In Vitro Tetraploid Induction from Leaf and Petiole Explants of Hybrid Sweetgum (Liquidambar styraciflua × Liquidambar formosana)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Establishment of Aseptic Seedlings and In Vitro Multiplication
2.3. Colchicine Application
2.4. Flow Cytometric Analysis of Ploidy Level
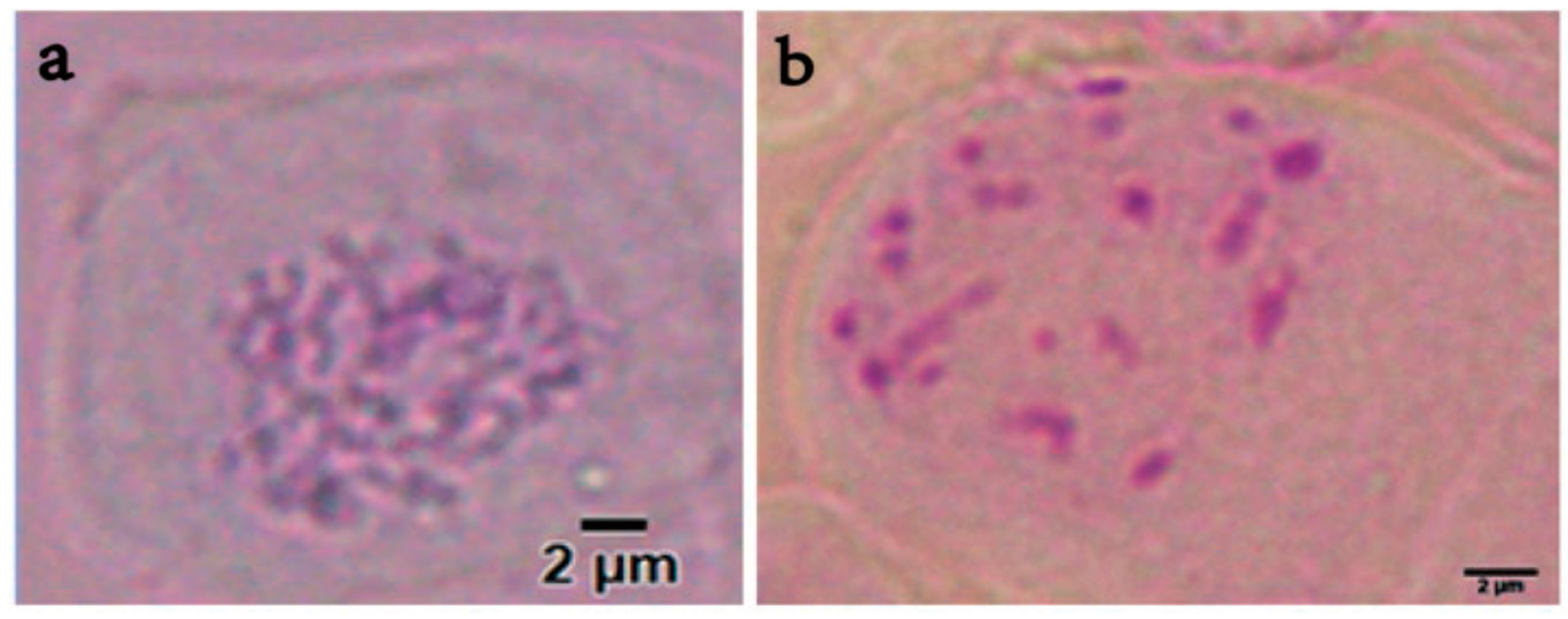
2.5. Chromosome Counting
2.6. Statistical Analysis
3. Results
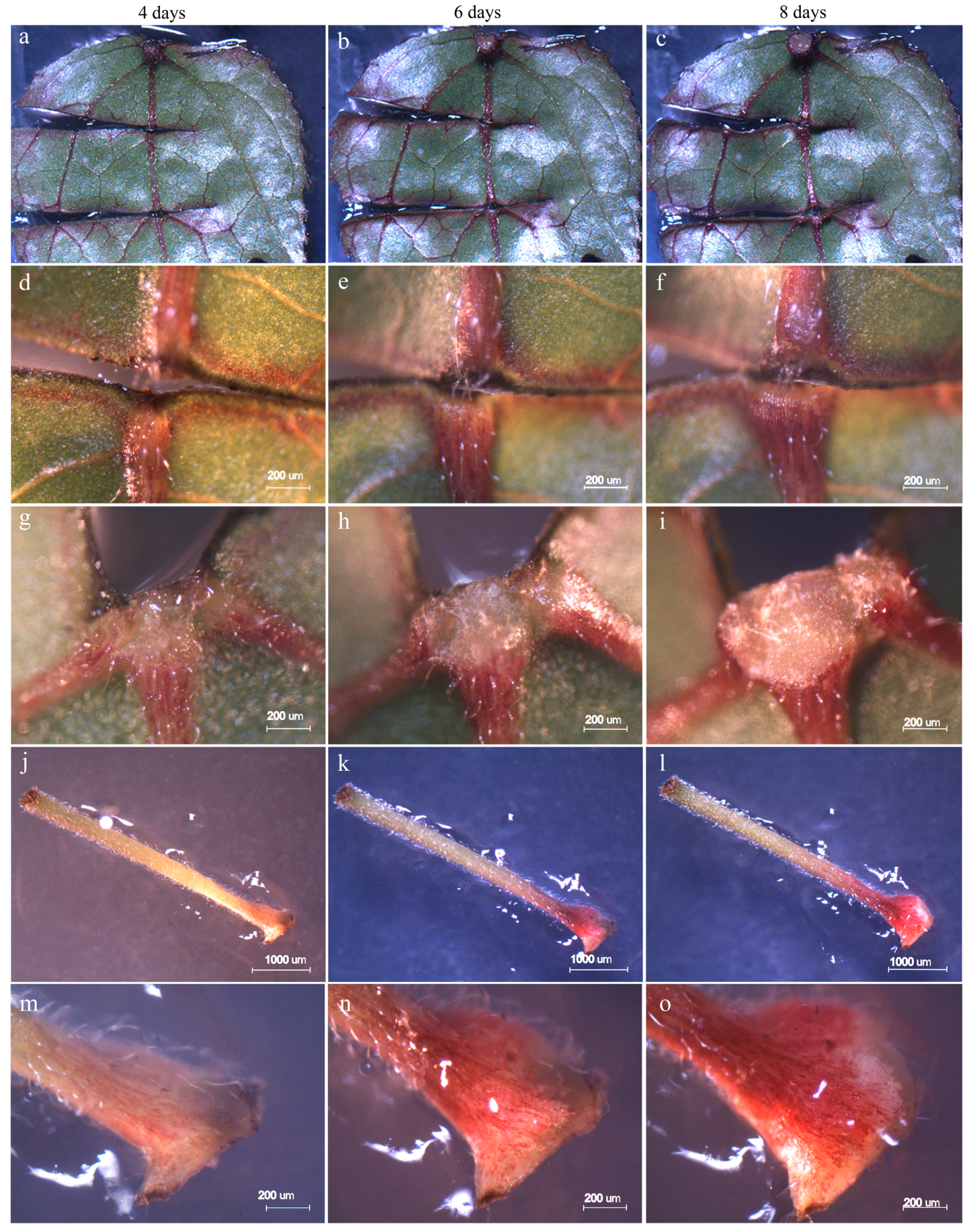
3.1. Morphologic Observations in the Initial Stage of Regeneration
3.2. Survival Rate and Regeneration of Colchicine-Treated Explants
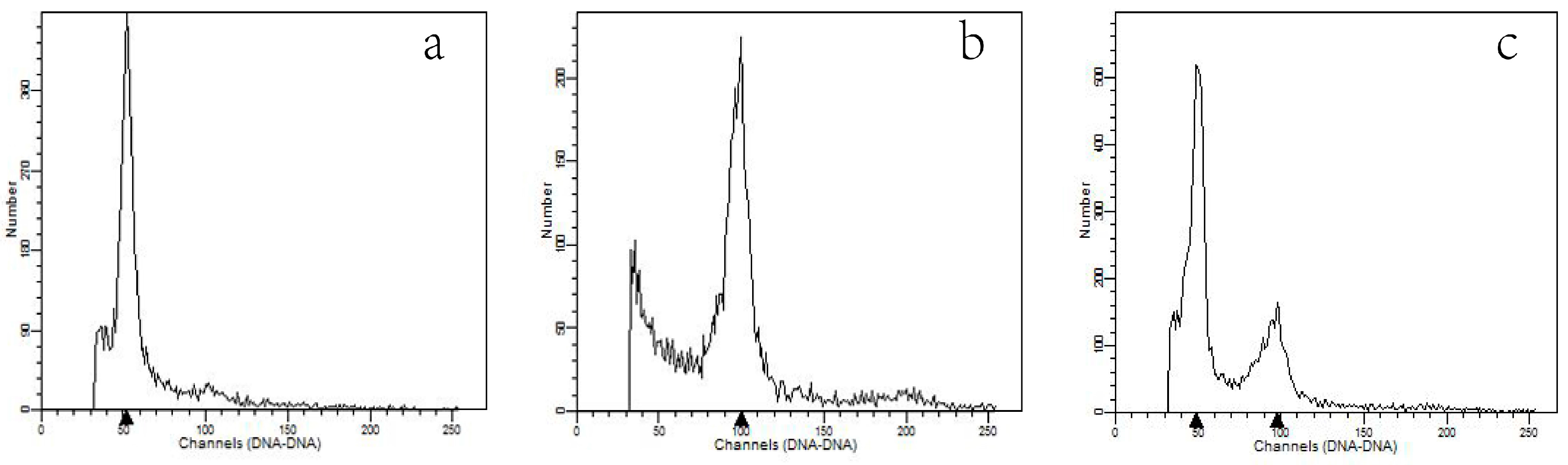
3.3. Analysis by Flow Cytometry and Polyploid Determination
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harlow, W.M.; Harrar, E.S.; Hardin, J.W.; White, F.M. Textbook of Dendrology, 8th ed.; McGraw-Hill: New York, NY, USA, 1996; p. 534. [Google Scholar]
- Merkle, S.A.; Neu, K.A.; Battle, P.J.; Bailey, R.L. Somatic embryogenesis and plantlet regeneration from immature and mature tissues of sweetgum (Liquidambar styraciflua). Plant Sci. 1998, 132, 169–178. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Pan, B.; Itohl, T. Chemical induction of traumatic gum ducts in Chinese sweetgum, Liquidambar formosana. IAWA J. 2015, 36, 58–68. [Google Scholar]
- Santamour, F.S. Interspecific hybridization in Liquidambar. For. Sci. 1972, 18, 23–26. [Google Scholar]
- Vendrame, W.A.; Holliday, C.P.; Merkle, S.A. Clonal propagation of hybrid sweetgum (Liquidambar styraciflua × L. formosana) by somatic embryogenesis. Plant Cell Rep. 2001, 20, 691–695. [Google Scholar]
