97 |
| P. nigra | 6.97 | ± | 2.20 | 4.52 | ± | 1.46 | 8.20 | ± | 1.29 | 7.54 | ± | 1.38 | 8.86 | ± | 0.77 |
| Similar | 6.87 | ± | 1.95 | 4.87 | ± | 0.81 | 7.87 | ± | 1.54 | 6.80 | ± | 1.47 | 8.93 | ± | 0.57 |
| More Humid | 6.97 | ± | 2.15 | 4.37 | ± | 1.22 | 8.27 | ± | 1.06 | 7.87 | ± | 1.12 | 8.67 | ± | 0.83 |
| Drier | 7.09 | ± | 2.46 | 4.33 | ± | 2.02 | 8.47 | ± | 1.15 | 7.97 | ± | 1.20 | 8.97 | ± | 0.84 |
| P. Pinaster | 4.59 | ± | 2.47 | 2.43 | ± | 1.79 | 5.68 | ± | 2.00 | 5.17 | ± | 1.72 | 6.18 | ± | 2.13 |
| More Humid | 4.44 | ± | 2.52 | 2.50 | ± | 2.14 | 5.42 | ± | 2.10 | 5.03 | ± | 1.72 | 5.80 | ± | 2.36 |
| Drier | 4.74 | ± | 2.40 | 2.37 | ± | 1.35 | 5.93 | ± | 1.87 | 5.30 | ± | 1.72 | 6.57 | ± | 1.80 |
| PPPH | 3.95 | ± | 2.60 | 1.63 | ± | 1.15 | 5.11 | ± | 2.34 | 4.33 | ± | 2.38 | 5.90 | ± | 2.01 |
| P. halepensis | 4.35 | ± | 2.78 | 1.65 | ± | 1.26 | 5.70 | ± | 2.31 | 4.57 | ± | 2.41 | 6.83 | ± | 1.52 |
| More Humid | 4.38 | ± | 2.92 | 1.77 | ± | 1.45 | 5.68 | ± | 2.57 | 4.40 | ± | 2.35 | 6.97 | ± | 2.09 |
| Drier | 4.32 | ± | 2.64 | 1.53 | ± | 1.02 | 5.72 | ± | 2.03 | 4.73 | ± | 2.46 | 6.70 | ± | 0.46 |
| P. Pinaster | 3.56 | ± | 2.35 | 1.62 | ± | 1.02 | 4.53 | ± | 2.22 | 4.08 | ± | 2.32 | 4.97 | ± | 2.02 |
| More Humid | 3.62 | ± | 2.51 | 1.63 | ± | 1.02 | 4.62 | ± | 2.44 | 4.03 | ± | 2.56 | 5.20 | ± | 2.15 |
| Drier | 3.49 | ± | 2.17 | 1.60 | ± | 1.02 | 4.43 | ± | 1.97 | 4.13 | ± | 2.05 | 4.73 | ± | 1.84 |
| Total Mean | 5.42 | ± | 2.77 | 3.01 | ± | 1.90 | 6.62 | ± | 2.32 | 6.04 | ± | 2.24 | 7.20 | ± | 2.26 |
References
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Maestre, F.T.; Cortina, J. Are Pinus halepensis plantations useful as a restoration tool in semiarid Mediterranean areas? For. Ecol. Manag. 2004, 198, 303–317. [Google Scholar] [CrossRef]
- Moreno, J.M. Forest fires: Trends and implications in desertification prone areas of southern Europe. In Mediterranean Desertification: Research Results and Policy Implications, Proceedings of the International Conference, Crete, Greece, 29 October–1 November 1996; Office for Official Publications of the European Communities: Luxembourg, 1999; Volume 1, pp. 115–150. [Google Scholar]
- Herranz, J.M. Aspectos botánicos y ecológicos del pino carrasco (Pinus halepensis Mill.). In Actas de la Reunión Sobre Selvicultura del Pino carrasco; Sociedad Española de las Ciencias Forestales: Madrid, Spain, 2000; Volume 10, pp. 13–17. [Google Scholar]
- Casals, P.; Valor, T.; Besalú, A.; Molina-Terrén, D. Understory fuel load and structure eight to nine years after prescribed burning in Mediterranean pine forests. For. Ecol. Manag. 2016, 362, 156–168. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Davies, G.M.; Ascoli, D.; Fernández, C.; Moreira, F.; Rigolot, E.; Stoof, C.A.; Vega, J.A.; Molina, D. Prescribed burning in southern Europe: Developing fire management in a dynamic landscape. Front. Ecol. Environ. 2013, 11, e4–e14. [Google Scholar] [CrossRef]
- Finney, M.A.; McHugh, C.W.; Grenfell, I.C. Stand and landscape-level effects of prescribed burning on two Arizona wildfires. Can. J. For. Res. 2005, 35, 1714–1722. [Google Scholar] [CrossRef]
- De las Heras, J.; Moya, D.; Vega, J.A.; Daskalakou, E.; Vallejo, V.R.; Grigoriadis, N.; Tsitsoni, T.; Baeza, J.; Valdecantos, A.; Fernández, C.; et al. Post-fire management of serotinous pine forests. In Post-Fire Management and Restoration of Southern European Forests; Springer: Dordrecht, The Netherlands, 2012; pp. 121–150. [Google Scholar]
