Fine Root Dynamics in Afromontane Forest and Adjacent Land Uses in the Northwest Ethiopian Highlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Descriptions
2.2. Root Sampling
2.2.1. Sequential Coring
2.2.2. In-Growth Core and Net Methods
2.3. Soil Sampling
2.4. Estimation of Annual Fine Root Production, Mortality and Decomposition
2.5. Calculation of Fine Root Turnover
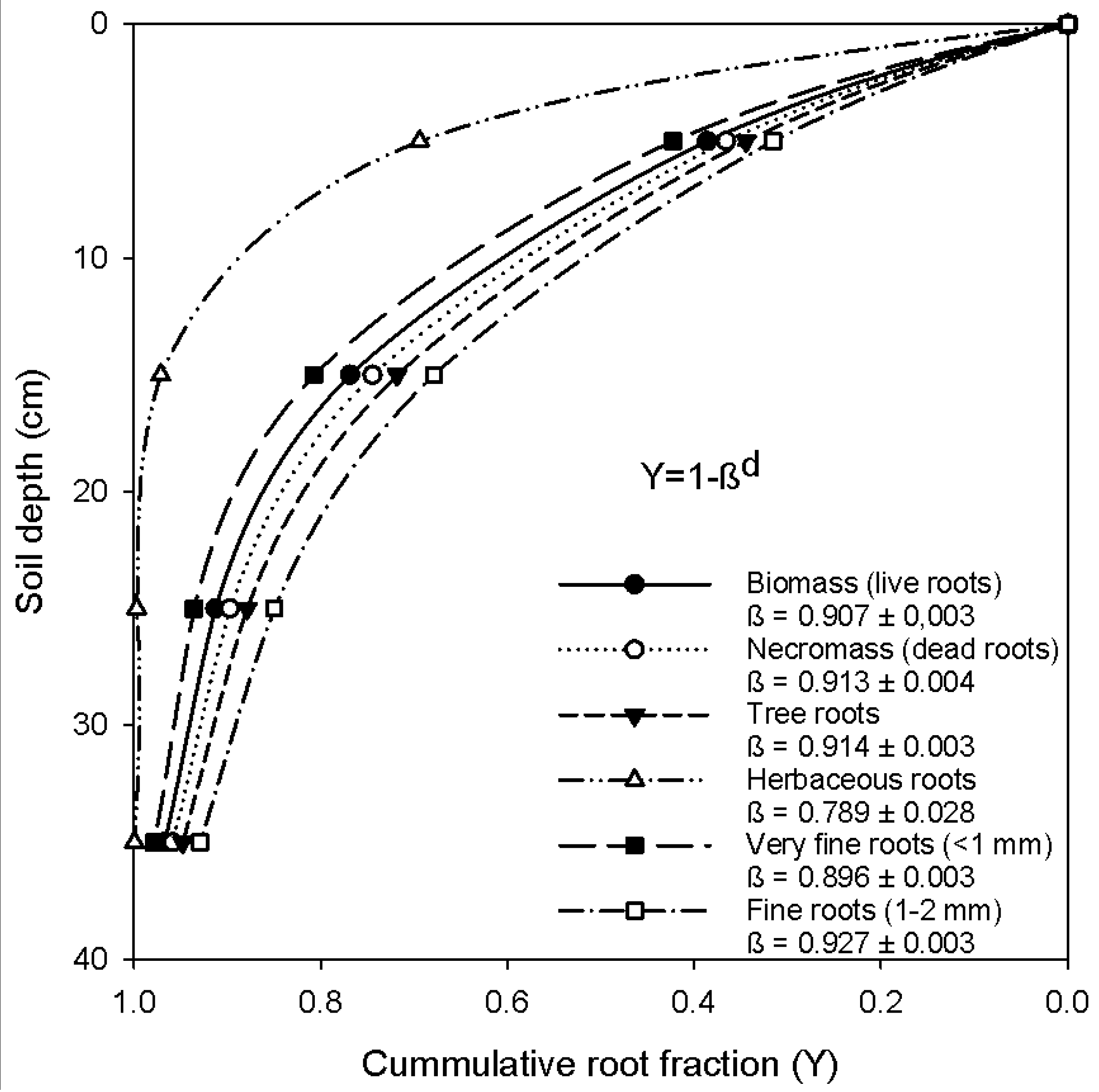
2.6. Fine Root Vertical Distribution
2.7. Carbon and Nitrogen Analysis
2.8. Statistical Analyses
3. Results
3.1. Fine Root Biomass, Necromass and Distribution with Depth
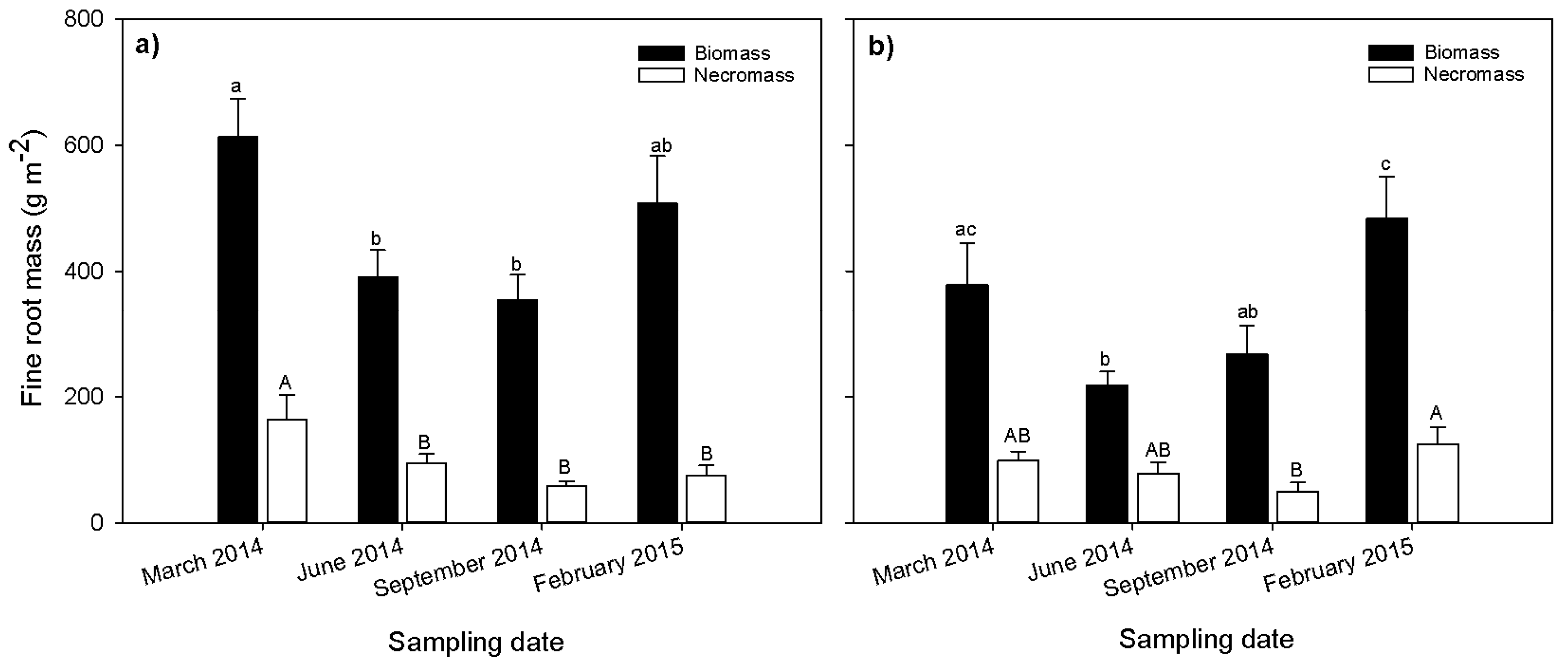
3.2. Seasonal Variation of Root Stocks
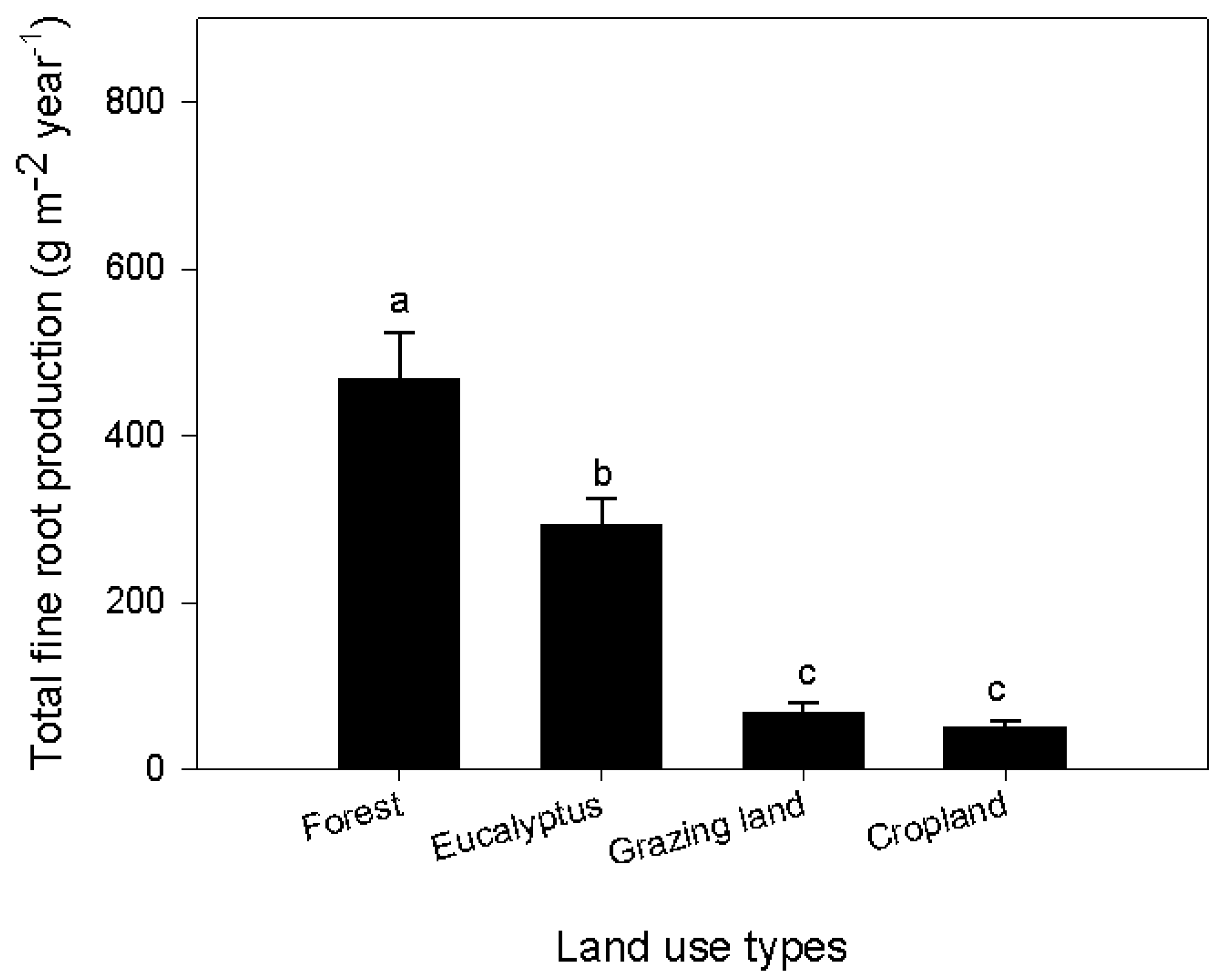
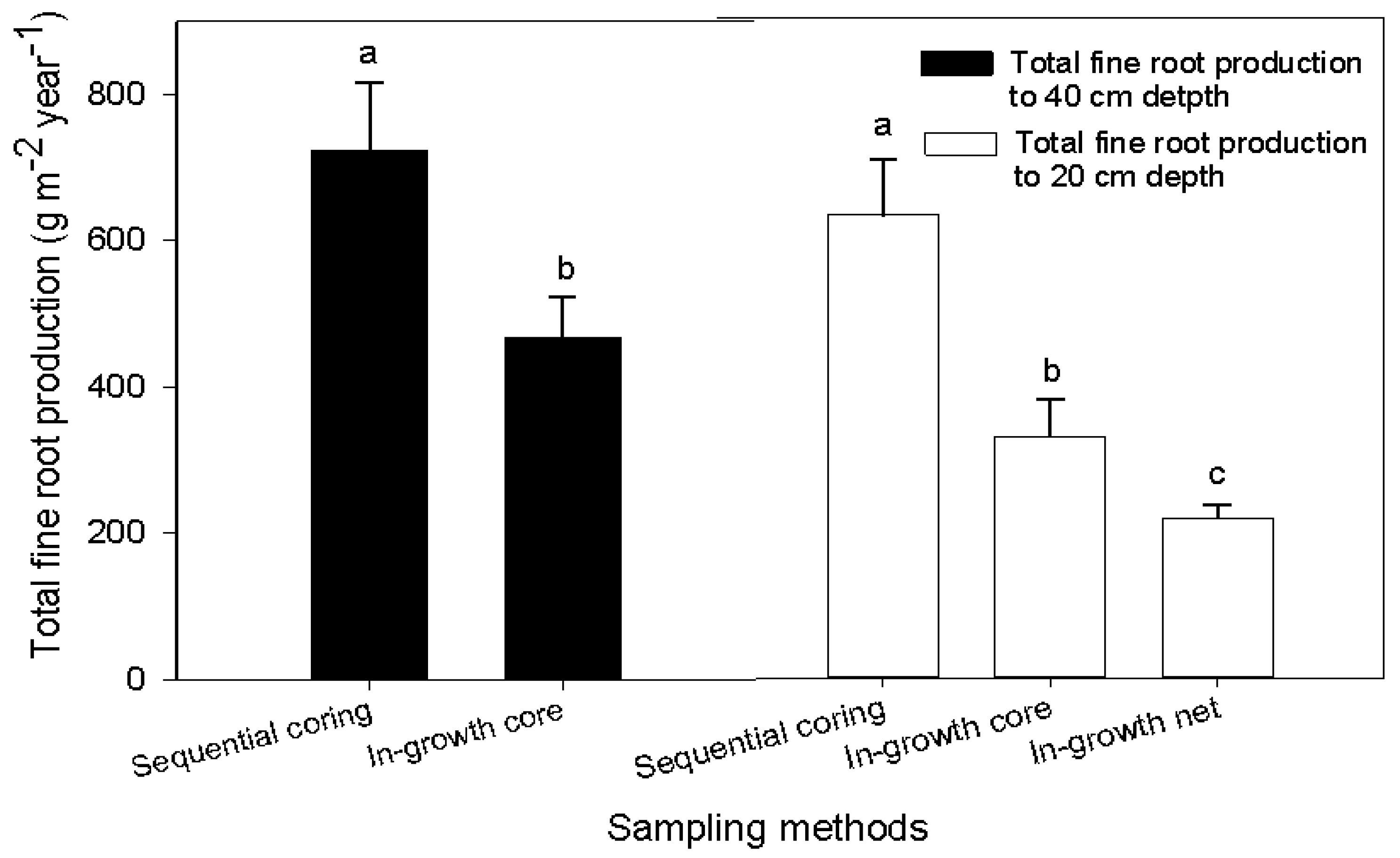
3.3. Fine Root Production, Mortality and Turnover
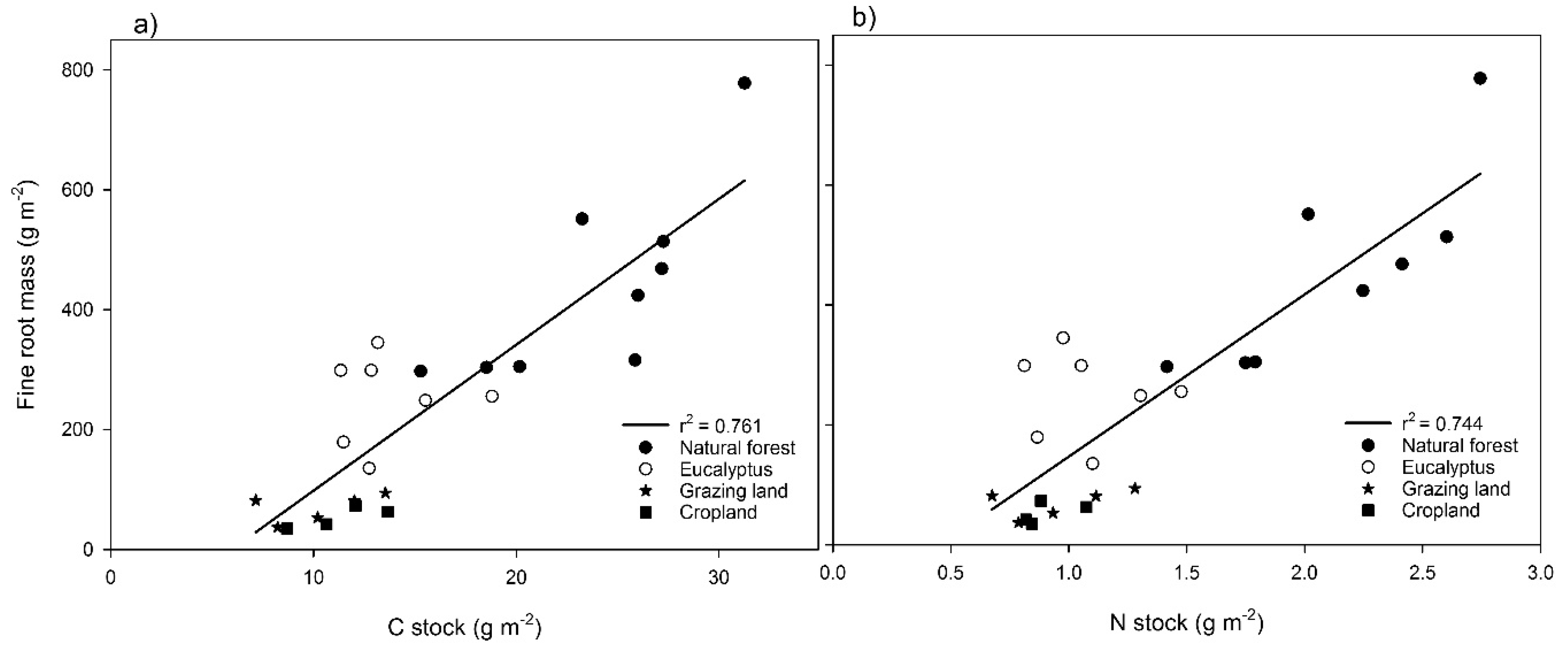
3.4. Annual C and N Flux into the Soil
4. Discussion
4.1. Effect of Land Use Change on Fine Root Stocks and Production
4.2. Seasonal Variation of Fine Root Mass
4.3. Limitations of Sampling Methods
4.4. Implications of Fine Root Turnover in Ecosystem Carbon Cycling
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Böhm, W. Methods of Studying Root Systems; Billings, W.D., Golley, F., Lange, O.L., Olson, J.S., Eds.; Springer: New York, NY, USA, 1979. [Google Scholar]
- Leuschner, C.; Hertel, D. Fine Root Biomass of Temperate Forests in Relation to Soil Acidity and Fertility, Climate, Age and Species. In Progress in Botany; Springer: Berlin, Germany, 2003; pp. 405–438. [Google Scholar]
- Pinno, B.D.; Wilson, S.D.; Steinaker, D.F.; Van Rees, K.C.J.; Mcdonald, S.A. Fine root dynamics of trembling aspen in boreal forest and aspen parkland in central Canada. Ann. For. Sci. 2010, 67, 1–6. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: Literature review and meta-analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Johnson, M.G.; Tingey, D.T.; Phillips, D.L.; Storm, M.J. Advancing fine root research with minirhizotrons. Environ. Exp. Bot. 2001, 45, 263–289. [Google Scholar] [CrossRef]
- Santantonio, D.; Grace, J.C. Estimating fine-root production and turnover from biomass and decomposition data: A compartment–flow model. Can. J. For. Res. 1987, 17, 900–908. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Bloomfield, J. Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 1998, 200, 71–89. [Google Scholar] [CrossRef]
- Lukac, M. Fine Root Turnover. In Measuring Roots; Springer: Berlin, Germany, 2012; pp. 363–373. [Google Scholar]
