Earth System Model Needs for Including the Interactive Representation of Nitrogen Deposition and Drought Effects on Forested Ecosystems
Abstract
:1. Introduction
2. Observations of N Impacts
3. Observations of Drought Impacts
4. Interactions between N and Drought
5. Earth System Models
5.1. Nitrogen
5.2. Allometry
5.3. Roots
6. Models Development Priorities
6.1. Flexibility of C:N Coupling in Models
6.2. Adaptive Dynamics Approach to C Allocation
6.3. Improving Form and Function of Roots
6.4. Succession
6.5. Competition
6.6. Trait-Based Modeling
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Friedlingstein, P.; Meinshausen, M.; Arora, V.K.; Jones, C.J.; Anav, A.; Liddicoat, S.K.; Knutti, R. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 2014, 27, 511–526. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; McInnes, K.; Rahimi, M.; et al. Changes in climate extremes and their impacts on the natural physical environment. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.-K., Allen, S.K., et al., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 109–230. [Google Scholar]
- Vitousek, P.M.; Aber, J.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, G.D. Human alteration of the global nitrogen cycle: Causes and consequences. Issues Ecol. 1997, 1, 1–17. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Dentener, F.; Drevet, J.; Lamarque, J.F.; Bey, I.; Eickhout, B.; Fiore, A.M.; Hauglustaine, D.; Horowitz, L.W.; Krol, M.; Kulshrestha, U.C.; et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef] [Green Version]
- Aber, J.D.; Goodale, C.L.; Ollinger, S.V.; Smith, M.-L.; Magill, A.H.; Martin, M.E.; Hallett, R.A.; Stoddard, J.L. Is Nitrogen deposition altering the nitrogen status of northeastern forests? Bioscience 2003, 53, 375–389. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, L.H.; Fenn, M.E.; Goodale, C.L.; Geiser, L.H.; Driscoll, C.T.; Allen, E.B.; Baron, J.S.; Bobbink, R.; Bowman, W.D.; Clark, C.M.; et al. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol. Appl. 2011, 21, 3049–3082. [Google Scholar] [CrossRef]
- Bobbink, R.; Roelofs, J.G.M. Nitrogen critical loads for natural and semi-natural ecosystems: The empirical approach. Water Air. Soil Pollut. 1995, 85, 2413–2418. [Google Scholar] [CrossRef]
- Melillo, J.; Steudler, P.; Aber, J.; Newkirk, K.; Lux, H.; Bowles, F.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.; Post, W. Response of northern forests to CO2-induced climate change. Nature 1988, 334, 55–58. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Melillo, J.M.; Steudler, P.A.; Newkirk, K.M.; Bowles, F.P.; Aber, J.D. Responses of trace gas fluxes and N availability to experimentailly elevated soil temperatures. Ecol. Appl. 1994, 4, 617–625. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Simkin, S.M.; Allen, E.B.; Bowman, W.D.; Clark, C.M.; Belnap, J.; Brooks, M.L.; Cade, B.S.; Collins, S.L.; Geiser, L.H.; Gilliam, F.S.; et al. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 4086–4091. [Google Scholar] [CrossRef] [PubMed]
- BassiriRad, H.; Lussenhop, J.F.; Sehtiya, H.L.; Borden, K.K. Nitrogen deposition potentially contributes to oak regeneration failure in the Midwestern temperate forests of the USA. Oecologia 2015, 177, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Turlapati, S.A.; Long, S.; McDowell, W.H.; Minocha, S.C. Long-term trends of changes in pine and oak foliar nitrogen metabolism in response to chronic nitrogen amendments at Harvard Forest, MA. Tree Physiol. 2015, 35, 894–909. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.; Lohse, K.A.; Hall, S.J. The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. Ambio 2002, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.T.; Yoh, M.; Mo, J.M.; Gundersen, P.; Zhou, G.Y. Response of nitrogen leaching to nitrogen deposition in disturbed and mature forests of southern China. Pedosphere 2009, 19, 111–120. [Google Scholar] [CrossRef]
- Dise, N.B.; Wright, R.F. Nitrogen leaching from European forests in relation to nitrogen deposition. For. Ecol. Manag. 1995, 71, 153–161. [Google Scholar] [CrossRef]
- Broeckx, L.S.; Verlinden, M.S.; Berhongaray, G.; Zona, D.; Fichot, R.; Ceulemans, R. The effect of a dry spring on seasonal carbon allocation and vegetation dynamics in a poplar bioenergy plantation. GCB Bioenergy 2014, 6, 473–487. [Google Scholar] [CrossRef]
- Hertel, D.; Strecker, T.; Müller-Haubold, H.; Leuschner, C. Fine root biomass and dynamics in beech forests across a precipitation gradient—Is optimal resource partitioning theory applicable to water-limited mature trees? J. Ecol. 2013, 101, 1183–1200. [Google Scholar] [CrossRef]
- Martin-Stpaul, N.K.; Limousin, J.M.; Vogt-Schilb, H.; Rodríguez-Calcerrada, J.; Rambal, S.; Longepierre, D.; Misson, L. The temporal response to drought in a Mediterranean evergreen tree: Comparing a regional precipitation gradient and a throughfall exclusion experiment. Glob. Chang. Biol. 2013, 19, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.M.; Miranda, J.D.; Jorquera, M.J.; Pugnaire, F.I. Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecol. 2009, 204, 261–270. [Google Scholar] [CrossRef]
- Friedrich, U.; von Oheimb, G.; Kriebitzsch, W.U.; Schleßelmann, K.; Weber, M.S.; Härdtle, W. Nitrogen deposition increases susceptibility to drought—Experimental evidence with the perennial grass Molinia caerulea (L.) Moench. Plant Soil 2012, 353, 59–71. [Google Scholar] [CrossRef]
- Kinugasa, T.; Tsunekawa, A.; Shinoda, M. Increasing nitrogen deposition enhances post-drought recovery of grassland productivity in the Mongolian steppe. Oecologia 2012, 170, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Boisier, J.P.; Ciais, P.; Ducharne, A.; Guimberteau, M. Projected strengthening of Amazonian dry season by constrained climate model simulations. Nat. Clim. Chang. 2015, 5, 656–660. [Google Scholar] [CrossRef]
