Short-Term Effects of Reduced-Impact Logging on Copaifera spp. (Fabaceae) Regeneration in Eastern Amazon
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Selection and Measurement of Copaifera
2.3. Regeneration of Copaifera
2.3.1. Demographic Rates
2.3.2. Modeling of Mortality and Survival
2.4. Forest Canopy Cover
3. Results
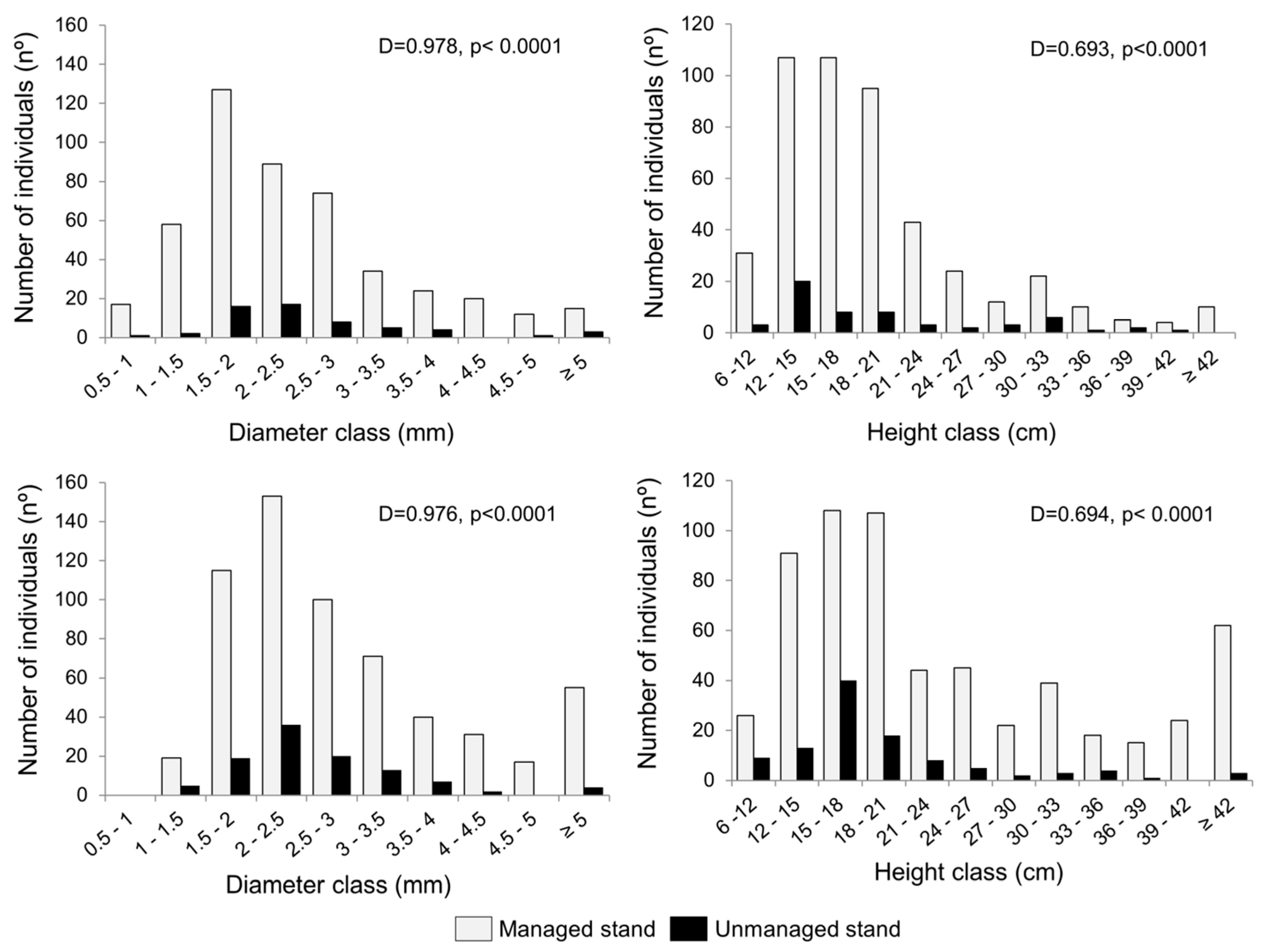
3.1. Copaifera Regeneration
3.2. Spatial Distribution and Modeling of Mortality and Survival