- Andreasson, F.; Gonzalez, M.; Augusto, L.; Bakker, M.R. Comparison of ingrowth cores and ingrowth meshes in root studies: 3 years of data on Pinus pinaster and its understory. Trees 2016, 30, 555–570. [Google Scholar] [CrossRef]
- Hertel, D.; Leuschner, C. A comparison of four different fine root production estimates with ecosystem carbon balance data in a Fagus-Quercus mixed forest. Plant Soil 2002, 239, 237–251. [Google Scholar] [CrossRef]
- Sun, T.; Dong, L.; Mao, Z.; Li, Y. Fine root dynamics of trees and understorey vegetation in a chronosequence of Betula platyphylla stands. For. Ecol. Manag. 2015, 346, 1–9. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Simplifying the decision matrix for estimating fine root production by the sequential soil coring approach. Acta Oecol. 2013, 48, 54–61. [Google Scholar] [CrossRef]
- Brunner, I.; Bakker, M.R.; Björk, R.G.; Hirano, Y.; Lukac, M.; Aranda, X.; Børja, I.; Eldhuset, T.D.; Helmisaari, H.S.; Jourdan, C.; et al. Fine-root turnover rates of European forests revisited: An analysis of data from sequential coring and ingrowth cores. Plant Soil 2012, 362, 357–372. [Google Scholar] [CrossRef]
- Hansson, K.; Helmisaari, H.-S.; Sah, S.P.; Lange, H. Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For. Ecol. Manag. 2013, 309, 58–65. [Google Scholar] [CrossRef]
- Lukac, M.; Godbold, D.L. Fine root biomass and turnover in southern taiga estimated by root inclusion nets. Plant Soil 2010, 331, 505–513. [Google Scholar] [CrossRef]
- Rewald, B.; Ephrath, J. Minirhizotron Techniques. In Plant Roots; Eshel, A., Beeckman, T., Eds.; CRC Press: New York, NY, USA, 2013; pp. 1–15. [Google Scholar]
- Makkonen, K.; Helmisaari, H.-S. Assessing fine-root biomass and production in a Scots pine stand–comparison of soil core and root ingrowth core methods. Plant Soil 1999, 210, 43–50. [Google Scholar] [CrossRef]
- Neill, C. Comparison of Soil Coring and Ingrowth Methods for Measuring Belowground Production. Ecology 1992, 73, 1918–1921. [Google Scholar] [CrossRef]
- Majdi, H.; Pregitzer, K.; Moren, A.-S.; Nylund, J.-E.; Ågren, G.I. Measuring fine root turnover in forest ecosystems. Plant Soil 2005, 276, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Chen, G.-S.; Lin, P.; Xie, J.-S.; Guo, J.-F. Fine root distribution, seasonal pattern and production in four plantations compared with a natural forest in subtropical China. Ann. For. Sci. 2004, 61, 617–627. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Helmisaari, H.-S.; Hallbäcken, L. Fine-root biomass and necromass in limed and fertilized Norway spruce (Picea abies (L.) Karst.) stands. For. Ecol. Manag. 1999, 119, 99–110. [Google Scholar] [CrossRef]
