1. Introduction
Evidence that the climate is already changing and prospects that it will continue to change even more rapidly in coming decades should be a major impetus to embrace a multi-scale perspective that will aid understanding of how forests and the services they provide are influenced by a continually changing climate [
1,
2]. Increased temperatures and changing precipitation regimes will directly and indirectly affect tree vitality and growth [
3,
4]. Rapid environmental changes directly alter tree phenology and physiology, and will ultimately affect species distributions [
5,
6]. Negative impacts of a changing climate on forests are most likely to be felt first through more frequent and more intense disturbances [
7,
8]. Altered temperature and precipitation regimes affect the ability of trees to resist insects and pathogens [
9,
10,
11]. Physical damage to trees may also occur when climatic thresholds are crossed (e.g., [
12]), for example heavy wet snow loading causing stem breakage and/or forking in trees [
1]. Interactions among multiple disturbance agents have the potential to cause large, nonlinear unexpected changes in ecosystem structure and function [
13], including reduced productivity in managed forests (e.g., [
14]). Forest productivity responses to interactions between climate and disturbance are difficult to predict, and entail large degrees of uncertainty.
Gradual increases in chronic stressors as the climate warms will likely alter individual tree survival and growth with unknown consequences for ecosystem services at larger geographic scales [
1,
15,
16]. This is especially the case for biotic and abiotic stressors such as insects, pathogens and extreme weather events, that cause damage and result in diffuse mortality in managed forests (e.g., [
14,
17]). The way that such damaging agents and events interact to cause a consistent response within a region and how such behavior is constrained or directed by climate is understood at only a general level [
18]. In combination, multiple stressors could result in a major loss of forest productivity due to cumulative effects of stem mortality, reduced growth rates, and physical damage that reduces timber quality and value [
19].
Forest pathogens are a prime example of chronic stressors that respond to climate change. Outbreak characteristics of many forest pathogens such as foliar diseases and rusts are sensitive to climatic conditions and are expected to increase in intensity or duration as the climate warms, especially if conditions become warmer and wetter [
20,
21,
22]. Those same organisms may decline or remain unchanged in severity where conditions become drier [
22]. In contrast, other pathogens such as root diseases and wound colonizers, that are primarily opportunistic and rely on poor host vigor may display greater virulence in areas where host trees become stressed by drought [
22,
23]. The pace of current climatic change coupled with the widening range in variability, particularly in precipitation, is creating environmental conditions where both drought and excess summer precipitation can occur in the same location, year over year.
As the climate changes, it is essential to understand the complex interactions occurring in managed forests as a result of changing disturbance drivers, which in turn determine long-term productivity. In our study, we examine the direct effects of chronic stressors on patterns of tree development in managed stands across a wide expanse of northern British Columbia (BC), Canada and explore the interactions that may be occurring between abiotic events, tree species, pests and pathogens under a changing climate. Our study area covers the northern most extent of large-scale commercial forest management in western North America and given that high-latitude ecosystems are expected to respond first to climate change [
24] it represents the front line of where we might first expect climate change interactions with chronic stressors in managed forests. Even-aged pine stands dominate this landscape and they are considered especially vulnerable to a wide range of foliar diseases and stem rusts that are expected to expand over the coming decades [
17,
21,
22].
In order to conclusively test a hypothesis as to whether or not the incidence and severity of disturbances across a managed forest landscape have increased over time due to climate change, a number of essential elements are required. Ideally, one would have a 60+ year time series of repeated measurements of managed stands replicated across the landscape following the same harvest techniques and management regime allowing for two 30-year periods (typical climate normals) of measure to compare and contrast. These data sets, in addition to tracking growth rates, would need to consistently monitor forest disturbances including the incidence and severity of attack of insects, disease and abiotic damage over the entire study period. Weather stations would also be required in each managed stand over that 60-year period, tracking daily temperature and precipitation, so that if a given insect, pathogen or abiotic event caused damage on that stand a relationship could be drawn between weather and the disturbance agent. Well documented relationships between each of the possible disturbance agents and weather would also need to exist, yet these remain for the most part poorly understood [
25]. In our study area it is impossible to currently meet these high standards of hypothesis testing but our situation is not unique. Terrestrial disturbances are accelerating globally, but their full impact is not quantified due to the lack of an adequate monitoring system [
26]. Based on over 100 years of monitoring, some of the most intensively studied managed forests, those of Central Europe, are reported to have experienced increased growth rates due to climate change [
27], yet this study explicitly excludes plots damaged by storms or bark beetle infestations. Such reductionist approaches to monitoring forest dynamics, where disturbance damaged plots are removed from data sets, are the norm in traditional forestry [
28].