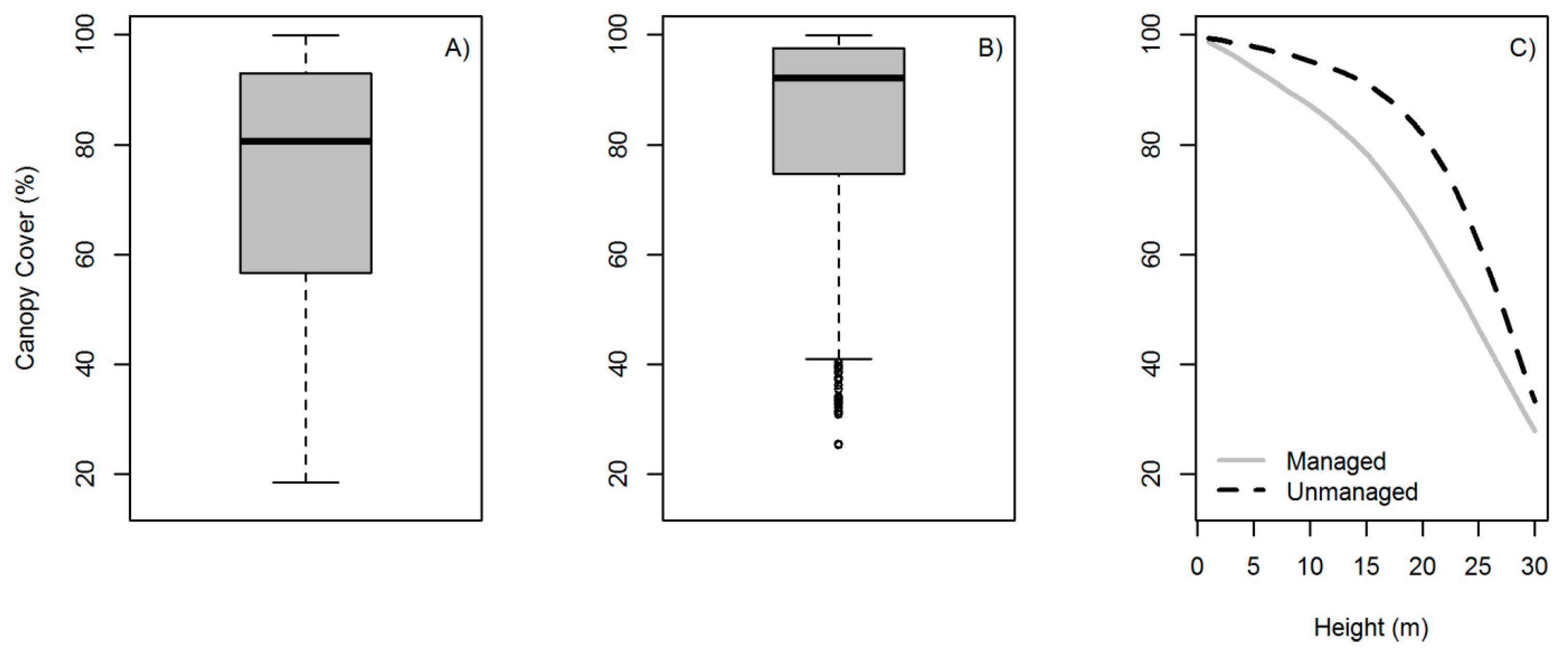
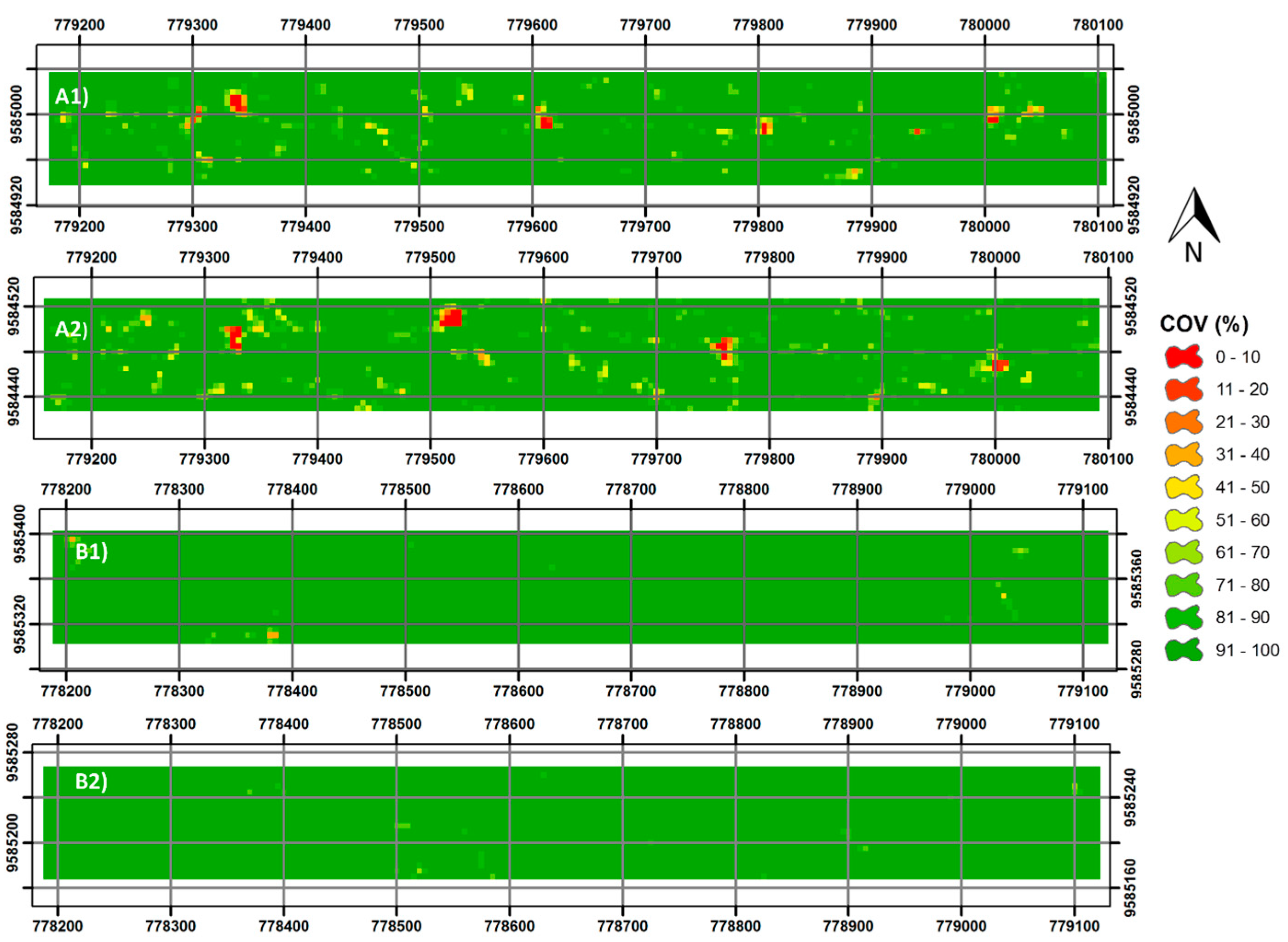
3.3. Forest Canopy Cover
4. Discussion
4.1. Effects of Reduced-Impact Logging on Population Dynamics of Copaifera
4.2. Effects of Reduced-Impact Logging on Diameter Distribution of Copaifera
4.3. Effects of Reduced-Impact Logging on Spatial Distribution of Copaifera
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeppel, H. Indigenous Ecotourism: Sustainable Development and Management; Ecotourism Series No. 3; CABI: London, UK, 2006; 295p. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Estudos e Pesquisas Informação Geográfica Número 9; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2012; 350p. Available online: http://geoftp.ibge.gov.br/documentos/recursos_naturais/indicadores_desenvolvimento_sustentavel/2012/ids2012.pdf (accessed on 18 February 2015).