- Cook, B.I.; Ault, T.R.; Smerdon, J.E. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci. Adv. 2015, 1, e1400082. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.J.; Weltzin, J.F. Drought disturbance from climate change: Repsonse of United States forests. Sci. Total Enivron. 2000, 262, 205–220. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Cui, X.; Wang, H.; Chen, A. Drought: The most important physical stress of terrestrial ecosystems. Acta Ecol. Sin. 2014, 34, 179–183. [Google Scholar] [CrossRef]
- Thornton, P.E.; Doney, S.C.; Lindsay, K.; Moore, J.K.; Mahowald, N.; Randerson, J.T.; Fung, I.; Lamarque, J.F.; Feddema, J.J.; Lee, Y.H. Carbon-nitrogen interactions regulate climate-carbon cycle feedbacks: Results from an atmosphere-ocean general circulation model. Biogeosciences 2009, 6, 2099–2120. [Google Scholar] [CrossRef]
- Koirala, S.; Yeh, P.J.F.; Hirabayashi, Y.; Kanae, S.; Oki, T. Global-scale land surface hydrologic modeling with the representation of water table dynamics. J. Geophys. Res. Atmos. 2014, 119, 75–89. [Google Scholar] [CrossRef]
- Yan, B.; Dickinson, R.E. Modeling hydraulic redistribution and ecosystem response to droughts over the Amazon Basin using Community Land Model 4.0 (CLM4). J. Geophys. Res. Biogeosci. 2014, 119, 2130–2143. [Google Scholar] [CrossRef]
- Leuzinger, S.; Quinn Thomas, R. How do we improve Earth system models? Integrating Earth system models, ecosystem models, experiments and long-term data. New Phytol. 2011, 191, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, A.M.; De Kauwe, M.G.; Pitman, A.J.; Best, M.J.; Abramowitz, G.; Haverd, V.; Decker, M.; Haughton, N. Land surface models systematically overestimate the intensity, duration and magnitude of seasonal-scale evaporative droughts. Environ. Res. Lett. 2016, 11. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Zhou, S.X.; Medlyn, B.E.; Pitman, A.J.; Wang, Y.P.; Duursma, R.A.; Prentice, I.C. Do land surface models need to include differential plant species responses to drought? Examining model predictions across a mesic-xeric gradient in Europe. Biogeosciences 2015, 12, 7503–7518. [Google Scholar] [CrossRef]
- Fisher, J.B.; Badgley, G.; Blyth, E. Global nutrient limitation in terrestrial vegetation. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Gerber, S.; Hedin, L.O.; Oppenheimer, M.; Pacala, S.W.; Shevliakova, E. Nitrogen cycling and feedbacks in a global dynamic land model. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationship in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Howarth, R.W. Nutrients in synergy. Nature 2007, 449, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Bala, G.; Devaraju, N.; Chaturvedi, R.K.; Caldeira, K.; Nemani, R. Nitrogen deposition: How important is it for global terrestrial carbon uptake? Biogeosciences 2013, 10, 7147–7160. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Burton, A.J.; Zak, D.R.; Talhelm, A.F. Simulated chronic nitrogen deposition increases carbon storage in northern temperate forests. Glob. Chang. Biol. 2008, 14, 142–153. [Google Scholar]
- Elvir, J.A.; Rustad, L.; Wiersrna, G.B.; Fernandez, I.; White, A.S.; White, G.J. Eleven-year response of foliar chemistry to chronic nitrogen and sulfur additions at the Bear Brook Watershed in Maine. Can. J. For. Res. 2005, 35, 1402–1410. [Google Scholar]
- Boggs, J.L.; Mcnulty, S.G.; Gavazzi, M.J.; Myers, J.M. Tree growth, foliar chemistry, and nitrogen cycling across a nitrogen deposition gradient in southern Applachian deciduous forests. Can. J. For. Res. 2005, 35, 1901–1913. [Google Scholar]
- Pitcairn, C.E.R.; Leith, I.D.; Fowler, D.; Hargreaves, K.J.; Moghaddam, M.; Kennedy, V.H.; Granat, L. Foliar nitrogen as an indicator of nitrogen deposition and critical loads exceedance on a European scale. Water Air. Soil Pollut. 2001, 130, 1037–1042. [Google Scholar]
- Fleischer, K.; Rebel, K.T.; Van Der Molen, M.K.; Erisman, J.W.; Wassen, M.J.; Van Loon, E.E.; Montagnani, L.; Gough, C.M.; Herbst, M.; Janssens, I.A.; et al. The contribution of nitrogen deposition to the photosynthetic capacity of forests. Glob. Biogeochem. Cycles 2013, 27, 187–199. [Google Scholar]
- Talhelm, A.F.; Pregitzer, K.S.; Burton, A.J. No evidence that chronic nitrogen additions increase photosynthesis in mature sugar maple forests. Ecol. Appl. 2011, 21, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Maughan, M.W.; Sun, J.; Feng, X.; Miguez, F.; Lee, D.; Dietze, M.C. Impact of nitrogen allocation on growth and photosynthesis of Miscanthus (Miscanthus × giganteus). GCB Bioenergy 2012, 4, 688–697. [Google Scholar]
- Quinn Thomas, R.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar]
- De Vries, W.; Du, E.; Butterbach-Bahl, K. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest ecosystems. Curr. Opin. Environ. Sustain. 2014, 9–10, 90–104. [Google Scholar]
- Xia, J.; Wan, S. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 2008, 179, 428–439. [Google Scholar] [PubMed]
- Ibáñez, I.; Zak, D.R.; Burton, A.J.; Pregitzer, K.S. Chronic nitrogen deposition alters tree allometric relationships: Implications for biomass production and carbon storage. Ecol. Appl. 2016, 26, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Fang, J. Weak growth response to nitrogen deposition in an old-growth boreal forest. Ecosphere 2014, 5. [Google Scholar] [CrossRef]
- Verma, P.; Sagar, R.; Verma, H.; Verma, P.; Singh, D.K. Changes in species composition, diversity and biomass of herbaceous plant traits due to N amendment in a dry tropical environment of India. J. Plant Ecol. 2014, 8, 321–332. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y. Influence of environmental variability on root dynamics in northern forests. CRC Crit. Rev. Plant Sci. 2009, 28, 179–197. [Google Scholar] [CrossRef]
