1. Introduction
The old-growth forests of the western U.S. that historically experienced low- to moderate-severity fire are undergoing changes in climate and competition that may affect carbon allocation to growth and defense in trees. The persistent legacy of fire exclusion practices has substantially increased the stand density in many of these forests [
1,
2,
3], which can cause greater competition for water and other resources [
4]. These conditions are potentially exacerbated by a warming climate [
5] that can further limit water resources and increase tree stress [
6]. The combination of these changes in climate and competition can substantially affect tree vigor by impacting tree growth and resin defenses [
7,
8] and contribute to observed increases in background tree mortality rates [
9,
10], large-scale tree die-off events [
11], and the decline of large diameter trees [
12,
13].
Large trees provide many valuable ecological functions and ecosystem services but are generally rare across most landscapes as a result of past logging practices [
14,
15]. While large trees typically have a competitive advantage, these trees can still be vulnerable to environmental stress and higher mortality [
12,
13,
15]. Additionally, large trees could be threatened by bark beetle activity, which has increased in a warmer drier climate coupled with denser forests [
16,
17]. Understanding the effects of how climate and competition changes growth and defense allocation can provide important information about large tree vigor responses to environmental stress with potential implications on forest dynamics.
There are multiple theoretical frameworks for communicating the trade-off between allocating carbon to growth or defense in trees with varying resource availability [
18]. The growth-differentiation balance hypothesis states that trees experiencing stressors that limit growth but not photosynthesis will allocate more carbon to secondary compounds (e.g., oleoresin) and structures (e.g., resin ducts) [
19,
20]. The resource availability hypothesis expands on the growth-differentiation balance hypothesis by stating that slower growing trees will allocate more to constitutive (i.e., continually produced) defenses when the cost of replacing tissues is high, while faster growing trees will allocate more to inducible (i.e., produced in reaction to stress) defenses [
21]. These hypotheses posit that reduced resource availability from elevated temperatures and increased competition may reduce growth and potentially shift carbon allocation patterns to defense. Alternatively, warmer and earlier growing seasons could promote conditions that activate photosynthesis earlier, allowing for greater carbon prioritization to growth.
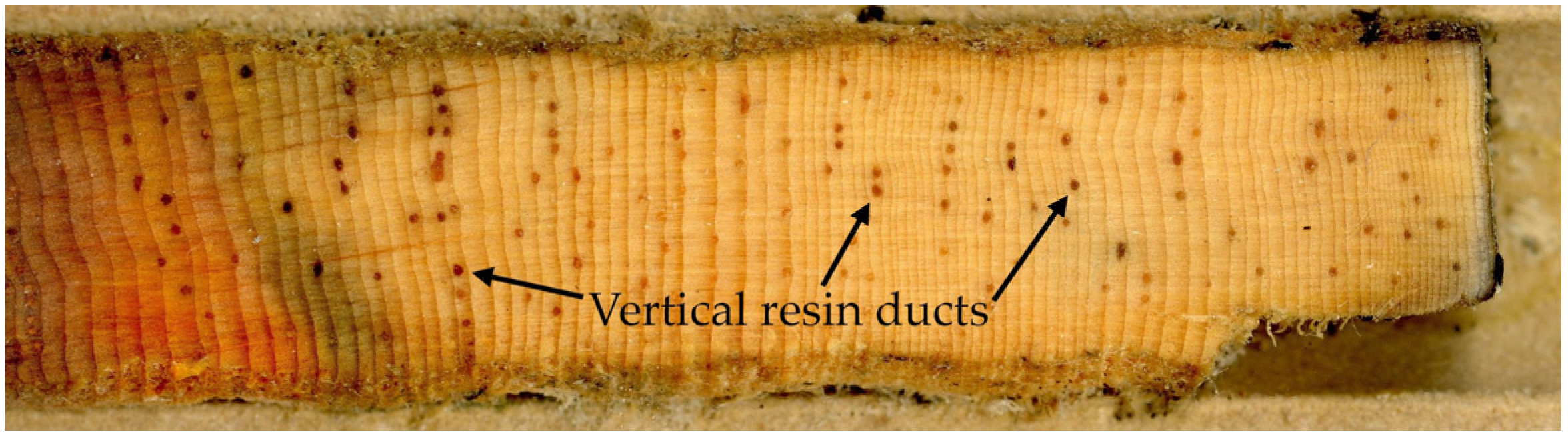
Tree growth and resin duct defenses provide a functional assessment of tree vigor that can be retrospectively assessed using dendrochronology methods. Measures of radial growth reflect the ability of a tree to acquire resources and allocate carbon to growth. Numerous studies have linked reduced radial growth and changes in growth patterns to increased mortality in many tree species [
22,
23,
24,
25]. Resin ducts are the network of conduits that produce and deliver oleoresins that chemically impair, isolate, and expel insects and pathogens [
26]. Greater allocation to resin ducts has been positively correlated with increased resin flow [
27] and decreased probability of tree mortality [
8,
28,
29,
30].