- Laurance, W.F.; Ferreira, L.V.; Merona, J.M.R.; Laurance, S.G. Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 1998, 79, 2032–2040. [Google Scholar] [CrossRef]
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.C.; Silva, J.N. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]
- Lavinsky, A.O.; Sant’Ana, C.S.; Mielke, M.S.; Almeida, A.A.F.; Gomes, F.P.; França, S.; Silva, D.C. Effects of light availability and soil flooding on growth and photosynthetic characteristics of Genipa americana L. seedlings. New For. 2007, 34, 41–50. [Google Scholar] [CrossRef]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia Ilustrado Para Identificação das Famílias Nativas e Exóticas no Brasil, Baseado em APG II, 2nd ed.; Instituto Plantarum: Nova Odessa, São Paul, 2008; 640p. [Google Scholar]
- Veiga Junior, V.F.; Pinto, A.C. The Copaifera L. genus. Quím. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Herrero-Jáuregui, C.; Sist, P.; Casado, M.A. Population structure of two low-density neotropical tree species under different management systems. For. Ecol. Manag. 2012, 280, 31–39. [Google Scholar] [CrossRef]
- Newton, P.; Peres, C.A.; Desmoulière, S.J.M.; Watkinson, A.R. Cross-scale variation in the density and spatial distribution of an Amazonian non-timber forest resource. For. Ecol. Manag. 2012, 276, 41–51. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Foster, R.B. Biology, chance and the history and structure of tropical rain Forest tree communities. In Community Ecology; Diamond, J.M., Case, T.J., Eds.; Harper and Row: New York, NY, USA, 1986; 329p. [Google Scholar]
- Leite, A.M.C.; Salomão, A.N. Estrutura populacional de copaíba (Copaifera langsdorffii Desf.) em mata ciliar do Distrito Federal. Acta Amazon. Bras. 1992, 6, 123–134. [Google Scholar] [CrossRef]
- Resende, J.C.F.; Klink, C.A.; Schiavini, I. Spatial heterogeneity and its influence on Copaifera langsdorffii Desf. (Caesalpiniaceae). Braz. Arch. Biol. Technol. 2003, 46, 405–414. [Google Scholar] [CrossRef]
- Gavin, D.G.; Peart, D.R. Spatial structure and regeneration of Tretamerista glabra in peat swamp rain forest in Indonesian Borneo. Plant Ecol. 1997, 131, 223–231. [Google Scholar] [CrossRef]
- Jakovac, A.; Bentos, T.V.; Mesquita, R.C.G.; Williamson, G.B. Age and light effects on seedling growth in two alternative secondary successions in central Amazonia. Plant Ecol. Divers. 2014, 7, 349–358. [Google Scholar] [CrossRef]
- Verissimo, A.; Barreto, P.; Mattos, M.; Tarifa, R.; Uhl, C. Logging impacts and prospects for sustainable forest management in an old Amazonian frontier: The case of Paragominas. For. Ecol. Manag. 1992, 55, 169–199. [Google Scholar] [CrossRef]
- Fredericksen, T.S.; Mostacedo, B. Regeneration of timber species following selection logging in a Bolivian tropical dry forest. For. Ecol. Manag. 2000, 131, 47–55. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Schulze, M.D.; Carter, D.R.; Vidal, E. A model for comparing reduced impact logging with conventional logging for an Eastern Amazonian Forest. For. Ecol. Manag. 2010, 250, 2002–2011. [Google Scholar] [CrossRef]
- Holmes, T.P.; Blate, G.M.; Zweede, J.C.; Pereira, R., Jr.; Barreto, P.; Boltz, F.; Bauch, R. Financial and ecological indicators of reduced impact logging performance in the eastern Amazon. For. Ecol. Manag. 2002, 163, 93–110. [Google Scholar] [CrossRef]