- Song, Q.; Chen, Z.J. Epigenetic and developmental regulation in plant polyploids. Curr. Opin. Plant Biol. 2015, 24, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Ordóñez, M.C.; Espinosa-Barrera, L.A.; Tamayo-Ordóñez, Y.J.; Ayil-Gutiérrez, B.; Sánchez-Teyer, L.F. Advances and perspectives in the generation of polyploidy plant species. Euphytica 2016, 209, 1–22. [Google Scholar] [CrossRef]
- Xu, C.P.; Huang, Z.; Liao, T.; Li, Y.; Kang, X.Y. In vitro tetraploid plants regeneration from leaf explants of multiple genotypes in Populus. Plant Cell Tissue Organ Cult. 2016, 125, 1–9. [Google Scholar] [CrossRef]
- Cai, X.; Kang, X.Y. In vitro tetraploid induction from leaf explants of Populus pseudo-simonii Kitag. Plant Cell Rep. 2011, 30, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Q.; Chen, D.L.; Song, Z.J.; He, Y.C.; Cai, D.T. In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tissue Organ Cult. 2010, 102, 213–220. [Google Scholar] [CrossRef]
- Wu, J.H.; Mooney, P. Autotetraploid tangor plant regeneration from in vitro Citrus somatic embryogenic callus treated with colchicine. Plant Cell Tissue Organ Cult. 2002, 70, 99–104. [Google Scholar] [CrossRef]
- Yang, X.M.; Cao, Z.Y.; An, L.Z.; Wang, Y.M.; Fang, X.W. In vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica 2006, 152, 217–224. [Google Scholar] [CrossRef]
- Stanys, V.; Weckman, A.; Staniene, G.; Duchovskis, P. In vitro induction of polyploidy in japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult. 2006, 84, 263–268. [Google Scholar] [CrossRef]
- Dhooghe, E.; Denis, S.; Eeckhaut, T.; Reheul, D.; Van Labeke, M. In vitro induction of tetraploids in ornamental Ranunculus. Euphytica 2009, 168, 33–40. [Google Scholar] [CrossRef]