Our objectives are to: (1) show how the climate of our study area has changed over the past century; (2) establish a baseline assessment of mid-rotation managed forests focusing on the variety, incidence, and severity of biotic and abiotic damage to individual tree species by size class considering climate change/disturbance interactions and their implications for future managed stand productivity; and (3) report warning signals associated with the degree to which chronic stressors under the influence of a changing climate may have already affected these forests so that, in the absence of conclusive evidence, forest managers make more proactive, informed decisions.
4. Discussion
A changing climate is probably one of the most critical external drivers of forest dynamics [
4]. The climate of northern latitude forests in British Columbia, Canada, has been steadily changing since monitoring began in our study area (1895) and increasingly so since the 1970s [
35]. Through our analysis of climatic data from over 40 weather stations we found that mean annual temperature has increased more than 1 °C since 1895 and annual and summer precipitation amounts have increased by 8% and 18%, respectively. We cannot say conclusively whether those climatic changes are largely responsible for the high levels of damage observed in 15–40 year-old managed stands because we do not have a baseline record of forest disturbance to compare to. A consistently monitored cohort of managed stands where disturbance agent incidence and impacts were tracked for decades does not exist. Our inability to directly compare our results to long-term records of disturbance is in part a reflection of a shortcoming found throughout traditional forestry where forest disturbances, if acknowledged at all, are often seen as insignificant (see [
36]). Science is, however, starting to view forest ecosystems as integrated social-ecological systems [
37] and adequate data on forest health status globally is increasingly being seen as a key information gap [
25].