Changes in climate and competition can have different effects on carbon allocation to tree growth and defense. Studies have demonstrated that tree growth in lower elevation forests is often negatively correlated with increasing temperatures, while growth in higher elevation forests is often positively correlated with increasing temperatures [
31,
32]. Greater competition has been shown to limit growth for many species [
33,
34,
35], but these relationships can vary depending on tree species [
36]. The role of climate and competition on resin duct defenses has generally been less well studied, though some have found more allocation to resin ducts with higher precipitation and temperature [
30,
37,
38,
39].
This study examined growth and defense relationships with climate and competition in large sugar pine (
Pinus lambertiana) in an old-growth, mixed-conifer forest of the central Sierra Nevada. Much of California has experienced increasing temperatures over the last few decades, punctuated by an extended and severe drought [
40]. Additionally, a century of fire exclusion has allowed shade tolerant species such as white fir (
Abies concolor) and incense cedar (
Calocedrus decurrens) to establish, resulting in denser stands with greater competition than historical conditions [
3,
9,
41]. Cumulatively, these conditions have likely contributed to the observed decline in sugar pine populations [
42] and threaten the persistence of large individuals. Here, we took a retrospective approach using common dendrochronological methods to analyze the influence of climate and competition on sugar pine growth and defense at an annual resolution. The primary objectives of this study were to: (1) develop growth and defense models that explore the relative importance of climate and competition and (2) examine the trends in growth and defense over the past 50 years. The results of this study highlight the potential drivers of carbon allocation to growth and defense, improving our understanding of how the combined stressors of a warming climate and increased competition affect large sugar pine vigor.
4. Discussion
The sugar pine growth and defense (resin duct size and total area) models were improved when both climate and competition variables were included, but the measures of climate and competition and the direction of these relationships differed. Climate and competition relationships with growth have been well established [
34,
35,
36], but their influence on defense structures has received markedly less attention [
37,
38]. Our results suggest that annual carbon allocation to defense structures is not fixed and that both climate and competition influence resin duct characteristics even when incorporating changes in growth. Consistently, we found that growth and defense were more strongly associated with competition than climate variables. Growth and resin duct size had contrasting relationships with measures of competition, wherein growth was negatively related to intraspecific competition and the resin duct total area was positively related to total competition.
We also observed that the trends of growth and defense over the past 50 years differed, highlighting that these measures of vigor are responding differently to changes in climate. Increasing temperature and climatic water deficit likely have contrasting impacts on growth, and increasing competition could favor carbon allocation to defense but had differing impacts on resin duct characteristics (e.g., size and total area). Below, we place our findings in context with previous research and discuss future implications for sugar pines with potential insight into other species.