- Wu, J.H.; Ferguson, A.R.; Murray, B.G. Manipulation of ploidy for kiwifruit breeding: In vitro chromosome doubling in diploid Actinidia chinensis. Planch. Plant Cell Tissue Organ Cult. 2011, 106, 503–511. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Luo, F.X.; Liu, L.; Guo, F.C. In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tissue Organ Cult. 2010, 101, 41–47. [Google Scholar]
- De Carvalho, J.F.R.P.; de Carvalho, C.R.; Otoni, W.C. In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tissue Organ Cult. 2005, 80, 69–75. [Google Scholar] [CrossRef]
- Nilanthi, D.; Chen, X.L.; Zhao, F.C.; Yang, Y.S.; Wu, H. Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. J. Biomed. Biotechnol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.H.; Lineberger, R.D. In vitro adventitious shoot formation on mature-phase leaves and petioles of Liquidambar styraciflua L. Plant Sci. 1988, 57, 173–179. [Google Scholar] [CrossRef]
- Kim, M.K.; Sommer, H.E.; Bongarten, B.C.; Merkle, S.A. High-frequency induction of adventitious shoots from hypocotyl segments of Liquidambar styraciflua L. by thidiazuron. Plant Cell Rep. 1997, 16, 536–540. [Google Scholar] [CrossRef]
- Xu, L.; Liu, G.F.; Bao, M.Z. Adventitious shoot regeneration from in vitro leaves of formosan sweetgum (Liquidambar formosana L.). Hortscience 2007, 42, 721723. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel; Kalmia latifolia; by use of shoot-tip culture. Proc. Int. Plant Propag. Soc. 1980, 30, 421–427. [Google Scholar]
- Erlanson, E.W. Cytological Conditions and Evidences for Hybridity in North American Wild Roses. Bot. Gaz. 1929, 87, 443–506. [Google Scholar] [CrossRef]