In the absence of a consistent long-term record of forest disturbances we turned to the growing body of literature that supports possible links between climatic changes and increased forest disturbance. Warmer and wetter conditions in spring and summer months favor hard pine rusts [
38,
39,
40], the dominant forest pathogens we observed, which combined affected 15% of all lodgepole pine trees. In the only prior extensive survey of forest disturbances in managed stands in BC, a study conducted in the early 1990s, 6% of pine trees were found to be affected by hard pine rusts across the SBS zone [
41]. This 1990s study along with other evidence has already been used to support the suggestion that the landscape level incidence of hard pine rusts in the central interior of BC is increasing [
42].
Modeling has shown that an increase of only 1 °C in the average temperature adequately warms the climate to convert many spruce leader weevil hazard zones to high hazard [
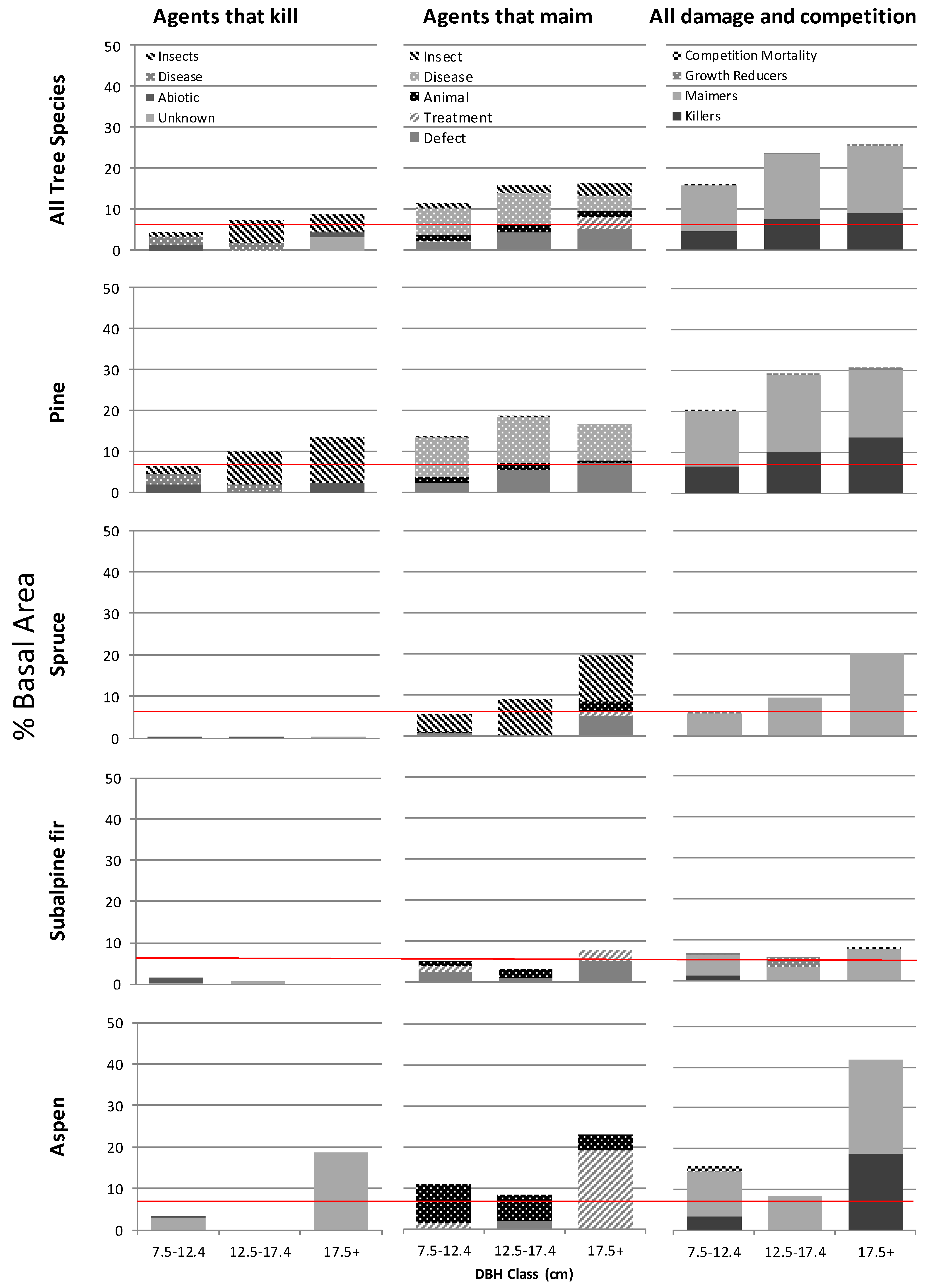
43] and we observed over 11% of the basal area of the largest spruce trees were unacceptably damaged by this insect. Warmer winters have been clearly linked to the mountain pine beetle epidemic in BC [
44], which was the leading large tree killer we observed. Extreme snowfall events damage and can even kill trees and the probability of their frequency increasing with climate change in some areas of western North America has been suggested [
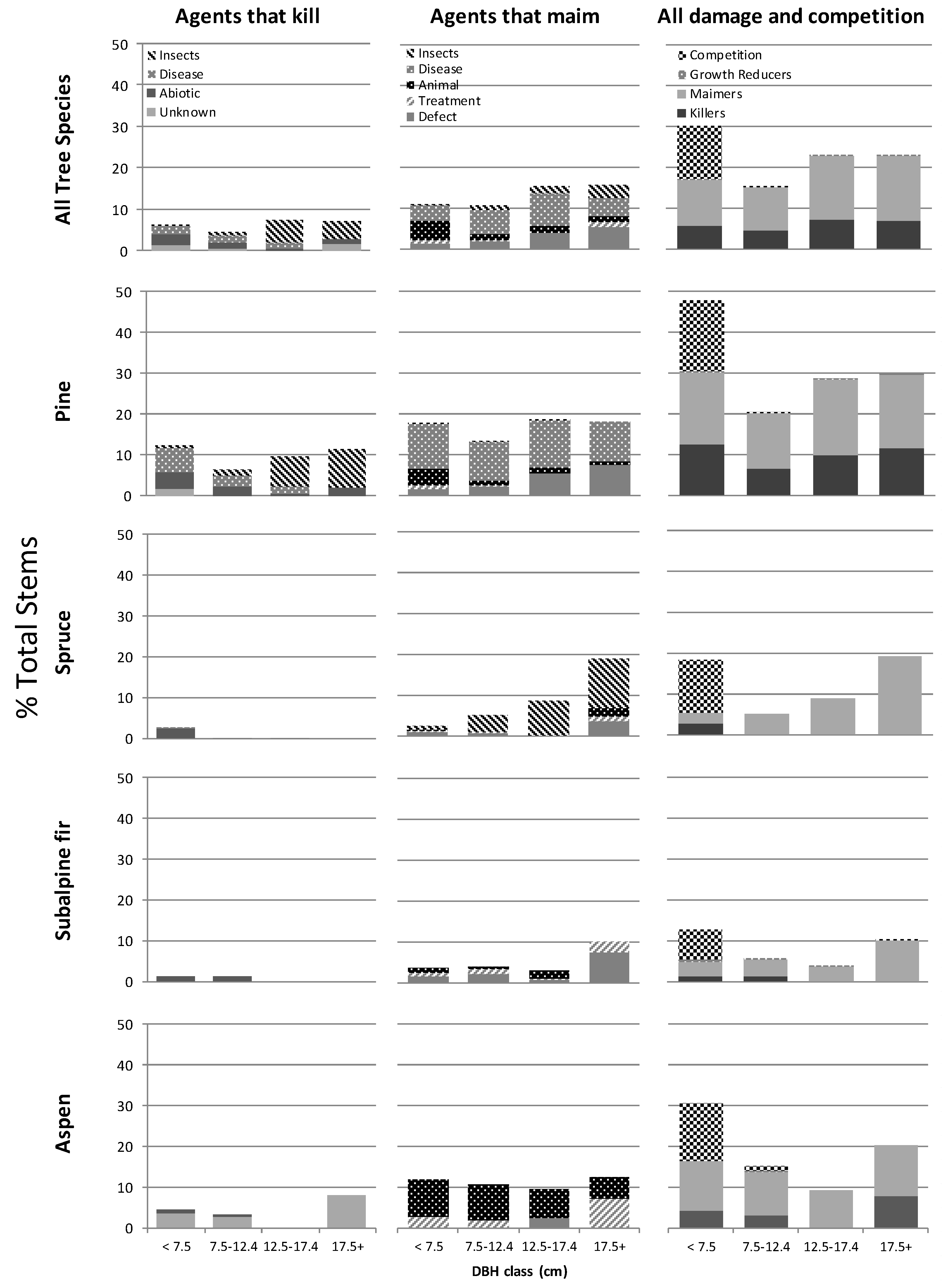
45]. We cannot compare our results of snow-pressed and broken topped trees to earlier records as once again they do not exist. However, given the magnitude and direction of climatic change already experienced and the documented relationships between these environmental changes and the behavior of chronic abiotic and biotic stressors, increased rates of disturbance and resulting damage would be expected. For the most dominant tree species, lodgepole pine, five of the top six damage agent categories (Killer Abiotics, Insects and Diseases and Maimer Defects and Diseases) may have been negatively influenced by the climatic changes that have already occurred across our study area.