4.1. Growth Relationships with Climate and Competition
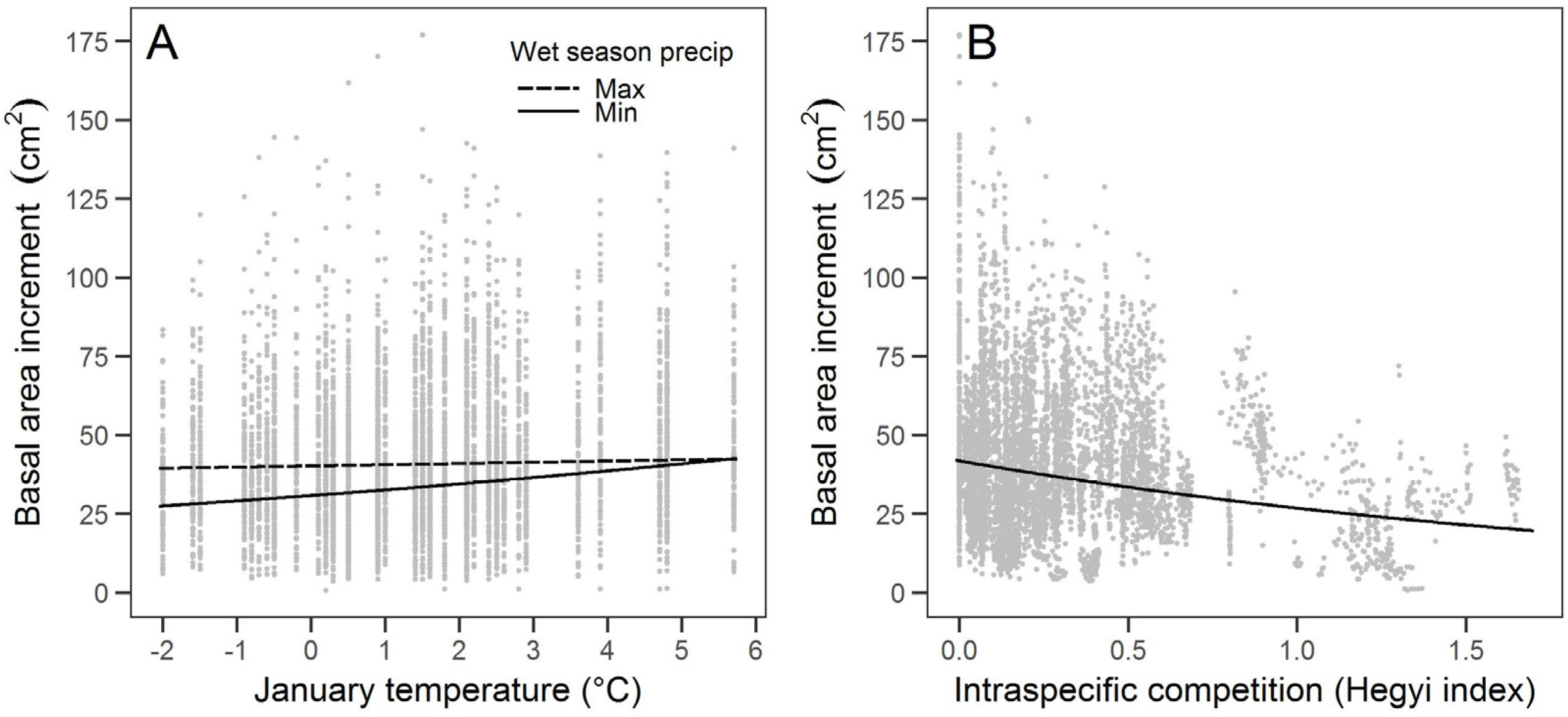
Sugar pine growth was associated with the climate variables of January temperature and wet season precipitation, though January temperature was more strongly associated with growth. The relationship between warmer January temperatures and higher growth suggests that sugar pines at our study site may be limited more by colder winter temperatures (i.e., limited by growing season) than by precipitation, at least for the years considered in this study. Growth that is limited by cold more than moisture has been found in high elevation conifers in California [
62]. However, this result was surprising considering that the elevation of the study site was 1700 m. Previous studies have suggested that the transition from low elevation, water-limited forests to high elevation, energy-limited forests occurs between 2000 and 2600 m in the southern Sierra Nevada [
31,
32].
Large sugar pine growth in our study was associated with intraspecific competition more than with total or interspecific competition, which is consistent with previous findings for the growth of sugar pine and other conifers [
36]. The influence of intraspecific competition may reflect that older sugar pine can be stronger competitors than younger shade-tolerant species such as white fir and incense cedar [
36]. The large sugar pine at our study site commonly occurred in clusters and were taller than most surrounding trees, likely resulting in greater intraspecific competition for light. Interspecific competition may have been less important because of the higher abundance of smaller incense cedar and white fir that likely did not compete as directly with large diameter sugar pines for aboveground and belowground resources.