- Smithwick, E.A.H.; Eissenstat, D.M.; Lovett, G.M.; Bowden, R.D.; Rustad, L.E.; Driscoll, C.T. Root stress and nitrogen deposition: Consequences and research priorities. New Phytol. 2013, 197, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, W.; Chen, H.; Mo, J. Impacts of nitrogen deposition on soil nitrogen cycle in forest ecosystems: A review. Acta Ecol. Sin. 2015, 35, 35–43. [Google Scholar] [CrossRef]
- Bobbink, R.; Lamers, L.P.M. Effects of increased nitrogen deposition. In Air Pollution and Plant Life; Bell, J.N.B., Treshow, M., Eds.; John Wiley and Sons: Chichester, UK, 2002; pp. 201–235. [Google Scholar]
- Grulke, N.E.; Minnich, R.A.; Paine, T.; Riggan, P. Air pollution increases forest susceptibility to wildfires: A case study for the San Bernardino Mountains in southern California. In Wildland Fires and Air Pollution; Bytnerowicz, A., Arbaugh, M., Riebau, A., Anderson, C., Eds.; Elsiver: New York, NY, USA, 2009; pp. 365–403. [Google Scholar]
- Mcdowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Weißhuhn, K.; Auge, H.; Prati, D. Geographic variation in the response to drought in nine grassland species. Basic Appl. Ecol. 2011, 12, 21–28. [Google Scholar] [CrossRef]
- Klein, T.; Cohen, S.; Yakir, D. Hydraulic adjustments underlying drought resistance of Pinus halepensis. Tree Physiol. 2011, 31, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Baker, I.T.; Prihodko, L.; Denning, A.S.; Goulden, M.; Miller, S.; Da Rocha, H.R. Seasonal drought stress in the amazon: Reconciling models and observations. J. Geophys. Res. Biogeosci. 2008, 113. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.A.; Pineda, M.; de la Cruz Jiménez, J.; Vergara, M.F.; Rao, I.M. Contrasting strategies to cope with drought conditions by two tropical forage C4 grasses. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.; da Costa, A.C.L.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.R.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Lotter, D.; Valentine, A.J.; Archer Van Garderen, E.; Tadross, M. Physiological responses of a fynbos legume, Aspalathus linearis to drought stress. S. Afr. J. Bot. 2014, 94, 218–223. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: Fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Hanson, P.J.; Tschaplinski, T.J.; Wullschleger, S.D.; Todd, D.E.; Augé, R.M. The Resilience of Upland-Oak Forest Canopy Trees to Chronic and Acute Precipitation Maniuplations; Buckley, D.S., Clatterbuck, W.K., Eds.; U.S. Department of Agriculture: Morgantown, WV, USA; Southern Research Station: Asheville, NC, USA, 2007. [Google Scholar]
- Prieto, I.; Armas, C.; Pugnaire, F.I. Water release through plant roots: New insights into its consequences at the plant and ecosystem level. New Phytol. 2012, 193, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.B.; Cardon, Z.G. The magnitude of hydraulic redistribution by plant roots: A review and synthesis of empirical and modeling studies. New Phytol. 2012, 194, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Kleczewski, N.M.; Herms, D.A.; Bonello, P. Nutrient and water availability alter belowground patterns of biomass allocation, carbon partitioning, and ectomycorrhizal abundance in Betula nigra. Trees 2012, 26, 525–533. [Google Scholar] [CrossRef]
- Meyer-Grünefeldt, M.; Friedrich, U.; Klotz, M.; Von Oheimb, G.; Härdtle, W. Nitrogen deposition and drought events have non-additive effects on plant growth—Evidence from greenhouse experiments. Plant Biosyst. 2013, 149, 424–432. [Google Scholar] [CrossRef]
- Meyer-Grünefeldt, M.; Calvo, L.; Marcos, E.; Von Oheimb, G.; Härdtle, W. Impacts of drought and nitrogen addition on Calluna heathlands differ with plant life-history stage. J. Ecol. 2015, 103, 1141–1152. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.; Long, J.; Wei, R.; Kjelgren, R.; Gong, C.; Zhao, J. Effects of soil water and nitrogen availability on photosynthesis and water use efficiency of Robinia pseudoacacia seedlings. J. Environ. Sci. 2013, 25, 585–595. [Google Scholar] [CrossRef]
- Wang, M.; Shi, S.; Lin, F.; Hao, Z.; Jiang, P.; Dai, G. Effects of soil water and nitrogen on growth and photosynthetic response of Manchurian Ash (Fraxinus mandshurica) seedlings in northeastern China. PLoS ONE 2012, 7, e30754. [Google Scholar] [CrossRef] [PubMed]
- Palátová, E. Effect of increased nitrogen depositions and drought stress on the development of young Norway spruce Picea abies (L.) Karst. stands. Dendrobiology 2004, 51, 41–45. [Google Scholar]
- Zhou, X.; Zhang, Y.; Ji, X.; Downing, A.; Serpe, M. Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in northwestern China. Environ. Exp. Bot. 2011, 74, 1–8. [Google Scholar] [CrossRef]
- Palátová, E. Effect of increased nitrogen depositions and drought stress on the development of scots pine (Pinus sylvestris L.)—II. Root system response. J. For. Sci. 2002, 48, 237–247. [Google Scholar]
- Albuquerque, W.G.; Severino, L.S.; Beltrao, N.E.M.; Azevedo, C.A.V.; Da Silva Filho, J.L. Growth and biomass allocation of Jutropha curcas plants as influenced by nitrogen under different soil moisture regimes. Res. Crop. 2013, 14, 928–934. [Google Scholar]
- Verburg, P.S.J.; Kapitzke, S.E.; Stevenson, B.A.; Bisiaux, M. Carbon allocation in Larrea tridentata plant-soil systems as affected by elevated soil moisture and N availability. Plant Soil 2014, 378, 227–238. [Google Scholar] [CrossRef]
- Oleson, K.W.; Lawrence, D.M.; Gordon, B.; Drewniak, B.; Huang, M.; Koven, C.D.; Levis, S.; Li, F.; Riley, W.J.; Subin, Z.M.; et al. Technical Description of Version 4.5 of the Community Land Model (CLM); National Center for Atmospheric Research: Boulder, CO, USA, 2013. [Google Scholar]
- Wang, Y.P.; Law, R.M.; Pak, B. A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences 2010, 7, 2261–2282. [Google Scholar] [CrossRef] [Green Version]
- Arora, V.K.; Boer, G.J. A parameterization of leaf phenology for the terrestrial ecosystem component of climate models. Glob. Chang. Biol. 2005, 11, 39–59. [Google Scholar] [CrossRef]