- Wright, S.J. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Falkowski, V.; Peña-Claros, M. Natural regeneration of tree species in the Eastern Amazon: Short-term responses after reduced-impact logging. For. Ecol. Manag. 2017, 385, 97–103. [Google Scholar] [CrossRef]
- Alencar, J.C. Estudos silviculturais de uma população natural de Copaifera multijulga Hayne–Leguminosae, na Amazônia Central. 1—Germinação. Acta Amazon. 1981, 11, 3–11. [Google Scholar] [CrossRef]
- Hosokawa, R.T.; Moura, J.B.; Cunha, U.S. Introdução ao Manejo e Economia de Florestas; Universidade Federal do Paraná: Curitiba, Paraná, 1998; 162p. [Google Scholar]
- Oliveira, R.E.; Engel, V.L. A restauração ecológica em destaque: Um retrato dos últimos vinte e oito anos de publicações na área. Oecol. Aust. 2011, 15, 303–315. [Google Scholar] [CrossRef]
- Schwartz, G.; Peña-Claros, M.; Lopes, J.C.A.; Mohren, G.M.J.; Kanashiro, M. Mid-term effects of reduced-impact logging on the regeneration of seven tree commercial species in the Eastern Amazon. For. Ecol. Manag. 2012, 274, 116–125. [Google Scholar] [CrossRef]
- RADAMBRASIL. Projeto RADAMBRASIL: 1973–1983, Levantamento de Recursos Naturais. v. 1–23; Departamento Nacional de Produção Mineral, Ministério das Minas e Energia: Rio de Janeiro, Brazil, 1983; 367p. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Manual Técnico da Vegetação Brasileira; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2012; 275p. [Google Scholar]
- Sheil, D.; May, R.M. Mortality and recruitment rate evaluations in heterogeneous tropical forests. J. Ecol. 1996, 84, 91–100. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw Hill Book: New York, NY, USA, 1980; p. 633. [Google Scholar]
- Hollander, M.; Wolfe, D.A. Nonparametric Statistical Methods, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1999; 787p. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; Wiley Series in Probability and Mathematical Statistics: New York, NY, USA, 2000; p. 307. [Google Scholar]
- Monserud, R.A.; Sterba, H. Modeling individual tree mortality for Austrian forest species. For. Ecol. Manag. 1999, 113, 109–123. [Google Scholar] [CrossRef]
- Mendenhall, W.; Scheaffer, R. Mathematical Statistics with Applications; Duxbury Press: Scituate, MA, USA, 1973. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org (accessed on 15 October 2016).
- Hudak, A.T.; Evans, J.S.; Smith, A.M.S. Review: LiDAR Utility for Natural Resource Managers. Remote Sens. 2009, 1, 934–951. [Google Scholar] [CrossRef]
- Silva, C.A.; Klauberg, C.; Hudak, A.T.; Carvalho, S.P.; Rodriguez, L.C.E. Mapping aboveground carbon stocks using LiDAR data in Eucalyptus spp. plantations in the state of São Paulo, Brazil. Sci. For. 2014, 42, 591–604. [Google Scholar]
- Silva, C.A.; Klauberg, C.; Hudak, A.T.; Vierling, L.A.; Liesenberg, V.; Carvalho, S.P.; Rodriguez, L.C. A principal component approach for predicting the stem volume in Eucalyptus plantations in Brazil using airborne Lidar data. Forestry 2016, 1–12. [Google Scholar] [CrossRef]
- McGaughey, R.J. FUSION/LDV3.42: Software for LiDAR Data Analysis and Visualization; USDA, Forest Service Pacific Northwest Research Station: Seattle, WA, USA, 2015; 150p. Available online: http://http://forsys.cfr.washington.edu/fusion/ FUSION_manual.pdf (accessed on 20 May 2014).