- Godbold, D.L.; Fritz, H.-W.; Jentschke, G.; Meesenburg, H.; Rademacher, P. Root turnover and root necromass accumulation of Norway spruce (Picea abies) are affected by soil acidity. Tree Physiol. 2003, 23, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Priess, J.; Then, C.; Fölster, H. Litter and fine-root production in three types of tropical premontane rain forest in SE Venezuela. Plant Ecol. 1999, 143, 171–187. [Google Scholar] [CrossRef]
- Ruess, R.W.; Hendrick, R.L.; Vogel, J.C.; Sveinbjornsson, B. The Role of Fine Roots in the Functioning of Alaskan Boreal Forests. In Alaska’s Changing Boreal Forest; Chapin, F.S., van Cleve, K., Viereck, L.A., Verbyla, D.L., Oswood, M.W., Eds.; Oxford University Press: New York, NY, USA, 2006; Volume 354. [Google Scholar]
- Nyssen, J.; Poesen, J.; Moeyersons, J.; Deckers, J.; Haile, M.; Lang, A. Human impact on the environment in the Ethiopian and Eritrean highlands—A state of the art. Earth-Sci. Rev. 2004, 64, 273–320. [Google Scholar] [CrossRef]
- Meshesha, D.T.; Tsunekawa, A.; Tsubo, M.; Ali, S.A.; Haregeweyn, N. Land-use change and its socio-environmental impact in Eastern Ethiopia’s highland. Reg. Environ. Chang. 2014, 14, 757–768. [Google Scholar] [CrossRef]
- Assefa, D.; Rewald, B.; Sandén, H.; Rosinger, C.; Abiyu, A.; Yitaferu, B.; Godbold, D.L. Deforestation and land use strongly effect soil organic carbon and nitrogen stock in Northwest Ethiopia. CATENA 2017, 153, 89–99. [Google Scholar] [CrossRef]
- Wassie, A.; Sterck, F.J.; Teketay, D.; Bongers, F. Tree regeneration in church forests of Ethiopia: Effects of microsites and management. Biotropica 2009, 41, 110–119. [Google Scholar] [CrossRef]
- Aerts, R.; Van Overtveld, K.; November, E.; Wassie, A.; Abiyu, A.; Demissew, S.; Daye, D.D.; Giday, K.; Haile, M.; TewoldeBerhan, S. Conservation of the Ethiopian church forests: Threats, opportunities and implications for their management. Sci. Total Environ. 2016, 551, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Desta, L.; Kassie, M.; Beninc, S.; Penderd, J. Land Degradation in the Highlands of Amhara Region and Strategies for Sustainable Land Management. In Policies for Sustainable Land Management in the Highlands of Ethiopia; Jabbar, M.A., Pender, J., Ehui, S.K., Eds.; International Livestock Research Institute: Addis Ababa, Eethiopia, 2000; Volume 30. [Google Scholar]
- Bekele, M. Forest Plantations and Woodlots in Ethiopia, 1st ed.; Regional Soil Conservation Unit, Swedish International Development Authority: Nairobi, Kenya, 2011; Volume 1. [Google Scholar]