- Carr, D.H.; Walker, J.E. Carbol fuchsin as a stain for human chromosomes. Stain Technol. 1961, 36, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, K.; Jeżowski, S.; Kaczmarek, Z. In vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind. Crops Prod. 2010, 32, 88–96. [Google Scholar] [CrossRef]
- Kadota, M.; Niimi, Y. In vitro induction of tetraploid plants from a diploid Japanese pear cultivar (Pyrus pyrifolia N. cv. Hosui). Plant Cell Rep. 2002, 21, 282–286. [Google Scholar]
- Sivolapov, A.I.; Blagodarova, T.A. Different levels of mixoploiy in hybrid poplars. In Cytogenetic Studies of Forest Trees and Shrub Species; Borzan, Z., Schlarbaum, S.E., Eds.; Faculty of Foresty Inc.: Zagreb, Croatia, 1997; pp. 311–316. [Google Scholar]
- Merkle, S.A.; Battle, P.J.; Ware, G.O. Factors influencing production of inflorescence-derived somatic seedlings of sweetgum. Plant Cell Tissue Organ Cult. 2003, 73, 95–99. [Google Scholar] [CrossRef]

| Levels | Factors | |||
|---|---|---|---|---|
| A | B | C | D | |
| Genotype | Colchicine Concentration (mg/L) | Pre-Culture Duration (day) | Exposure Time (day) | |
| 1 | Z1 | 120 | 4 | 3 |
| 2 | Z2 | 160 | 6 | 4 |
| 3 | Z3 | 200 | 8 | 5 |
| Treatment | Factors | Number of Shoots Examined | Survival Rate % | No. of Tetraploid | No. of Mixoploid | Tetraploid Induction % | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| c1 | 1 | 1 | 1 | 1 | 50 | 95.00 | 1 | 1 | 2.00 |
| c2 | 1 | 2 | 2 | 2 | 50 | 75.00 | 0 | 1 | 0.00 |
| c3 | 1 | 3 | 3 | 3 | 30 | 41.67 | 1 | 2 | 3.33 |
| c4 | 2 | 1 | 2 | 3 | 15 | 8.33 | 0 | 0 | 0.00 |
| c5 | 2 | 2 | 3 | 1 | 50 | 81.67 | 1 | 1 | 2.00 |
| c6 | 2 | 3 | 1 | 2 | 50 | 90.00 | 0 | 1 | 0.00 |
| c7 | 3 | 1 | 3 | 2 | 50 | 75.00 | 0 | 1 | 0.00 |
| c8 | 3 | 2 | 1 | 3 | 50 | 70.00 | 0 | 0 | 0.00 |
| c9 | 3 | 3 | 2 | 1 | 50 | 85.00 | 1 | 2 | 2.00 |