4.2. Defense Relationships with Climate and Competition
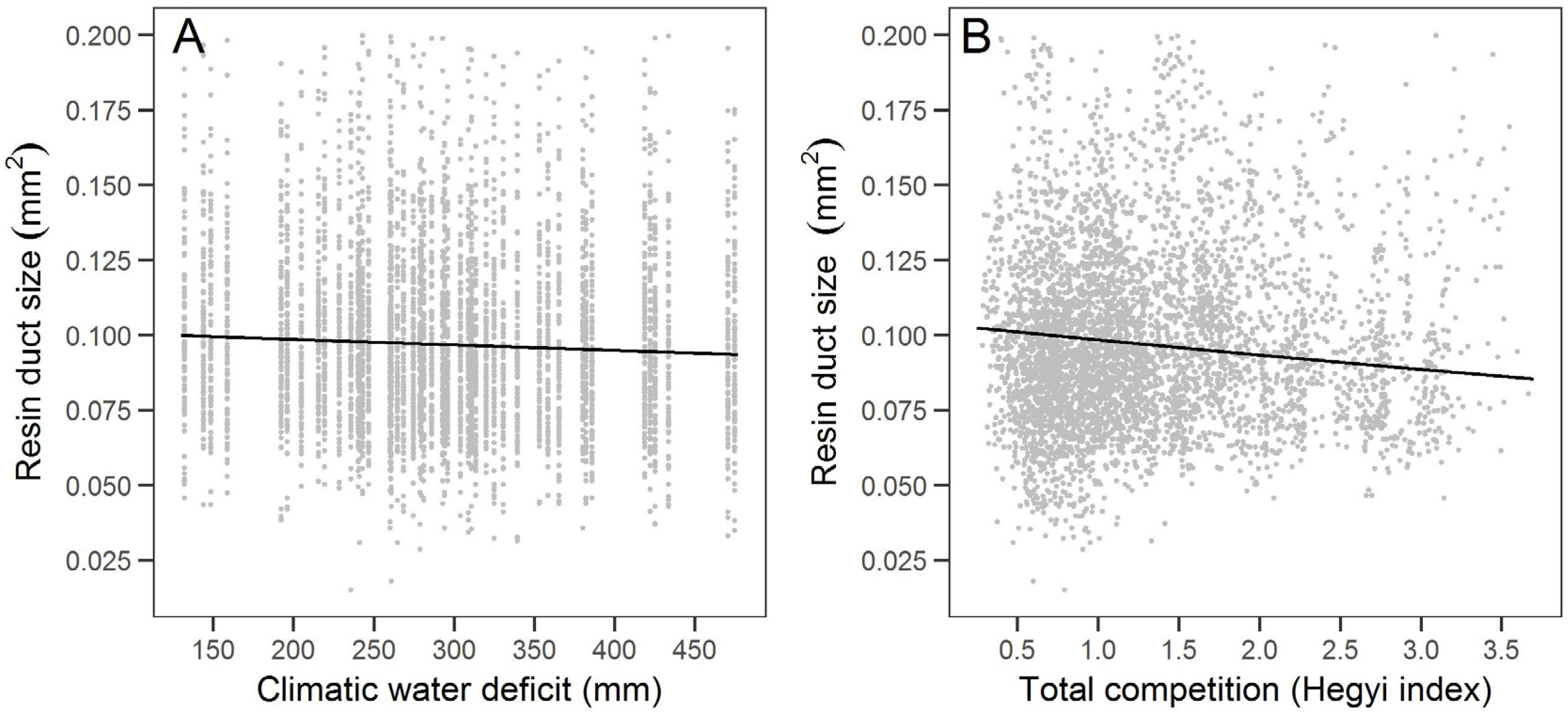
The variation in large sugar pine resin duct defense in our study was partially associated with climate factors. In particular, the relationship of defense measures with total wet season precipitation and climatic water deficit suggests that years with lower snowpack in the Sierra Nevada may limit large sugar pine carbon allocation to defense. Years with lower wet season temperatures and greater water stress may lead to less carbon allocation to resin ducts. Warmer temperatures have been associated with earlier spring snowmelt [
63], and reduced snowpack development along with earlier snowmelt can contribute to lower soil moisture and drier conditions [
64]. The relationship between climate and resin duct defense characteristics has been observed in both ponderosa pine and longleaf pine (
P. palustris); however, the strength of the relationships and variables of importance were not consistent with our findings. One study in Montana found that ponderosa pine resin duct total area was greater when temperatures were warmer and precipitation was higher during the month of July [
30], while another study examining longleaf pine in Florida found that resin duct total area was lower following a dry winter and did not show a significant relationship with temperature [
38]. These differences indicate that the relationship between resin duct defense and climate likely varies depending on the species and the climatic regions examined. Recent research has observed substantial variation in defense characteristics in pines across sites of differing climate [
65] and along elevational gradients [
66], but more direct examination with climate across these scales is needed.