- Pohjonen, V.; Pukkala, T. Eucalyptus globulus in Ethiopian forestry. For. Ecol. Manag. 1990, 36, 19–31. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Palmiotto, P.A.; Boon, P.; O’Hara, J.; Asbjornsen, H. Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 1996, 187, 159–219. [Google Scholar] [CrossRef]
- Jackson, R.; Mooney, H.A.; Schulze, E.-D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.H.-S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Doughty, C.; Galbraith, D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc. Lond. B 2011, 366, 3225–3245. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Talhelm, A.F.; Pregitzer, K.S. Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytol. 2015, 208, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Leppälammi-Kujansuu, J. Norway Spruce Fine Root Dynamics and Carbon Input into Soil in Relation to Environmental Factors. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2014. [Google Scholar]
- King, J.; Pregitzer, K.; Zak, D.; Sober, J.; Isebrands, J.; Dickson, R.; Hendrey, G.; Karnosky, D. Fine-root biomass and fluxes of soil carbon in young stands of paper birch and trembling aspen as affected by elevated atmospheric CO2 and tropospheric O3. Oecologia 2001, 128, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, S.S.; Kitajima, K.; Wright, S.J. Plant physiological ecology of tropical forest canopies. Trends Ecol. Evol. 1996, 11, 408–412. [Google Scholar] [CrossRef]
- Steinaker, D.F.; Wilson, S.D. Phenology of fine roots and leaves in forest and grassland. J. Ecol. 2008, 96, 1222–1229. [Google Scholar] [CrossRef]
- Joslin, J.D.; Wolfe, M.H.; Hanson, P.J. Factors controlling the timing of root elongation intensity in a mature upland oak stand. Plant Soil 2001, 228, 201–212. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. 2007, 4, 439–473. [Google Scholar] [CrossRef]
- WRB. World Reference Base for Soil Resources 2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Leppälammi-Kujansuu, J.; Aro, L.; Salemaa, M.; Hansson, K.; Kleja, D.B.; Helmisaari, H.-S. Fine root longevity and carbon input into soil from below- and aboveground litter in climatically contrasting forests. For. Ecol. Manag. 2014, 326, 79–90. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Lange, H.; Han, S. A modified ingrowth core method for measuring fine root production, mortality and decomposition in forests. Tree Physiol. 2013, 33, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Osawa, A.; Aizawa, R. A new approach to estimate fine root production, mortality, and decomposition using litter bag experiments and soil core techniques. Plant Soil 2012, 355, 167–181. [Google Scholar] [CrossRef]
- McClaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef]
- Fairley, R.; Alexander, I. Methods of Calculating Fine Root Production in Forests. In Ecological Interactions in Soil; Fitter, A., Ed.; British Ecological Society: London, UK, 1985; pp. 37–42. [Google Scholar]
- Aerts, R.; Bakker, C.; De Caluwe, H. Root turnover as determinant of the cycling of C, N, and P in a dry heathland ecosystem. Biogeochemistry 1992, 15, 175–190. [Google Scholar] [CrossRef]
- Gale, M.R.; Grigal, D.F. Vertical root distributions of northern tree species in relation to successional status. Can. J. For. Res. 1987, 17, 829–834. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Raich, J.W.; Russell, A.E. Fine-root mass, growth and nitrogen content for six tropical tree species. Plant Soil 2007, 290, 357–370. [Google Scholar] [CrossRef]
- Kucbel, S.; Jaloviar, P.; Špišák, J. Quantity, vertical distribution and morphology of fine roots in Norway spruce stands with different stem density. Plant Root 2011, 5, 46–55. [Google Scholar] [CrossRef]
- Finér, L.; Helmisaari, H.-S.; Lõhmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, T.; Godbold, D.; Grebenc, T.; Konôpka, B. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Cavard, X.; Laganière, J.; Reich, P.B.; Bergeron, Y.; Paré, D.; Yuan, Z.; Laganiere, J.; Reich, P.B.; et al. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 101, 210–219. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. The Global Biogeography of Roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.-D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Eamus, D.; Hutley, L.B. Seasonal patterns of fine-root productivity and turnover in a tropical savanna of northern Australia. J. Trop. Ecol. 2004, 20, 221–224. [Google Scholar] [CrossRef]
- Contador, M.L.; Comas, L.H.; Metcalf, S.G.; Stewart, W.L.; Porris Gomez, I.; Negron, C.; Lampinen, B.D. Root growth dynamics linked to above-ground growth in walnut (Juglans regia). Ann. Bot. 2015, 116, 49–60. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Sabaté, S.; Gracia, C.A. Annual and seasonal changes in fine root biomass of a Quercus ilex L. forest. Plant Soil 2001, 230, 125–134. [Google Scholar] [CrossRef]
- Teskey, R.O.; Hinckley, T.M. Influence of temperature and water potential on root growth of white oak. Physiol. Plant. 1981, 52, 363–369. [Google Scholar] [CrossRef]