- Retana, J.; Arnan, X.; Arianoutsou, M.; Barbati, A.; Kazanis, D.; Rodrigo, A. Post-fire management of non-serotinous pine forests. In Post-Fire Management and Restoration of Southern European Forests; Springer: New York, NY, USA, 2012; pp. 151–170. [Google Scholar]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G. Incendios Forestales. Una Visión desde la Ecología; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2012. [Google Scholar]
- Knapp, E.E.; Estes, B.L.; Skinner, C.N. Ecological Effects of Prescribed Fire Season: A Literature Review and Synthesis for Managers; General Technical Report; United States Department of Agriculture (USDA): Albany, NY, USA, 2009.
- Hadri, H.; Tschinkel, H. La Régénération de Pinus halepensis Après Coupe Rase et Sous Peuplement; Institut National de Recherches Forestières: Tunisia, 1975; research note. [Google Scholar]
- Schupp, E.W. Annual variation in seedfall, postdispersal predation, and recruitment of a neotropical tree. Ecology 1990, 71, 504–515. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A. Mechanisms blocking Pinus sylvestris colonization of Mediterranean mountain meadows. J. Veg. Sci. 2002, 13, 725–731. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Candel-Pérez, D.; Onkelinx, T.; Fule, P.Z.; Moya, D.; Gómez, R.; de las Heras, J. Early Mediterranean pine recruitment in burned and unburned Pinus nigra Arn. ssp. salzmannii stands of central Spain: Influence of species identity, provenances and post-dispersal predation. For. Ecol. Manag. 2017, 390, 203–211. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Retana, J. Early reduction of post-fire recruitment of Pinus nigra by post-dispersal seed predation in different time-since-fire habitats. Ecography 2004, 27, 449–458. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Madrigal, J.; Candel-Pérez, D.; Jiménez, E.; Moya, D.; de las Heras, J.; Guijarro, M.; Vega, J.A.; Fernández, C.; Hernando, C. Effects of prescribed burning, vegetation treatment and seed predation on natural regeneration of Spanish black pine (Pinus nigra Arn. ssp. salzmannii) in pure and mixed forest stands. For. Ecol. Manag. 2016, 378, 24–30. [Google Scholar] [CrossRef]
- Castro, J.; Gómez, J.M.; García, D.; Zamora, R.; Hódar, J.A. Seed predation and dispersal in relict Scots pine forests in southern Spain. Plant Ecol. 1999, 145, 115–123. [Google Scholar] [CrossRef]
- Acherar, M.; Lepart, J.; Debussche, M. La colonisation des frisches par le pin d’Alep (Pinus halepensis). Languedoc mediterraneen. Acta Oecol. 1984, 5, 179–189. [Google Scholar]
- Nathan, R.; Ne’eman, G. Serotinity, seed dispersal and seed predation in Pinus halepensis. In Ecology, Biogeography and Management of Pinus halepensis and P. brutia Forest Ecosystems in the Mediterranean Basin; Backhuys: Leiden, The Netherlands, 2000; pp. 105–118. [Google Scholar]
- McShea, W.J. The influence of acorn crops on annual variation in rodent and bird populations. Ecology 2000, 81, 228–238. [Google Scholar] [CrossRef]
- Nystrand, O.; Granström, A. Predation on Pinus sylvestris seeds and juvenile seedlings in Swedish boreal forest in relation to stand disturbance by logging. J. Appl. Ecol. 2000, 37, 449–463. [Google Scholar] [CrossRef]
- Hulme, P.; Hunt, M. Rodent post-dispersal seed predation in deciduous woodland: Predator response to absolute and relative abundance of prey. J. Anim. Ecol. 1999, 68, 417–428. [Google Scholar] [CrossRef]