- Arora, V.K.; Boer, G.J.; Christian, J.R.; Curry, C.L.; Denman, K.L.; Zahariev, K.; Flato, G.M.; Scinocca, J.F.; Merryfield, W.J.; Lee, W.G. The effect of terrestrial photosynthesis down regulation on the twentieth-century carbon budget simulated with the CCCma Earth System Model. J. Clim. 2009, 22, 6066–6088. [Google Scholar] [CrossRef]
- Shevliakova, E.; Pacala, S.W.; Malyshev, S.; Hurtt, G.C.; Milly, P.C.D.; Caspersen, J.P.; Sentman, L.T.; Fisk, J.P.; Wirth, C.; Crevoisier, C. Carbon cycling under 300 years of land use change: Importance of the secondary vegetation sink. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Collatz, G.J.; Ball, J.T.; Grivet, C.; Berry, J.A. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agric. For. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- Zeng, X. Global vegetation root distribution for land modeling. J. Hydrometeorol. 2001, 2, 525–530. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.K.; Boer, G.J. A representation of variable root distribution in dynamic vegetation models. Earth Interact. 2003, 7, 1–19. [Google Scholar] [CrossRef]
- Krinner, G.; Viovy, N.; de Noblet-Ducoudré, N.; Ogée, J.; Polcher, J.; Friedlingstein, P.; Ciais, P.; Sitch, S.; Prentice, I.C. A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Zaehle, S.; Friend, A.D. Carbon and nitrogen cycle dynamics in the O-CN land surface model: Model description, site-scale evaluation, and sensitivity to parameter estimates. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Best, M.J.; Pryor, M.; Clark, D.B.; Rooney, G.G.; Essery, R.L.H.; Ménard, C.B.; Edwards, J.M.; Hendry, M.A.; Porson, A.; Gedney, N.; et al. The Joint UK Land Environment Simulator (JULES), model description—Part 1: Energy and water fluxes. Geosci. Model Dev. 2011, 4, 677–699. [Google Scholar] [CrossRef]
- Clark, D.B.; Mercado, L.M.; Sitch, S.; Jones, C.D.; Gedney, N.; Best, M.J.; Pryor, M.; Rooney, G.G.; Essery, R.L.H.; Blyth, E.; et al. The Joint UK Land Environment Simulator (JULES), model description—Part 2: Carbon fluxes and vegetation dynamics. Geosci. Model Dev. 2011, 4, 701–722. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.; Prentice, I.C.; Sykes, M.T. Representation of vegetation dynamics in the modelling of terrestrial ecosystems: Comparing two contrasting approaches within European climate space. Glob. Ecol. Biogeogr. 2001, 10, 621–637. [Google Scholar] [CrossRef]
- Smith, B.; Wärlind, D.; Arneth, A.; Hickler, T.; Leadley, P.; Siltberg, J.; Zaehle, S. Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences 2014, 11, 2027–2054. [Google Scholar] [CrossRef] [Green Version]
- Friend, A.D.; Kiang, N.Y. Land surface model development for the GISS GCM: Effects of improved canopy physiology on simulated climate. J. Clim. 2005, 18, 2883–2902. [Google Scholar] [CrossRef]
- Collatz, G.J.; Ribas-Carbo, M.; Berry, J.A. Coupled photosynthesis-stomatal conductance model for leaves of C4 plants. Aust. J. Plant Physiol. 1992, 19, 519–538. [Google Scholar] [CrossRef]
- Zaehle, S.; Jones, C.D.; Houlton, B.; Lamarque, J.F.; Robertson, E. Nitrogen availability reduces CMIP5 projections of twenty-first-century land carbon uptake. J. Clim. 2015, 28, 2494–2511. [Google Scholar] [CrossRef]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Su, S.; Currie, W.S.; Dukes, J.S.; Finzi, A.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.; Parton, W.J.; et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 2004, 54, 731–739. [Google Scholar] [CrossRef]
- Zaehle, S.; Medlyn, B.E.; De Kauwe, M.G.; Walker, A.P.; Dietze, M.C.; Hickler, T.; Luo, Y.; Wang, Y.-P.; El-masri, B.; Thornton, P.; et al. Evaluation of 11 terrestrial carbon-nitrogen cycle models against observations from two temperate free-air CO2 enrichment studies. New Phytol. 2014, 202, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.; Riley, W.J.; Koven, C.D.; Mu, M.; Randerson, J.T. Representing leaf and root physiological traits in CLM improves global carbon and nitrogen cycling predictions. J. Adv. Model. Earth Syst. 2016, 8, 598–613. [Google Scholar] [CrossRef]
- Parida, B.R. The Influence of Plant Nitrogen Availability on the Global Carbon Cycle and NO Emissions. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2011. [Google Scholar]
- Fisher, J.B.; Sitch, S.; Malhi, Y.; Fisher, R.A.; Huntingford, C.; Tan, S.Y. Carbon cost of plant nitrogen acquisition: A mechanistic, globally applicable model of plant nitrogen uptake, retranslocation, and fixation. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Ali, A.A.; Xu, C.; Rogers, A.; McDowell, N.G.; Medlyn, B.E.; Fisher, R.A.; Wullschleger, S.D.; Reich, P.B.; Vrugt, J.A.; Bauerle, W.L.; et al. Global-scale environmental control of plant photosynthetic capacity. Ecol. Appl. 2015, 25, 2349–2365. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.Q.; Zaehle, S.; Templer, P.H.; Goodale, C.L. Global patterns of nitrogen limitation: Confronting two global biogeochemical models with observations. Glob. Chang. Biol. 2013, 19, 2986–2998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Riley, W.J.; Tang, J. A new theory of plant-microbe nutrient competition resolves inconsistencies between observations and model predictions. Ecol. Appl. 2017, 27, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Riley, W.J. A total quasi-steady-state formulation of substrate uptake kinetics in complex networks and an example application to microbial litter decomposition. Biogeosciences 2013, 10, 8329–8351. [Google Scholar] [CrossRef]
- Tjiputra, J.F.; Roelandt, C.; Bentsen, M.; Lawrence, D.M.; Lorentzen, T.; Schwinger, J.; Seland, Ø.; Heinze, C. Evaluation of the carbon cycle components in the Norwegian Earth System Model (NorESM). Geosci. Model Dev. 2013, 6, 301–325. [Google Scholar] [CrossRef]