- Priestley, C.A.; Catlin, P.B.; Olsson, E.A. The Distribution of 14C-Labelled Assimilates in Young Apple Trees as Influenced by Doses of Supplementary Nitrogen. Ann. Bot. 1976, 40, 1163–1170. [Google Scholar] [CrossRef]
- Harteveld, M.; Hertel, D.; Wiens, M.; Leuschner, C. Spatial and temporal variability fo fine root abundance and growth in tropical moist forests and agroforestry systems (Sulawesi, Indonesia). ECOTROPICA 2007, 13, 111–120. [Google Scholar]
- Lima, T.T.S.; Miranda, I.S.; Vasconcelos, S.S. Effects of water and nutrient availability on fine root growth in eastern Amazonian forest regrowth, Brazil. New Phytol. 2010, 187, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Kätterer, T.; Fabião, A.; Madeira, M.; Ribeiro, C.; Steen, E. Fine-root dynamics, soil moisture and soil carbon content in a Eucalyptus globulus plantation under different irrigation and fertilisation regimes. For. Ecol. Manag. 1995, 74, 1–12. [Google Scholar] [CrossRef]
- Yavitt, J.B.; Wright, S.J. Drought and Irrigation Effects on Fine Root Dynamics in a Tropical Moist Forest, Panama. Biotropica 2001, 33, 421–434. [Google Scholar] [CrossRef]
- Hertel, D.; Strecker, T.; Müller-Haubold, H.; Leuschner, C. Fine root biomass and dynamics in beech forests across a precipitation gradient—Is optimal resource partitioning theory applicable to water-limited mature trees? J. Ecol. 2013, 101, 1183–1200. [Google Scholar] [CrossRef]
- Joslin, J.D.; Wolfe, M.H.; Hanson, P.J. Effects of altered water regimes on forest root systems. New Phytol. 2000, 147, 117–129. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Meir, P.; Aragão, L.E.O.C.; da Costa, A.C.L.; Braga, A.P.; Gonçalves, P.H.L.; de Athaydes Silva Junior, J.; de Almeida, S.S.; Dawson, L.A.; Malhi, Y.; et al. The effects of water availability on root growth and morphology in an Amazon rainforest. Plant Soil 2008, 311, 189–199. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The Ecology of Root Lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Gilbert, K.J.; Fahey, T.J.; Maerz, J.C.; Sherman, R.E.; Bohlen, P.; Dombroskie, J.J.; Groffman, P.M.; Yavitt, J.B. Exploring carbon flow through the root channel in a temperate forest soil food web. Soil Biol. Biochem. 2014, 76, 45–52. [Google Scholar] [CrossRef]
- Hertel, D.; Harteveld, M.A.; Leuschner, C. Conversion of a tropical forest into agroforest alters the fine root-related carbon flux to the soil. Soil Biol. Biochem. 2008, 41, 481–490. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Eckert, G.E.; Coleman, D.C. Soil carbon differences among forest, agriculture, and secondary vegetation in lower montane Ecuador. Ecol. Appl. 2000, 10, 497–505. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