- Alcántara, J.M.; Rey, P.J.; Sánchez-Lafuente, A.M.; Valera, F. Early effects of rodent post-dispersal seed predation on the outcome of the plant–seed disperser interaction. Oikos 2000, 88, 362–370. [Google Scholar] [CrossRef]
- Retana, J.; Espelta, J.M.; Habrouk, A.; Ordonez, J.L.; Sola-Morales, F. Regeneration patterns of three Mediterranean pines and forest changes after a large wildfire in northeastern Spain. Ecoscience 2002, 9, 89–97. [Google Scholar] [CrossRef]
- Fedriani, J.M.; Manzaneda, A.J. Pre-and postdispersal seed predation by rodents: Balance of food and safety. Behav. Ecol. 2005, 16, 1018–1024. [Google Scholar] [CrossRef]
- Habrouk, A.; Retana, J.; Espelta, J.M. Role of heat tolerance and cone protection of seeds in the response of three pine species to wildfires. Plant Ecol. 1999, 145, 91–99. [Google Scholar] [CrossRef]
- Tapias, R.; Gil, L.; Fuentes-Utrilla, P.; Pardos, J.A. Canopy seed banks in Mediterranean pines of south-eastern Spain: A comparison between Pinus halepensis Mill., P. pinaster Ait., P. nigra Arn. and P. pinea L. J. Ecol. 2001, 89, 629–638. [Google Scholar] [CrossRef]
- Vander Wall, S.B. Masting in animal-dispersed pines facilitates seed dispersal. Ecology 2002, 83, 3508–3516. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Silva-Santos, P.; Fonseca, T.; López-Serrano, F.R.; Tiscar, P.A.; Martínez-García, E.; Abellán, M.A.; Del Cerro, A. Modelling Spanish black pine postdispersal seed predation in Central-eastern Spain. For. Syst. 2010, 19, 393–403. [Google Scholar] [CrossRef]
- Flores-Peredo, R.; Cimé, B.B. Pine seed predation by mice: An experimental assessment of preference. Anim. Biodivers. Conserv. 2016, 39, 173–184. [Google Scholar]
- Doblas-Miranda, E.; Martínez-Vilalta, J.; Lloret, F.; Álvarez, A.; Ávila, A.; Bonet, F.J.; Brotons, L.; Castro, J.; Curiel Yuste, J.; Díaz, M.; et al. Reassessing global change research priorities in mediterranean terrestrial ecosystems: How far have we come and where do we go from here? Glob. Ecol. Biogeogr. 2015, 24. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Alonso, R.; Arnan, X.; Bermejo, V.; Brotons, L.; de las Heras, J.; Lopez-Serrano, F.R. A review of the combination among global change factors in forests, shrublands and pastures of the Mediterranean Region: Beyond drought effects. Glob. Planet. Chang. 2017, 148, 42–54. [Google Scholar] [CrossRef]
- Watt, A.D.; Leather, S.R. The pine beauty in Scottish lodgepole pine plantations. In Dynamics of Forest Insect Populations; Berryman, A.A., Ed.; Springer: Dorcrecht, The Netherlands, 1988; pp. 243–266. [Google Scholar]
- Reinhardt, K.; Castanha, C.; Germino, M.J.; Kueppers, L.M.; Pereira, J. Ecophysiological variation in two provenances of Pinus flexilis seedlings across an elevation gradient from forest to alpine. Tree Physiol. 2011, 31, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Castanha, C.; Torn, M.S.; Germino, M.J.; Weibel, B.; Kueppers, L.M. Conifer seedling recruitment across a gradient from forest to alpine tundra: Effects of species, provenance, and site. Plant Ecol. Divers. 2013, 6, 307–318. [Google Scholar] [CrossRef]
- Miranda, R.A.; del Barrio, J.M.G.; Sauce, S.I.; Núñez, J.A.M.; de Miguel, J.; Peragón, J.L.N.; de Ron, D.S. Regiones de Procedencia de Especies Forestales en España; Organismo Autónomo Parques Nacionales: Madrid, Spain, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 1 February 2017).