- Franklin, O.; Johansson, J.; Dewar, R.C.; Dieckmann, U.; McMurtrie, R.E.; Brännström, A.K.; Dybzinski, R. Modeling carbon allocation in trees: A search for principles. Tree Physiol. 2012, 32, 648–666. [Google Scholar] [CrossRef] [PubMed]
- Goll, D.S.; Brovkin, V.; Parida, B.R.; Reick, C.H.; Kattge, J.; Reich, P.B.; Van Bodegom, P.M.; Niinemets, U. Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences 2012, 9, 3547–3569. [Google Scholar] [CrossRef] [Green Version]
- Zaehle, S.; Dalmonech, D. Carbon-nitrogen interactions on land at global scales: Current understanding in modelling climate biosphere feedbacks. Curr. Opin. Environ. Sustain. 2011, 3, 311–320. [Google Scholar] [CrossRef]
- Thornton, P.E.; Lamarque, J.F.; Rosenbloom, N.A.; Mahowald, N.M. Influence of carbon-nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Zaehle, S.; De Kauwe, M.G.; Walker, A.P.; Dietze, M.C.; Hanson, P.J.; Hickler, T.; Jain, A.K.; Luo, Y.; Parton, W.; et al. Using ecosystem experiments to improve vegetation models. Nat. Clim. Chang. 2015, 5, 528–534. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Zaehle, S.; Walker, A.P.; Dietze, M.C.; Wang, Y.P.; Luo, Y.; Jain, A.K.; El-Masri, B.; Hickler, T.; et al. Where does the carbon go? A model-data intercomparison of vegetation carbon allocation and turnover processes at two temperate forest free-air CO2 enrichment sites. New Phytol. 2014, 203, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; Joel, G.; Field, C.B.; Fung, I.Y. Toward an allocation scheme for global terrestrial carbon models. Glob. Chang. Biol. 1999, 5, 755–770. [Google Scholar] [CrossRef]
- Warren, J.M.; Hanson, P.J.; Iversen, C.M.; Kumar, J.; Walker, A.P.; Wullschleger, S.D. Root structural and functional dynamics in terrestrial biosphere models—Evaluation and recommendations. New Phytol. 2015, 205, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Riley, W.J.; Niu, J. Incorporating root hydraulic redistribution in CLM4.5: Effects on predicted site and global evapotranspiration, soil moisture, and water storage. J. Adv. Model. Earth Syst. 2015, 7, 1828–1848. [Google Scholar] [CrossRef]
- Sivandran, G.; Bras, R.L. Dynamic root distributions in ecohydrological modeling: A case study at Walnut Gulch Experimental Watershed. Water Resour. Res. 2013, 49, 3292–3305. [Google Scholar] [CrossRef]
- McMurtrie, R.E.; Iversen, C.M.; Dewar, R.C.; Medlyn, B.E.; Näsholm, T.; Pepper, D.A.; Norby, R.J. Plant root distributions and nitrogen uptake predicted by a hypothesis of optimal root foraging. Ecol. Evol. 2012, 2, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- El Masri, B.; Shu, S.; Jain, A.K. Implementation of a dynamic rooting depth and phenology into a land surface model: Evaluation of carbon, water, and energy fluxes in the high latitude ecosystems. Agric. For. Meteorol. 2015, 211–212, 85–99. [Google Scholar] [CrossRef]
- Fisher, R.A.; Muszala, S.; Verteinstein, M.; Lawrence, P.; Xu, C.; McDowell, N.G.; Knox, R.G.; Koven, C.; Holm, J.; Rogers, B.M.; et al. Taking off the training wheels: The properties of a dynamic vegetation model without climate envelopes, CLM4.5(ED). Geosci. Model Dev. 2015, 8, 3593–3619. [Google Scholar] [CrossRef]
- Mcnickle, G.G.; Gonzalez-meler, M.A.; Lynch, D.J.; Baltzer, J.L.; Brown, J.S. The world’s biomes and primary production as a triple tragedy of the commons foraging game played among plants. Proc. R. Soc. B 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Scheiter, S.; Langan, L.; Higgins, S.I. Next-generation dynamic global vegetation models: Learning from community ecology. New Phytol. 2013, 198, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, R.; Drewry, D.T.; Bohn, K.; Reu, B.; Kleidon, A. The Jena Diversity-Dynamic Global Vegetation Model (JeDi-DGVM): A diverse approach to representing terrestrial biogeography and biogeochemistry based on plant functional trade-offs. Biogeosciences 2013, 10, 4137–4177. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.-P.; Wright, I.J.; Reich, P.B.; Shi, Z.; Dai, Y. Incorporation of plant traits in a land surface model helps explain the global biogeographical distribution of major forest functional types. Glob. Ecol. Biogeogr. 2016, 26, 304–317. [Google Scholar] [CrossRef]
- Lynch, D.J. Impacts of Fine-Roots on Terrestrial Net Primary Productivity and Soil Nutrient Cycling. Ph.D. Thesis, University of Illinois at Chicago, Chicago, IL, USA, 2015. [Google Scholar]
- Nippert, J.B.; Holdo, R.M. Challenging the maximum rooting depth paradigm in grasslands and savannas. Funct. Ecol. 2015, 29, 739–745. [Google Scholar] [CrossRef]
- Smithwick, E.A.H.; Lucash, M.S.; McCormack, M.L.; Sivandran, G. Improving the representation of roots in terrestrial models. Ecol. Model. 2014, 291, 193–204. [Google Scholar] [CrossRef]
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Mcdowell, N.; Purves, D.; Moorcroft, P.; Sitch, S.; Cox, P.; Huntingford, C.; Meir, P.; Woodward, F.I. Assessing uncertainties in a second-generation dynamic vegetation model caused by ecological scale limitations. New Phytol. 2010, 187, 666–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quillet, A.; Peng, C.H.; Garneau, M. Toward dynamic global vegetation models for simulating vegetation-climate interactions and feedbacks: Recent developments, limitations, and future challenges. Environ. Rev. 2010, 18, 333–353. [Google Scholar] [CrossRef]
- Arora, V.K.; Boer, G.J. Simulating competition and coexistence between plant functional types in a dynamic vegetation model. Earth Interact. 2006, 10, 1–30. [Google Scholar] [CrossRef]
- McNickle, G.G.; Brown, J.S. Evolutionarily stable strategies for nutrient foraging and below-ground competition in plants. Evol. Ecol. Res. 2012, 14, 667–687. [Google Scholar]
- Department of Energy. Available online: https://climatemodeling.science.energy.gov/sites/default/files/ACME_Overview_Brochure.pdf (accessed on 2 May 2017).