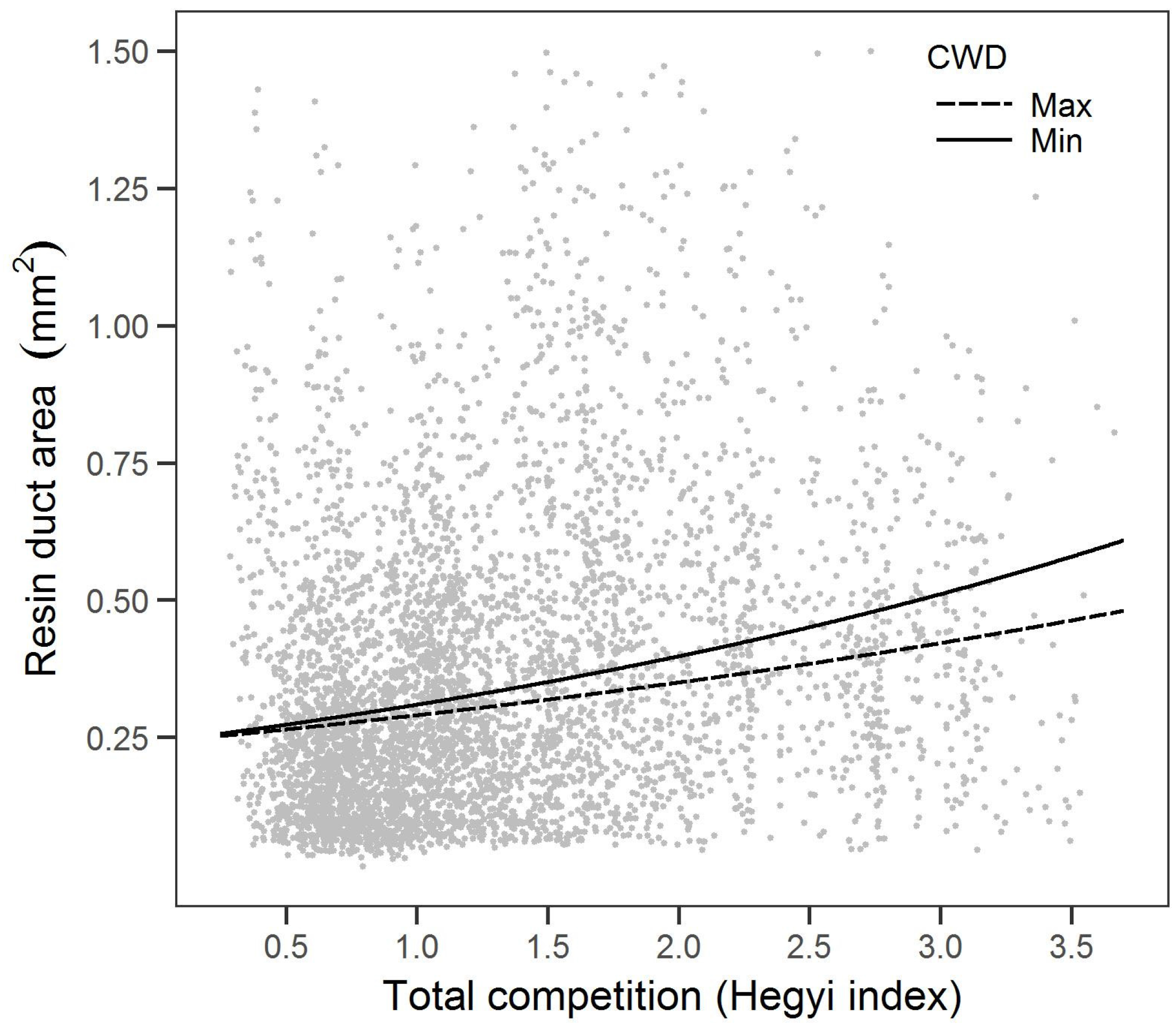
Notably, this is the first study we know of that examined the relationship between resin duct defense and competition. We found that higher total competition was associated with smaller resin ducts but resulted in greater total resin duct area. Increases in resin duct total area with competition are consistent with the growth-differentiation balance hypothesis [
20]. Specifically, competition-induced reductions in growth potentially allowed more carbohydrates to be allocated to resin ducts. The growth-differentiation balance hypothesis predicts that when environmental factors (e.g., water or nutrients) limit growth more than photosynthesis, carbon is directed towards the secondary metabolism, including defense. However, we also observed a reduction in resin duct size with more competition, which is counter to expectations of the growth-differentiation balance hypothesis. Despite more carbon allocation to resin duct defenses, specific resin duct characteristics (e.g., size and total area) may respond differently when the secondary metabolism is prioritized, although more research is needed. Conversely, trees with higher competition may limit resources sufficiently to reduce carbon allocation to both growth and defense. These conflicting results highlight the difficulty of directly examining the growth-differentiation balance hypothesis [
67]. We only examined xylem resin duct structures in our study, which represents a small subset of the defense characteristics of the whole tree (e.g., oleoresin, resin ducts in the branches and needles).