| Treatment | Factors | Number of Shoots Examined | Survival Rate % | No. of Tetraploid | No. of Mixoploid | Tetraploid Induction % | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| c1 | 1 | 1 | 1 | 1 | 50 | 85.00 | 1 | 0 | 2.00 |
| c2 | 1 | 2 | 2 | 2 | 50 | 56.67 | 2 | 1 | 4.00 |
| c3 | 1 | 3 | 3 | 3 | 30 | 28.33 | 1 | 0 | 3.33 |
| c4 | 2 | 1 | 2 | 3 | 15 | 10.00 | 0 | 0 | 0.00 |
| c5 | 2 | 2 | 3 | 1 | 50 | 56.67 | 3 | 2 | 6.00 |
| c6 | 2 | 3 | 1 | 2 | 50 | 75.00 | 0 | 0 | 0.00 |
| c7 | 3 | 1 | 3 | 2 | 50 | 48.33 | 0 | 0 | 0.00 |
| c8 | 3 | 2 | 1 | 3 | 50 | 51.67 | 0 | 0 | 0.00 |
| c9 | 3 | 3 | 2 | 1 | 50 | 70.00 | 4 | 2 | 8.00 |
| Variation Source | df | MS | F | Sig. |
|---|---|---|---|---|
| Genotype | 2 | 294.147 | 4.876 | 0.020 * |
| Concentration | 2 | 193.362 | 3.205 | 0.064 |
| Pre-culture duration | 2 | 1087.422 | 18.027 | 0.000 * |
| Exposure time | 2 | 2769.584 | 45.913 | 0.000 * |
| Error | 18 | 60.322 | ||
| Total | 27 |
| Variation Source | df | MS | F | Sig. |
|---|---|---|---|---|
| Genotype | 2 | 138.765 | 6.238 | 0.009 * |
| Concentration | 2 | 97.631 | 4.389 | 0.028 * |
| Pre-culture duration | 2 | 811.981 | 36.502 | 0.000 * |
| Exposure time | 2 | 1612.270 | 72.478 | 0.000 * |
| Error | 18 | 22.245 | ||
| Total | 27 |
| Explant Type | A | B | C | D | |
|---|---|---|---|---|---|
| Leaf | K1 | 211.67 | 178.33 | 255.00 | 261.67 |
| K2 | 180.00 | 226.67 | 168.33 | 240.00 | |
| K3 | 230.00 | 216.67 | 198.33 | 120.00 | |
| k1 | 70.56 | 59.44 | 85.00 | 87.22 | |
| k2 | 60.00 | 75.56 | 56.11 | 80.00 | |
| k3 | 76.67 | 72.22 | 66.11 | 40.00 | |
| R | 16.67 | 16.11 | 28.89 | 47.22 | |
| Petiole | K1 | 170.00 | 143.33 | 211.67 | 211.67 |
| K2 | 141.67 | 165.00 | 136.67 | 180.00 | |