- Swaine, M.D.; Hall, J.B.; Alexander, L.J. Tree population dynamics at Kade, Ghana. J. Trop. Ecol. 1987, 3, 331–345. [Google Scholar] [CrossRef]
- Wright, S.J.; Muller-Landau, H.C.; Condit, R.; Hubbell, S.P. Gap-dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology 2003, 84, 3174–3185. [Google Scholar] [CrossRef]
- Alencar, J.C. Estudos silviculturais de uma população natural de Copaifera multijulga Hayne–Leguminosae, na Amazônia Central. 3—Distribuição espacial da regeneração natural pré-existente. Acta Amazon. 1984, 14, 225–279. [Google Scholar] [CrossRef]
- Alencar, J.C.; Almeida, R.A.; Fernandes, N.P. Fenologia de espécies florestais em floresta tropical úmida de terra firme na Amazônia Central. Acta Amazon. 1979, 9, 163–198. [Google Scholar] [CrossRef]
- Pedroni, F.; Sanchez, M.; Santos, F.A.M. Fenologia da copaíba (Copaifera langsdorffii Desf.—Leguminosae, Caesalpinioideae) em uma floresta semidecídua no sudeste do Brasil. Rev. Bras. Bot. 2002, 25, 183–194. [Google Scholar] [CrossRef]
- Elias, M.E.A. Estabelecimento de Plântulas de Copaifera Multijuga Hayne—(Caesalpiniaceae) em Fragmentos Florestais e Estádios de Sucessão. Master’s Thesis, National Institute for Amazonian Research, Manaus, Brazil, 1997. [Google Scholar]
- Montgomery, R.A.; Chazdon, R.L. Light gradient partitioning by tropical seedlings in the absence of canopy gaps. Oecologia 2002, 131, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.H.M.; Silva, J.N.M.; Carvalho, J.O.P. Crescimento de árvores em uma área de terra firme na Floresta Nacional do Tapajós após a colheita de madeira. Rev. Cienc. Agrar. 2008, 50, 63–76. [Google Scholar]
- Rigamonte-Azevedo, O.C.; Wadt, P.G.S.; Wadt, L.H.O. Copaíba: Ecologia e Produção de óleo-resina, 1st ed.; (Documentos 91); Embrapa Acre: Rio Branco, Brazil, 2004; p. 28. [Google Scholar]
- Rubin, B.D.; Manion, P.D.; Faber-Langendoen, D. Diameter distributions and structural sustainability in forests. For. Ecol. Manag. 2006, 222, 427–438. [Google Scholar] [CrossRef]
- Freitas, C.V.; Oliveira, P.E. Biologia reprodutiva de Copaifera langsdorffii Desf. (Leguminosae, Caesalpinioedeae). Rev. Bras. Bot. 2002, 25, 311–321. [Google Scholar] [CrossRef]
- Garcia, L.C.; Lima, D. Comportamento de sementes de Copaifera multijuga durante o armazenamento. Acta Amazon. 2000, 30, 369–375. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in prevent competitive exclusion in some marine animal and rainforest trees. In Dynamics of Populations, Proceedings of the Advanced Study Institute on Dynamics of Numbers in Populations; den Boer, K.A., Gradwell, G.R., Eds.; PUDOC: Wageningen, The Netherlands, 1971; pp. 298–312. [Google Scholar]
- Condit, R.; Ashton, P.; Baker, P. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Denslow, J.S. Tropical rainforest gaps and tree species diversity. Ann. Rev. Ecol. Syst. 1987, 18, 431–451. [Google Scholar] [CrossRef]
- De Steven, D. Tropical tree seedling dynamics: recruitment patterns and their population consequences for three canopy species in Panama. J. Trop. Ecol. 1994, 10, 369–383. [Google Scholar] [CrossRef]