A more competitive environment that results in more resin duct total area suggests a potential induced response that may be similar to the induced response to disturbances (e.g., fire and pathogens) [
30,
68]. However, an increase in the total resin duct area and a decrease in resin duct size may confer different effects on tree defensibility through resin production and delivery. More resin duct area may result in a larger reservoir of resin available to pitch out invading pests [
27], but small resin ducts may also result in reduced resin flow (i.e., rate of resin delivered). Following Poiseuille’s law, decreases in resin duct size should decrease resin flow by the fourth power [
69]. This increase in resistance to resin flow may partially explain observations of decreased resin flow with greater competition [
70] but requires more direct examination.
4.3. Recent Trends and Future Implications
The differing long-term trends in the growth and defense of large sugar pines over the past 50 years were associated with concurrent increases in temperature and climate water deficit. Growth was clearly partitioned into two trends, a negative trend punctuated by a severe drought in 1977 and a positive trend since the late 1970s. This shift in growth pattern may partially reflect shifts in climate teleconnections such as the Pacific Decadal Oscillation (PDO), which had a phase shift from warm to cool during the late 1970’s [
71]. However, the persistent increase in growth also coincided with rapid temperature increases in the Sierra Nevada during the same period [
72]. The temporal pattern of resin duct size differed from growth and consisted of a more subtle but persistent decrease over the time period examined. This trend suggests that resin duct size was likely more responsive to increased competition over time.
Climate projections indicate that increased temperature and climatic water deficit will likely continue [
5] and, based on our findings, may result in increased growth and decreased resin duct size in large sugar pines. However, the positive relationship between growth and temperature may eventually reach a threshold at which rising temperatures will contribute to sufficient drought stress that begins to limit growth. Our data did not indicate that such a threshold between temperature and growth had been reached during the past 50 years. Temperature-growth relationship thresholds have not been established for sugar pines but have been observed in conifers at high latitudes and elevations [
73,
74].
Climate changes can also affect bark beetle outbreaks and the ability of large sugar pines to defend against attack. Warmer winter temperatures have been linked to higher bark beetle populations, leading to an increase in the occurrence of outbreaks [
16,
17]. Climatic water deficit is projected to increase by as much as 23% by 2050 due to climate change-induced warming [
54], which may result in smaller resin ducts that may further reduce defense against bark beetle attack. This and other potential climate changes could have a compounding effect on bark beetle-related mortality for sugar pines by creating favorable conditions for bark beetle outbreaks and weakened resin duct defenses.
In forests where fire exclusion continues to persist, competition could have a contrasting influence on resin duct defense, wherein resin duct size decreases but resin duct total area increases through the production of a higher density of smaller resin ducts [
8,
27]. In other words, there could be more resin available with higher resin duct total area but reduced resin flow due to increased resistance in resin flow independent of direct impacts to resin production [
27]. However, if higher tree densities persists due to continued fire exclusion, some large sugar pines may experience reduced competition due to increased mortality rates (e.g., density dependent mortality, bark beetle attacks, and drought) [
42], leading to fewer resin ducts that cover less area but are larger in size.
The impacts of climate and competition on large sugar pine growth and defense have potential implications for tree mortality. Slow growth and other patterns often indicate a higher probability of mortality [
23,
24,
25,
75]. In some cases, resin duct variables are better predictors of mortality than growth [
8,
29]. Trees with smaller resin ducts and less total resin duct area have been associated with greater probability of mortality [
28,
30]. Conversely, our observations of decreased resin duct size over time may indicate greater mortality risk. Regardless, continued increases in climatic water deficit may cause growth and defense to reach a threshold that could contribute to increases in large sugar pine mortality.
This study highlights that large sugar pine growth and defense had contrasting relationships with climate and competition, suggesting that measures of tree vigor may have differing responses to future changes. Competitive stress related to fire exclusion could result in the allocation of more carbon to resin duct defenses but could decrease the size of resin ducts, reducing resin flow. While the effects of climate and competition on tree vigor are complex, our research provides a better understanding on the factors related to large sugar pine vigor with potential implications for forest health modeling and forest management treatments (e.g., thinning and burning).