| Biomass | |||
|---|---|---|---|
| Increase | Decrease | ||
| Necromass | Increase | P = ΔB + ΔN | P = ΔB + ΔN or 0 |
| M = ΔN | M = ΔN or |−ΔB| | ||
| D = 0 | D = |−ΔB| − ΔN or 0 | ||
| Decrease | P = ΔB | P = 0 | |
| M = ΔB | M = |−ΔB| | ||
| D = |−ΔN| | D = |−ΔB| + |−ΔN| | ||
| Land Use | Depth | Total Root Mass | Live and Dead Roots | Diameter Class | Plant Type | |||
|---|---|---|---|---|---|---|---|---|
| Biomass | Necromass | Roots < 1 mm | Roots 1–2 mm | Tree Roots | Herbaceous Roots | |||
| Forest | Total | 564.2 ± 41.7 | 466.5 ± 31.6 | 97.7 ± 12.8 | 272.9 ± 18.2 | 291.4 ± 27.7 | 555.8 ± 42.2 | 8.4 ± 3.2 |
| 0–10 | 239.5 ± 18.5 | 195.5 ± 14.7 | 46.4 ± 6.9 | 137.5 ± 9.5 | 108.1 ± 12.1 | 232.1 ± 18.9 | 7.5 ± 2.9 | |
| 10–20 | 174.2 ± 15.2 | 153.3 ± 11.8 | 30.1 ± 4.5 | 81.4 ± 6.5 | 101.9 ± 10.5 | 177.9 ± 15.0 | 0.7 ± 0.4 | |
| 20–30 | 90.0 ± 11.3 | 74.0 ± 8.4 | 17.8 ± 3.9 | 37.2 ± 2.8 | 61.4 ± 10.4 | 89.8 ± 11.3 | 0.2 ± 0.2 | |
| 30–40 | 60.5 ± 8.4 | 55.6 ± 8.3 | 12.6 ± 2.2 | 27.1 ± 3.7 | 44.3 ± 6.8 | 65.4 ± 8.6 | na | |
| Eucalyptus | Total | 424.7 ± 36.9 | 336.8 ± 30.5 | 87.9 ± 10.2 | 211.8 ± 17.1 | 223.0 ± 22.8 | 302.6 ± 28.9 | 122.1 ± 15.1 |
| 0–10 | 231.4 ± 19.8 | 176.2 ± 16.5 | 55.2 ± 6.7 | 123.6 ± 12.1 | 107.8 ± 11.4 | 140.5 ± 11.8 | 90.9 ± 13.2 | |
| 10–20 | 93.6 ± 12.7 | 75.8 ± 11.0 | 20.9 ± 4.0 | 45.4 ± 4.5 | 60.3 ± 10.9 | 74.2 ± 12.1 | 19.4 ± 3.1 | |
| 20–30 | 58.1 ± 7.5 | 48.7 ± 7.0 | 12.5 ± 1.6 | 27.1 ± 2.9 | 41.3 ± 6.7 | 50.6 ± 7.7 | 8.7 ± 1.7 | |
| 30–40 | 41.6 ± 14.3 | 41.2 ± 15.5 | 9.7 ± 1.9 | 17.4 ± 2.8 | 47.2 ± 21.7 | 44.0 ± 16.2 | 3.1 ± 0.7 | |
| Grazing land | Total | 55.5 ± 13.3 | 53.2 ± 12.7 | 3.4 ± 1.6 | 55.5 ± 13.3 | na | na | 55.5 ± 13.3 |
| 0–10 | 31.9 ± 7.8 | 29.8 ± 7.1 | 2.1 ± 1.3 | 31.9 ± 7.8 | na | na | 31.9 ± 7.8 | |
| 10–20 | 13.3 ± 5.6 | 13.3 ± 5.6 | na | 13.3 ± 5.6 | na | na | 13.3 ± 5.6 | |
| 20–30 | 6.4 ± 1.7 | 5.9 ± 1.6 | 0.5 ± 0.0 | 6.4 ± 1.7 | na | na | 6.4 ± 1.7 | |
| 30–40 | 4.9 ± 2.1 | 4.1 ± 1.5 | 0.8 ± 1.3 | 4.9 ± 2.1 | na | na | 4.9 ± 2.1 | |
| Cropland | Total | 46.1 ± 4.4 | 45.3 ± 3.9 | 0.8 ± 0.6 | 44.8 ± 3.4 | 1.3 | na | 46.1 ± 4.4 |
| 0–10 | 31.2 ± 3.2 | 30.6 ± 6.3 | 0.7 ± 0.0 | 31.2 ± 3.2 | na | na | 31.2 ± 3.2 | |
| 10–20 | 13.2 ± 4.2 | 13.0 ± 4.2 | 0.2 ± 0.0 | 11.9 ± 3.1 | 1.3 | na | 13.2 ± 4.2 | |
| 20–30 | 1.7 ± 1.1 | 1.7 ± 1.1 | na | 1.7 ± 1.1 | na | na | 1.7 ± 1.1 | |
| 30–40 | na * | na | na | na | na | na | na | |