- Forget, P.M. Spatial patterns of two rodent-dispersed rain forest trees Carapa procera (Meliaceae) and Vouacapoua americana (Caesalpiniaceae) at Paracou, French Guiana. Biotropica 1994, 26, 408–419. [Google Scholar] [CrossRef]


| Features | Datum |
|---|---|
| Sensor | Optech ALTM 3100 |
| Date of acquisition | 27–29 July 2012 |
| Maximum flight altitude (relative to ground) | 850 m |
| Maximum off-nadir scan angle | 11° |
| Pulse frequency | 100 kHz |
| Pulse density | 13.8 pulses·m−2 |
| Classes | Managed Stand | Unmanaged Stand |
|---|---|---|
| Regeneration in 2011 (ind. ha−1) | 250.53 ** 1 | 30.00 ** 1 |
| Regeneration in 2013 (ind. ha−1) | 316.32 *** 2 | 55.79 *** 2 |
| Mortality rate (%·ano−1) | 32.02 n.s. 3 | 31.17 n.s. 3 |
| Recruitment rate (%·ano−1) | 39.49 n.s. 4 | 13.67 n.s. 4 |
| Average growth DGH (mm·month−1) | 0.049 (±0.640) | 0.029 (±0.241) |
| Maximum growth DGH (mm·month−1) | 0.255 | 0.075 |
| Average growth Ht (cm·month−1) | 0.640 (±0.532) | 0.241 (±0.200) |
| Maximum growth Ht (cm·month−1) | 3.906 | 0.667 |
| Classes | Managed Stand | Unmanaged Stand |
|---|---|---|
| Regeneration—2011 | ||
| Average DGH (mm) | 2.50 (±1.40) | 2.50 (±1.05) |
| Maximum DGH (mm) | 20.40 | 6.20 |
| Average Ht (cm) | 19.50 (±11.31) | 19.30 (±7.90) |
| Maximum Ht (cm) | 180.00 | 40.00 |
| Spearman correlation (ρ) | 0.685 * | 0.731 * |
| Regeneration—2013 | ||
| Average DGH (mm) | 3.00 (±1.70) | 2.60 (±1.15) |
| Maximum DGH (mm) | 21.10 | 9.10 |
| Average Ht (cm) | 25.40 (±18.20) | 18.90 (±7.61) |
| Maximum Ht (cm) | 230.00 | 53.90 |
| Spearman correlation (ρ) | 0.703 * | 0.529 * |
| Variables | Coefficient | Estimate | SE | Wald X2 | p-Value |
|---|---|---|---|---|---|
| Managed stand | |||||
| Intercept | −1.647 | 0.296 | −5.558 | 2.73·10−8 *** | |
| Diameter (mm) | 0.235 | 0.143 | 1.647 | 0.099 . | |
| Height (cm) | 0.039 | 0.021 | 1.861 | 0.063 . | |
| Distance (m) | 0.019 | 0.009 | 2.180 | 0.029 * | |
| Unmanaged stand | |||||
| Intercept | −0.935 | 0.799 | −1.169 | 0.242 n.s. | |
| Diameter (mm) | 0.146 | 0.379 | 0.386 | 0.700 n.s. | |
| Height (cm) | 0.0415 | 0.049 | 0.840 | 0.401 n.s. | |
| Distance (m) | −0.011 | 0.025 | −0.441 | 0.659 n.s. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klauberg, C.; Vidal, E.; Silva, C.A.; Hudak, A.T.; Oliveira, M.; Higuchi, P. Short-Term Effects of Reduced-Impact Logging on Copaifera spp. (Fabaceae) Regeneration in Eastern Amazon. Forests 2017, 8, 257. https://doi.org/10.3390/f8070257
Klauberg C, Vidal E, Silva CA, Hudak AT, Oliveira M, Higuchi P. Short-Term Effects of Reduced-Impact Logging on Copaifera spp. (Fabaceae) Regeneration in Eastern Amazon. Forests. 2017; 8(7):257. https://doi.org/10.3390/f8070257
Chicago/Turabian StyleKlauberg, Carine, Edson Vidal, Carlos Alberto Silva, Andrew Thomas Hudak, Manuela Oliveira, and Pedro Higuchi. 2017. "Short-Term Effects of Reduced-Impact Logging on Copaifera spp. (Fabaceae) Regeneration in Eastern Amazon" Forests 8, no. 7: 257. https://doi.org/10.3390/f8070257