- Baraza, E.; Zamora, R.; Hódar, J.A. Conditional outcomes in plant-herbivore interactions: Neighbours matters. Oikos 2006, 113, 148–156. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Zamora, R.; Castro, J.; Hódar, J.A. Facilitation of tree saplings by nurse plants: Microhabitat amelioration or protection against herbivores? J. Veg. Sci. 2008, 19, 161–172. [Google Scholar] [CrossRef]
- Bock, C.E.; Bock, J.H. Effects of fire on wildlife in southwestern lowland habitats. In Effects of Fire Management of Southwestern Natural Resources; US Forest Service: Fort Collins, CO, USA, 1990; pp. 15–17. [Google Scholar]
- Prodon, R.; Pons, P. Postfire bird studies: Methods, questions and perspectives. In Fire in Mediterranean Ecosystems. Ecosystems Research Report; Cambridge University Press: Cambridge, UK, 1993; Volume 5, pp. 332–343. [Google Scholar]
- Martínez-Sánchez, J.J.; Marín, A.; Herranz, J.M.; Ferrandis, P.; Heras, J. Effects of high temperatures on germination of Pinus halepensis Mill. and P. pinaster Aiton seeds in southeast Spain. Plant Ecol. 1995, 116, 69–72. [Google Scholar]
- Bond, W.J. Fire survival of Cape Proteaceae-influence of fire season and seed predators. Plant Ecol. 1984, 56, 65–74. [Google Scholar]
- Pausas, J.G.; Bladé, C.; Valdecantos, A.; Seva, J.P.; Fuentes, D.; Alloza, J.A.; Vilagrosa, A.; Bautista, S.; Cortina, J.; Vallejo, R. Pines and oaks in the restoration of Mediterranean landscapes of Spain: New perspectives for an old practice—A review. Plant Ecol. 2004, 171, 209–220. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Castro, J.; Puerta-Piñero, C.; Rey Benayas, J.M. Suitability of the management of habitat complexity, acorn burial depth, and a chemical repellent for post-fire reforestation of oaks. Ecol. Eng. 2013, 53, 15–22. [Google Scholar] [CrossRef]
- Lay, D.W. Browse palatability and the effects of prescribed burning in southern pine forests. J. For. 1967, 65, 826–828. [Google Scholar]
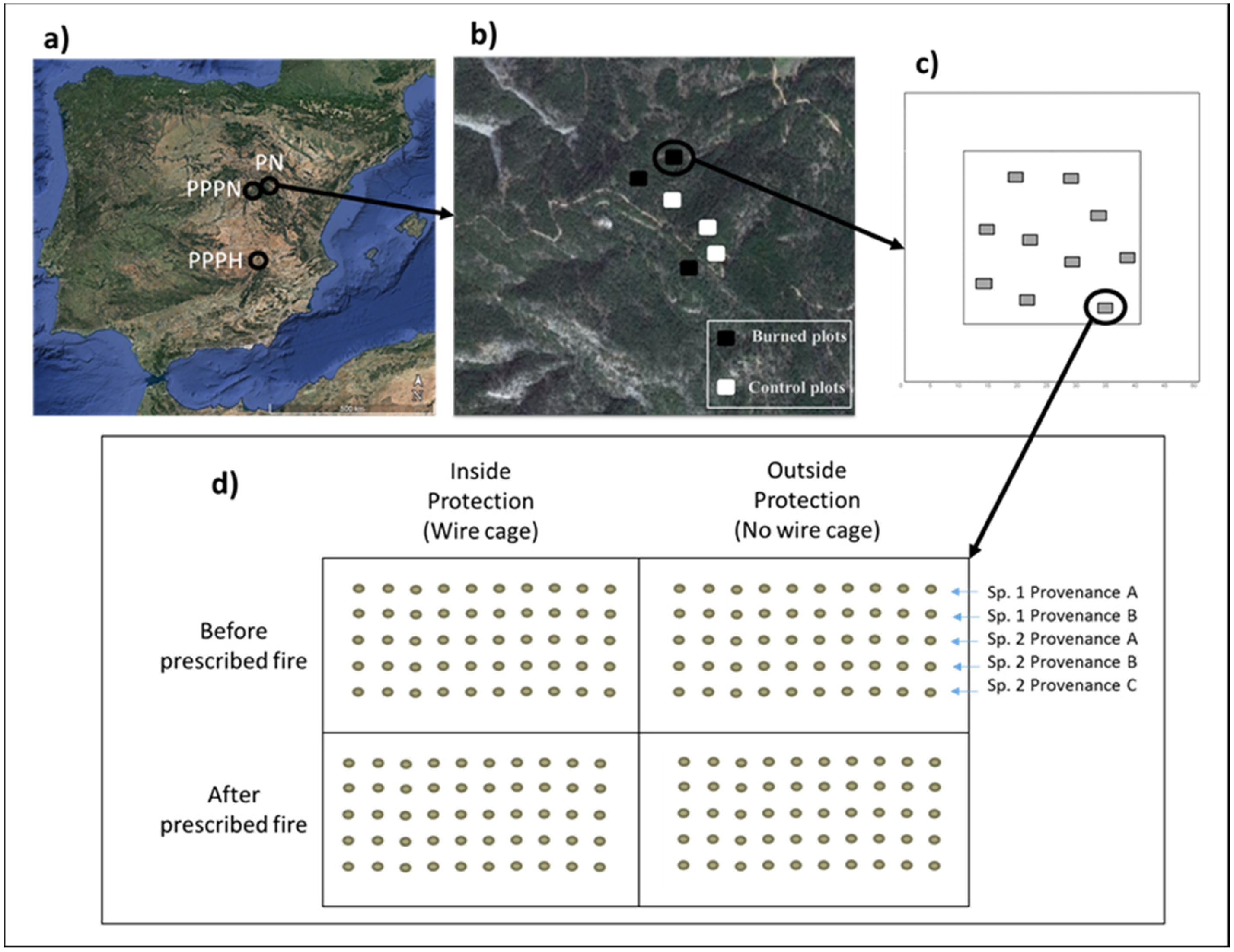
| Location | Species | Variation | Provenance | Code | Viability Test (%) |
|---|---|---|---|---|---|
| PPPN and PN | P. nigra | Drier | Baza, South Spain | ES08 | 92 |
| Similar | Cuenca, Central Spain | ES07 | 86 | ||
| More Humid | Burgos, North Spain | ES10 | 94 | ||
| P. pinaster | Drier | Moratalla, Southeast Spain | ES18 | 98 | |