| Land Use | Biomass | Decision Matrix | Max-Min | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (g·m−2) | Max. (g·m−2) | Production (g·m−2) | Turnover Rate | Production (g·m−2) | Turnover Rate | ||||||||
| B | N | D | TP | Bmean (year−1) | Bmax (year−1) | B | N | TP | Bmean (year−1) | Bmax (year−1) | |||
| Forest | 496.6 ± 41.0 | 684.4 ± 59.2 | 256.9 ± 43.8 | 50.1 ± 10.4 | 415.9 ± 66.2 | 722.9 ± 92.8 | 1.5 | 1.1 | 330.7 ± 40.5 | 128.7 ± 36.1 | 459.4 ± 63.8 | 0.93 | 0.67 |
| Eucalyptus | 336.8 ± 28.6 | 559.1 ± 70.1 | 313.3 ± 61.4 | 98.8 ± 28.2 | 282.2 ± 71.2 | 694.4 ± 94.8 | 2.1 | 1.2 | 376.6 ± 57.6 | 110.4 ± 26.4 | 486.9 ± 77.3 | 1.5 | 0.87 |
| Sampling Method and Land Use Type | Root production (g·m−2) | |||
|---|---|---|---|---|
| Biomass | Necromass | Decomposed | Total Production | |
| In-growth core 20-cm depth | ||||
| Forest | 173.8 ± 13.6 aA | 34.2 ± 6.2 aA | 121.0 ± 27.8 aA | 329.0 ± 50.6 aA |
| Eucalyptus | 96.7 ± 14.0 bA | 38.8 ± 12.5 aA | 95.1 ± 21.1 aA | 230.6 ± 30.6 aA |
| Grazing land | 43.1 ± 11.4 cA | 2.1 ± 0.9 bA | 1.8 ± 0.6 bA | 47.8 ± 11.5 bA |
| Cropland | 43.6 ± 3.2 cA | 0.8 ± 0.6 bA | 2.5 ± 1.5 bA | 46.2 ± 4.2 bA |
| In-growth net method 20-cm depth | ||||
| Forest | 71.1 ± 12.2 aB | 25.9 ± 5.6 aA | 120.6 ± 19.7 aA | 217.7 ± 17.7 aB |
| Eucalyptus | 78.6 ± 20.2 aA | 17.7 ± 4.2 aB | 140.3 ± 22.1 aA | 226.9 ± 19.2 aA |
| Grazing land | 39.9 ± 22.0 bA | 2.6 ± 1.5 bA | 2.1 ± 1.1 bA | 44.6 ± 23.1 bA |
| Cropland | 54.7 ± 4.6 bA | 0.9 ± 0.6 bA | 0.2 ± 0.2 bB | 55.8 ± 4.6 bA |
| Land Use Type | C% in Roots | N% in Roots | Element Flux (g·m−2·year−1) | |
|---|---|---|---|---|
| C | N | |||
| Forest | 48.4 ± 0.9 (n = 9) | 1.6 ± 0.3 (n = 9) | 226.4 | 7.5 |
| Eucalyptus | 45.4 ± 0.8 (n = 9) | 0.9 ± 0.1 (n = 9) | 132.9 | 2.6 |
| Grazing land | 46.3 ± 1.2 (n = 3) | 1.0 ± 0.0 (n = 3) | 32.2 | 0.7 |
| Cropland | 46.3 ± 1.2 (n = 3) | 1.0 ± 0.0 (n = 3) | 23.9 | 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assefa, D.; Rewald, B.; Sandén, H.; Godbold, D.L. Fine Root Dynamics in Afromontane Forest and Adjacent Land Uses in the Northwest Ethiopian Highlands. Forests 2017, 8, 249. https://doi.org/10.3390/f8070249
Assefa D, Rewald B, Sandén H, Godbold DL. Fine Root Dynamics in Afromontane Forest and Adjacent Land Uses in the Northwest Ethiopian Highlands. Forests. 2017; 8(7):249. https://doi.org/10.3390/f8070249
Chicago/Turabian StyleAssefa, Dessie, Boris Rewald, Hans Sandén, and Douglas L. Godbold. 2017. "Fine Root Dynamics in Afromontane Forest and Adjacent Land Uses in the Northwest Ethiopian Highlands" Forests 8, no. 7: 249. https://doi.org/10.3390/f8070249