| K3 | 170.00 | 173.33 | 133.33 | 90.00 | |
| k1 | 56.67 | 47.78 | 70.56 | 70.56 | |
| k2 | 47.22 | 55.00 | 45.56 | 60.00 | |
| k3 | 56.67 | 57.78 | 44.44 | 30.00 | |
| R | 9.44 | 10.00 | 26.11 | 40.56 |
| Explant Type | A | B | C | D | |
|---|---|---|---|---|---|
| Leaf | K1 | 5.33 | 2.00 | 2.00 | 6.00 |
| K2 | 2.00 | 2.00 | 2.00 | 0.00 | |
| K3 | 2.00 | 5.33 | 5.33 | 3.33 | |
| k1 | 1.78 | 0.67 | 0.67 | 2.00 | |
| k2 | 0.67 | 0.67 | 0.67 | 0.00 | |
| k3 | 0.67 | 1.78 | 1.78 | 1.11 | |
| R | 1.11 | 1.11 | 1.11 | 2.00 | |
| Petiole | K1 | 9.33 | 2.00 | 2.00 | 16.00 |
| K2 | 6.00 | 10.00 | 12.00 | 4.00 | |
| K3 | 8.00 | 11.33 | 9.33 | 3.33 | |
| k1 | 3.11 | 0.67 | 0.67 | 5.33 | |
| k2 | 2.00 | 3.33 | 4.00 | 1.33 | |
| k3 | 2.67 | 3.78 | 3.11 | 1.11 | |
| R | 1.11 | 3.11 | 3.33 | 4.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Z.; Qi, S.; Wang, X.; Zhao, J.; Zhang, J.; Li, B.; Zhang, Y.; Liu, X.; Yuan, W. In Vitro Tetraploid Induction from Leaf and Petiole Explants of Hybrid Sweetgum (Liquidambar styraciflua × Liquidambar formosana). Forests 2017, 8, 264. https://doi.org/10.3390/f8080264
Zhang Y, Wang Z, Qi S, Wang X, Zhao J, Zhang J, Li B, Zhang Y, Liu X, Yuan W. In Vitro Tetraploid Induction from Leaf and Petiole Explants of Hybrid Sweetgum (Liquidambar styraciflua × Liquidambar formosana). Forests. 2017; 8(8):264. https://doi.org/10.3390/f8080264
Chicago/Turabian StyleZhang, Yan, Zewei Wang, Shuaizheng Qi, Xiaoqi Wang, Jian Zhao, Jinfeng Zhang, Bailian Li, Yadong Zhang, Xuezeng Liu, and Wei Yuan. 2017. "In Vitro Tetraploid Induction from Leaf and Petiole Explants of Hybrid Sweetgum (Liquidambar styraciflua × Liquidambar formosana)" Forests 8, no. 8: 264. https://doi.org/10.3390/f8080264