| More Humid | Soria, North Spain | ES09 | 82 | ||
| PPPH | P. halepensis | Drier | Almeria, South Spain | ES13 | 90 |
| More Humid | Cazorla, Southeast Spain | ES16 | 94 | ||
| P. pinaster | Drier | Moratalla Southeast Spain | ES18 | 98 | |
| More Humid | Soria, North Spain | ES09 | 82 |
| Factor | R2 | Variable | Res. Dev. | df | p |
|---|---|---|---|---|---|
| PN | 54.88 | Model | 605.97 | 449 | |
| Treatment | 221.84 | 448 | 2.00 × 10−6 | ||
| Species | 105.05 | 447 | 2.00 × 10−6 | ||
| Species * Treatment | 5.66 | 446 | 0.017 | ||
| PNPP | 53.65 | Model | 632.51 | 449 | |
| Treatment | 266.36 | 448 | 2.00 × 10−6 | ||
| Species | 69.53 | 447 | 2.00 × 10−6 | ||
| Species * Treatment | 3.46 | 446 | 0.062 | ||
| PHPP | 41.71 | Model | 721.88 | 359 | |
| Treatment | 284.89 | 358 | 2.20 × 10−6 | ||
| Species | 14.39 | 357 | 0.0001 | ||
| Species * Treatment | 1.86 | 356 | 0.172099 | ||
| PN | 57.34 | Model | 605.97 | 449 | |
| Specie | 236.71 | 448 | 2.00 × 10−6 | ||
| Management method | 105.05 | 447 | 2.00 × 10−6 | ||
| Species * Management method | 5.71 | 446 | 0.0576 | ||
| PNPP | 54.75 | Model | 632.51 | 449 | |
| Species | 272.55 | 448 | <2 × 10−6 | ||
| Management method | 69.54 | 447 | <2 × 10−6 | ||
| Species * Management method | 4.24 | 446 | 0.1201 | ||
| PHPP | 46.20 | Model | 721.88 | 359 | |
| Species | 314.12 | 358 | 2.20 × 10−6 | ||
| Management method | 14.39 | 357 | 0.00012 | ||
| Species * Management method | 5.03 | 356 | 0.0804 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagra, J.; Moya, D.; Plaza-Álvarez, P.A.; Lucas-Borja, M.E.; Alfaro-Sánchez, R.; De Las Heras, J.; Ferrandis, P. Predation on Early Recruitment in Mediterranean Forests after Prescribed Fires. Forests 2017, 8, 243. https://doi.org/10.3390/f8070243
Sagra J, Moya D, Plaza-Álvarez PA, Lucas-Borja ME, Alfaro-Sánchez R, De Las Heras J, Ferrandis P. Predation on Early Recruitment in Mediterranean Forests after Prescribed Fires. Forests. 2017; 8(7):243. https://doi.org/10.3390/f8070243
Chicago/Turabian StyleSagra, Javier, Daniel Moya, Pedro Antonio Plaza-Álvarez, Manuel Esteban Lucas-Borja, Raquel Alfaro-Sánchez, Jorge De Las Heras, and Pablo Ferrandis. 2017. "Predation on Early Recruitment in Mediterranean Forests after Prescribed Fires" Forests 8, no. 7: 243. https://doi.org/10.3390/f8070243