- Van Bodegom, P.M.; Douma, J.C.; Witte, J.P.M.; Ordoñez, J.C.; Bartholomeus, R.P.; Aerts, R. Going beyond limitations of plant functional types when predicting global ecosystem-atmosphere fluxes: Exploring the merits of traits-based approaches. Glob. Ecol. Biogeogr. 2012, 21, 625–636. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology. A review. Agron. Sustain. Dev. 2012, 32, 365–399. [Google Scholar] [CrossRef]
- Matheny, A.M.; Mirfenderesgi, G.; Bohrer, G. Trait-based representation of hydrological functional properties of plants in weather and ecosystem models. Plant Divers. 2016, 39, 1–12. [Google Scholar] [CrossRef]
- McNeil, B.E.; Read, J.M.; Driscoll, C.T. Foliar nitrogen responses to elevated atmospheric nitrogen deposition in nine temperate forest canopy species. Environ. Sci. Technol. 2007, 41, 5191–5197. [Google Scholar] [CrossRef] [PubMed]
- Throop, H.L. Nitrogen deposition and herbivory affect biomass production and allocation in an annual plant. Oikos 2005, 111, 91–100. [Google Scholar] [CrossRef]
- Roy, J.; Picon-Cochard, C.; Augusti, A.; Benot, M.-L.; Thiery, L.; Darsonville, O.; Landais, D.; Piel, C.; Defossez, M.; Devidal, S.; et al. Elevated CO2 maintains grassland net carbon uptake under a future heat and drought extreme. Proc. Natl. Acad. Sci. USA 2016, 113, 6224–6229. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Hobbie, S.E.; Lee, T.D. Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat. Geosci. 2014, 7, 920–924. [Google Scholar] [CrossRef]
- Los, S.O. Analysis of trends in fused AVHRR and MODIS NDVI data for 1982-2006: Indication for a CO2 fertilization effect in global vegetation. Glob. Biogeochem. Cycles 2013, 27, 318–330. [Google Scholar] [CrossRef]
- Zhao, M.; Running, S.W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Meler, M.A.; Rucks, J.S.; Aubanell, G. Mechanistic insights on the responses of plant and ecosystem gas exchange to global environmental change: Lessons from Biosphere 2. Plant Sci. 2014, 226, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Randerson, J.T.; Canadell, J.G.; Anderson, R.G.; Avissar, R.; Baldocchi, D.D.; Bonan, G.B.; Caldeira, K.; Diffenbaugh, N.S.; Field, C.B. Protecting climate with forests. Environ. Res. Lett. 2008, 3. [Google Scholar] [CrossRef]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.G.; Canadell, J.G.; Randerson, J.T.; Jackson, R.B.; Hungate, B.A.; Baldocchi, D.D.; Ban-Weiss, G.A.; Bonan, G.B.; Caldeira, K.; Cao, L.; et al. Biophysical considerations in forestry for climate protection. Front. Ecol. Environ. 2011, 9, 174–182. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Peñuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Gonzalez-Meler, M.A.; Silva, L.B.C.; Dias-De-Oliveira, E.; Flower, C.E.; Martinez, C.A. Experimental air warming of a Stylosanthes capitata, vogel dominated tropical pasture affects soil respiration and nitrogen dynamics. Front. Plant Sci. 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
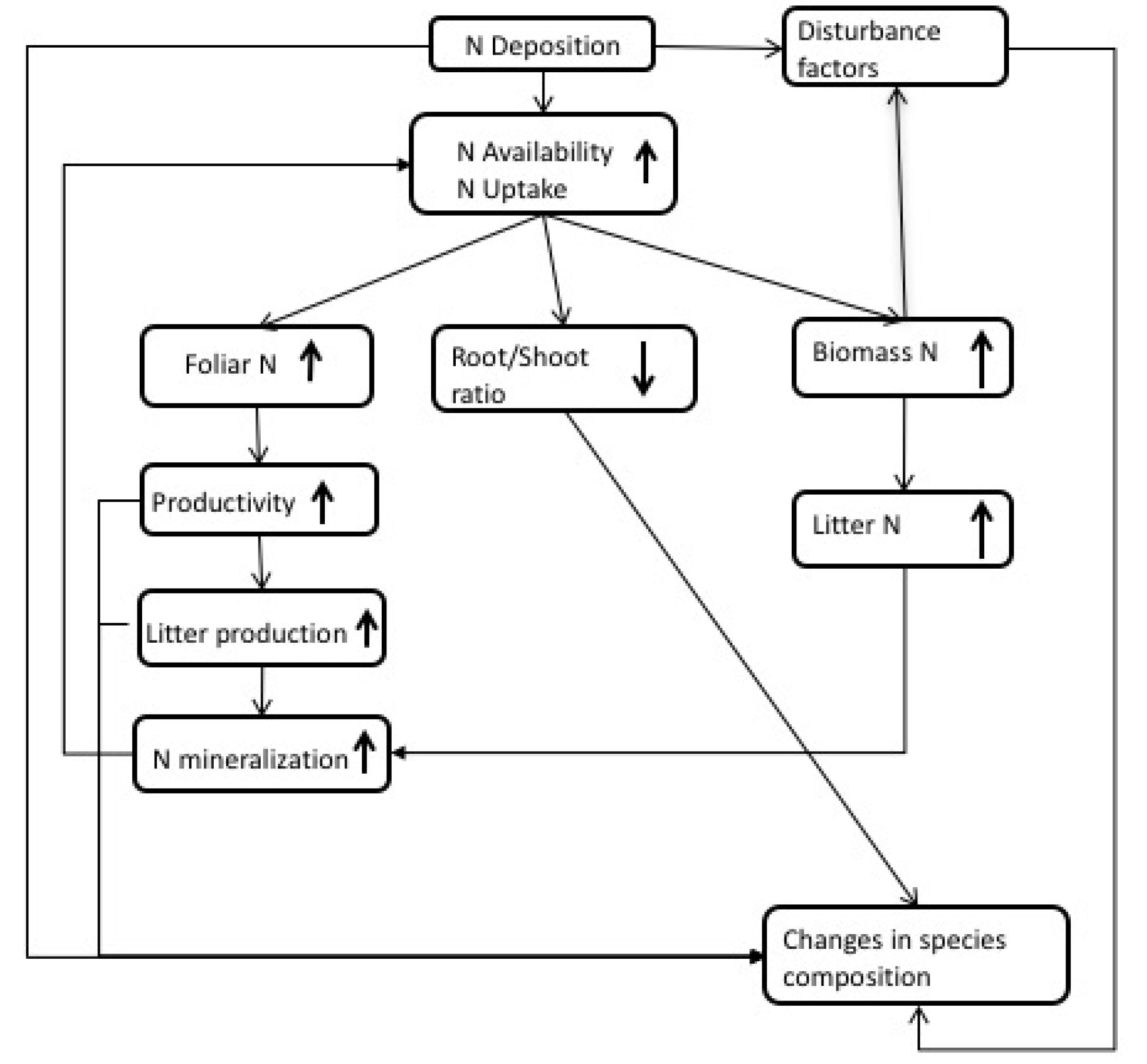
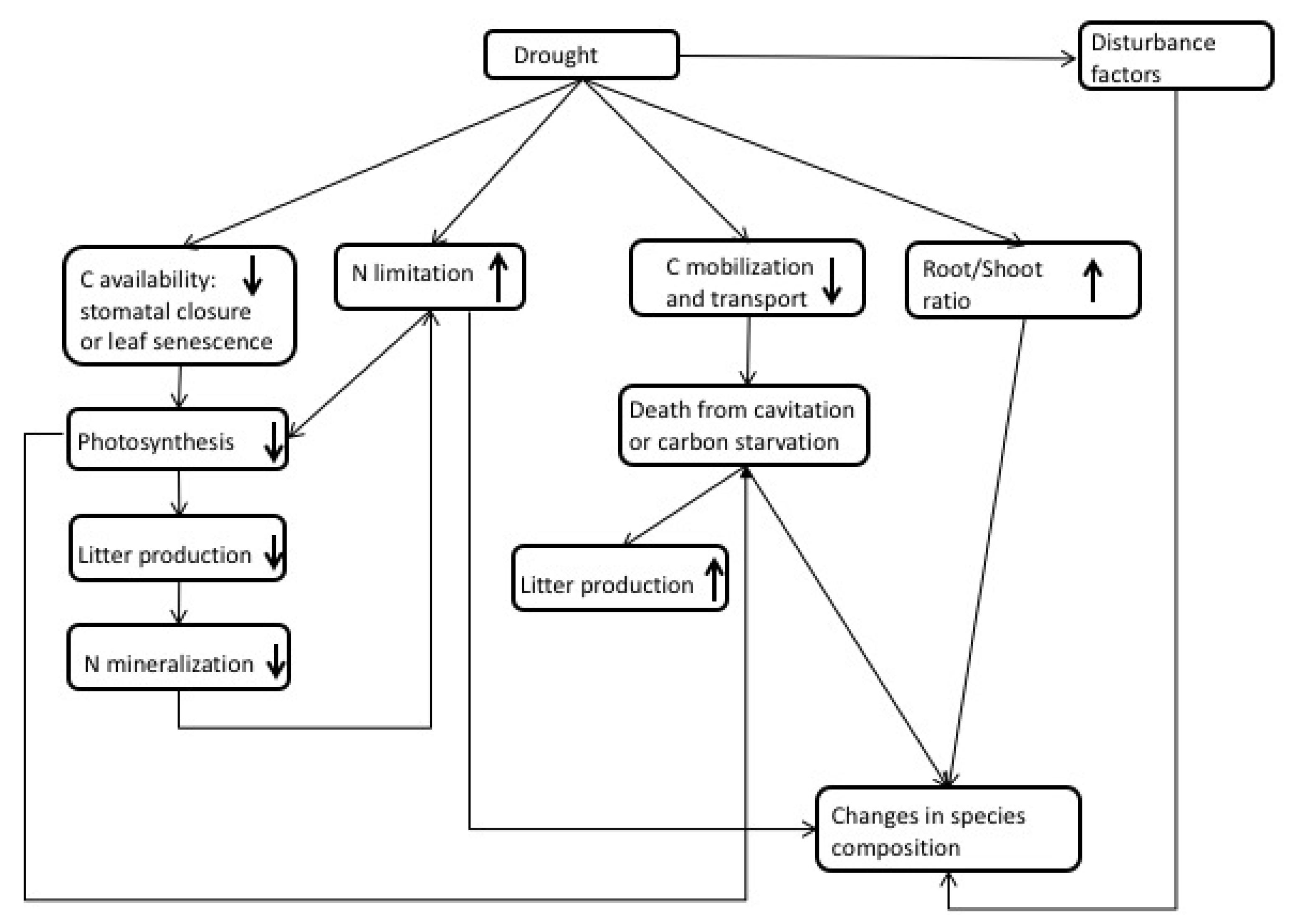
| Process | Model | |||
| CLM4.5 | CABLE | CTEM | LM3 | |
| Reference | [84] | [85] | [86,87] | [38,88] |
| Time step | 30 min to one hour | 30 min | 30 min to one day | 30 min |
| Plant Functional Type (PFTs) | 14 natural and two generic crop types | 15 natural and one crop type | seven natural and two crop types (C3 and C4) | five natural |
| Dynamic vegetation | Dependent on climate or prescribed | NA | Dependent on climate or prescribed | Dependent on climate and light |
| Plant C | ||||
| Photosynthesis | [89,90] | [89] | [89,90] | [89,90] |
| Phenology | Evergreen, stress deciduous, seasonal deciduous, and crop | Biome dependent, four states, input from remote sensing | Four leaf states: maximum growth, normal growth, leaf fall, and dormancy | Drought and cold deciduous seasonal |
| Allocation | Fixed fraction | Fixed fraction | Dependent on light, water, phenological status | Functional balance to maintain root-to-shoot ratio |
| Plant N | ||||
| Uptake | Dependent on N pool size, plant demand | Dependent on N pool size, plant demand | NA | Michaelis-Menten kinetics, dependent on N pool size and root biomass; priority given to immobilization |
| Fixation | Function of Net Primary Productivity (NPP) | External input | NA | Dependent on plant N demand, NPP, and light availability; C cost paid for biological nitrogen fixation (BNF) |
| Stoichiometry (C:N) | Flexible (within 0.8 N:C) | Fixed (PFT dependent) | NA | Fixed (PFT dependent) |
| Plant water | ||||
| Uptake | Dependent on plant demand, root profile, and soil matric potential | Dependent on plant demand, root fraction, and soil water content | Dependent on soil moisture content | NA |
| Root architecture | Double exponential for water uptake [91]; single exponential for soil C/N cycling [92] | Exponential [86] | Prescribed maximum rooting depth, root distribution dependent on time and PFT [93] | NA |
| Process | Model | |||
| ORCHIDEE | O-CN | JULES | LPJ-GUESS | |
| Reference | [94] | [94,95] | [96,97] | [98,99] |
| Time step | 30 min to one day | 30 min to one day | 30 min to one day | 1 day |
| PFTs | 10 natural and two agricultural grasses | 10 natural and two agricultural grasses | 5 natural | 11 natural |
| Dynamic vegetation | Dependent on climate, stand structure, and light | Dependent on climate, stand structure, and light | Dependent on NPP and tree-shrub-grass hierarchy from the Lotka-Volterra competition approach | Dependent on climate, stand structure, light and soil resources, disturbance, and succession |
| Plant C | ||||
| Photosynthesis | [89,90] | [100] | [90,101] | [90,101] |
| Phenology | Drought and cold deciduous seasonal | Drought and cold deciduous seasonal | Cold deciduous | Evergreen, drought, and cold deciduous |
| Allocation | Rule-based response to external limits; dependent on light, water, and N | Pipe model to maintain root-to-shoot ratio | Fixed fraction | Functional balance to maintain root-to-shoot ratio |
| Plant N | ||||
| Uptake | Implicit, dependent on soil humidity and soil temperature | Michaelis-Menten kinetics, dependent on fine root biomass, plant N status, N pool size, and soil temperature | NA | Dependent on N pool size, plant demand, root mass, and soil temperature |
| Fixation | NA | Calculate potential N fixation from evapotranspiration | NA | Calculate potential N fixation from evapotranspiration |
| Stoichiometry (C:N) | Prescribed | Flexible (provided range) | Fixed fraction | Flexible (provided range) |
| Plant water | ||||
| Uptake | Dependent on plant demand, root fraction, and soil water content | Dependent on plant demand, root fraction, and soil water content | Dependent on plant demand, root fraction, and available soil moisture | Dependent on plant demand and soil water in root zone |
| Root architecture | Exponential root profile | Exponential root profile | Double exponential | Two soil layers; more roots in lower layer (except grass) |
| Recommendation | Description | Impact | Example(s) |
|---|---|---|---|
| Flexibility of CN coupling | • Allows C:N ratios in the leaf to vary with N availability • Dynamic partitioning of N in the plant | • Effects N in the leaf with influences on photosynthesis | Fixation and Uptake of Nitrogen (FUN) model [108] Leaf Utilization of Nitrogen for Assimilation (LUNA) [109] Community Land Model (CLM)/Accelerated Climate Model for Energy (ACME) [106] |
| Adaptive dynamics approach to C partitioning | • Flexibility in C allocation to account for plant plasticity across environmental conditions | • Optimize nutrient uptake • Increase tissue allocation to respond to limiting resource | CLM/ACME [106] |
| Improve form and function of roots | • Time varying root structure (depth and distribution) • Variable root depth, traits, plasticity, and hydraulics that scale across space and time | • Adapt to heterogeneity of water and nutrients in soil • Optimizes below ground resource uptake | Dynamic root depth [125] Maximize N [124] Maximize evapotranspiration (ET) [123] |
| Succession | • Representing age class • Variable growth dynamics and response to stress with age | • Capture disturbance and recovery • Heterogeneity in plant distribution, improved canopy light dynamics | Ecosystem Demography (ED) model [126] |
| Competition | • Inter- and intra-species competition for resources (e.g., light, water, N, etc.) • Allows competition both within and between PFTs | • Alters allocation of resources to outcompete neighbors • Possibly altering productivity or shift vegetation distribution | Triple Tragedy of Commons [127] Competition with consumers [111] |
| Trait-based modeling | • Varying morphology, physiology, or phenology characteristics of individuals across an environmental gradient • Environment acts as filter for trait composition | • Adaptation and evolution of species to environmental conditions • Dynamic vegetation moves beyond simple rules of existence and/or establishment | Adaptive Dynamic Global Vegetation Model (aDGVM) [128] Jena Diversity-Dynamic Global Vegetation Model (JeDi-DGVM) [129] CSIRO Atmospheric Biosphere Land Exchange (CABLE) [130] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewniak, B.; Gonzalez-Meler, M.A. Earth System Model Needs for Including the Interactive Representation of Nitrogen Deposition and Drought Effects on Forested Ecosystems. Forests 2017, 8, 267. https://doi.org/10.3390/f8080267
Drewniak B, Gonzalez-Meler MA. Earth System Model Needs for Including the Interactive Representation of Nitrogen Deposition and Drought Effects on Forested Ecosystems. Forests. 2017; 8(8):267. https://doi.org/10.3390/f8080267
Chicago/Turabian StyleDrewniak, Beth, and Miquel A. Gonzalez-Meler. 2017. "Earth System Model Needs for Including the Interactive Representation of Nitrogen Deposition and Drought Effects on Forested Ecosystems" Forests 8, no. 8: 267. https://doi.org/10.3390/f8080267