We observed particularly high levels of damage in lodgepole pine dominated stands where, on average, 27% of the trees were unacceptably damaged. Extensive damage to individual pine trees in older operational plantations or in replicated experiments has also been observed in other BC studies [
14,
19,
40,
46] and has been forecast to intensify under climate change [
17]. We found that rates of loss for pine due to killing agents alone either matched the expected rate of loss (TIPSY projections [
34]), or were as much as twice as high as expected in the largest diameter trees. When losses due to maiming agents were included we found overall rates of loss more than four times greater than expected. Adding to the uncertainty in future stand productivity forecasts is the fact that not all maimed tree volume is lost. Damaged trees with poor stem form significantly reduce saw-log lumber recovery and from a timber productivity viewpoint represent a loss of growth, similar to non-lethal growth reductions associated with pathogens such as root disease, which are difficult to model [
47]. We cannot state with certainty that the higher than expected rates of loss we observed have been influenced by climatic changes. We do not know if the TIPSY loss projections used to be correct and that damage used to be at a lower level because the manner in which those loss estimates were derived is poorly documented and again, we lack long-term records of damage. The average age of our sample stands was 23 years and the average site index was 19.2 m so the fact that just over 1000 sph of the trees in these stands were greater than 7.5 cm dbh is consistent with TIPSY projections [
34], but just over 700 sph of lodgepole pine and interior spruce trees of that size were considered undamaged and this is cause for concern.
Competition has been long considered the primary driver of forest dynamics in even-aged stands [
48] but few long-term studies exist to test this assumption [
49]. As forest scientists perceive that climate change is significantly impacting forests, there is now a debate as to whether competition or climate is the most important driver of forest dynamics. For example, Zhang et al. [
50] concluded, after examining extensive permanent sample plot data across western Canada, that competition was the primary factor causing long-term changes in tree mortality, growth and recruitment and that external climate factors were less important. Price et al. [
51] replied, among other concerns, that Zhang et al. [
50] overlooked the critical role of climate-sensitive disturbance in their conclusions. We believe this debate cannot be resolved without an actual examination of the causal agents of damage in stands. In the 15–40-year-old stands we studied, it was clear that disturbance agents, and not competition, were the dominant driver of forest dynamics (mortality, growth and physical damage), especially for the dominant trees most counted upon for stand productivity and timber supply. Similar to the work of Lutz and Halpern [
49] we found losses to competition were only a factor in the smallest trees.
Our results also contribute to the emerging observation of increasing levels of damage as tree size increases among the larger sized trees in plantations [
14,
46,
52]. This interaction between tree size and damage among the larger trees was most obvious for pine, spruce and aspen and less so for subalpine fir. The trend of increasing damage in larger diameter trees was clearest when the cumulative effect of all disturbance agents was examined. Individual disturbance agents may or may not have damaged larger trees more severely. Reid et al. [
46] suggested several mechanisms that might explain why larger or faster growing trees in plantations are more prone to damage from disturbance agents including resource allocation targeted at tree growth at the expense of defense. Faster growth in lodgepole pine has been related to a larger surface area of host tissue susceptible to western gall rust infections [
53]. We agree with Burma [
13] that disturbance interactions should be studied as emergent phenomena, separate from studies of individual disturbance events and disturbance types. As climate changes, trees might be expected to grow faster at northern latitudes [
35], where summers are projected to be, and our data shows, are warmer and wetter. These same environmental changes have, however, been linked to increases in negative interactions among chronic biotic stressors such as foliar diseases and rusts [
39].
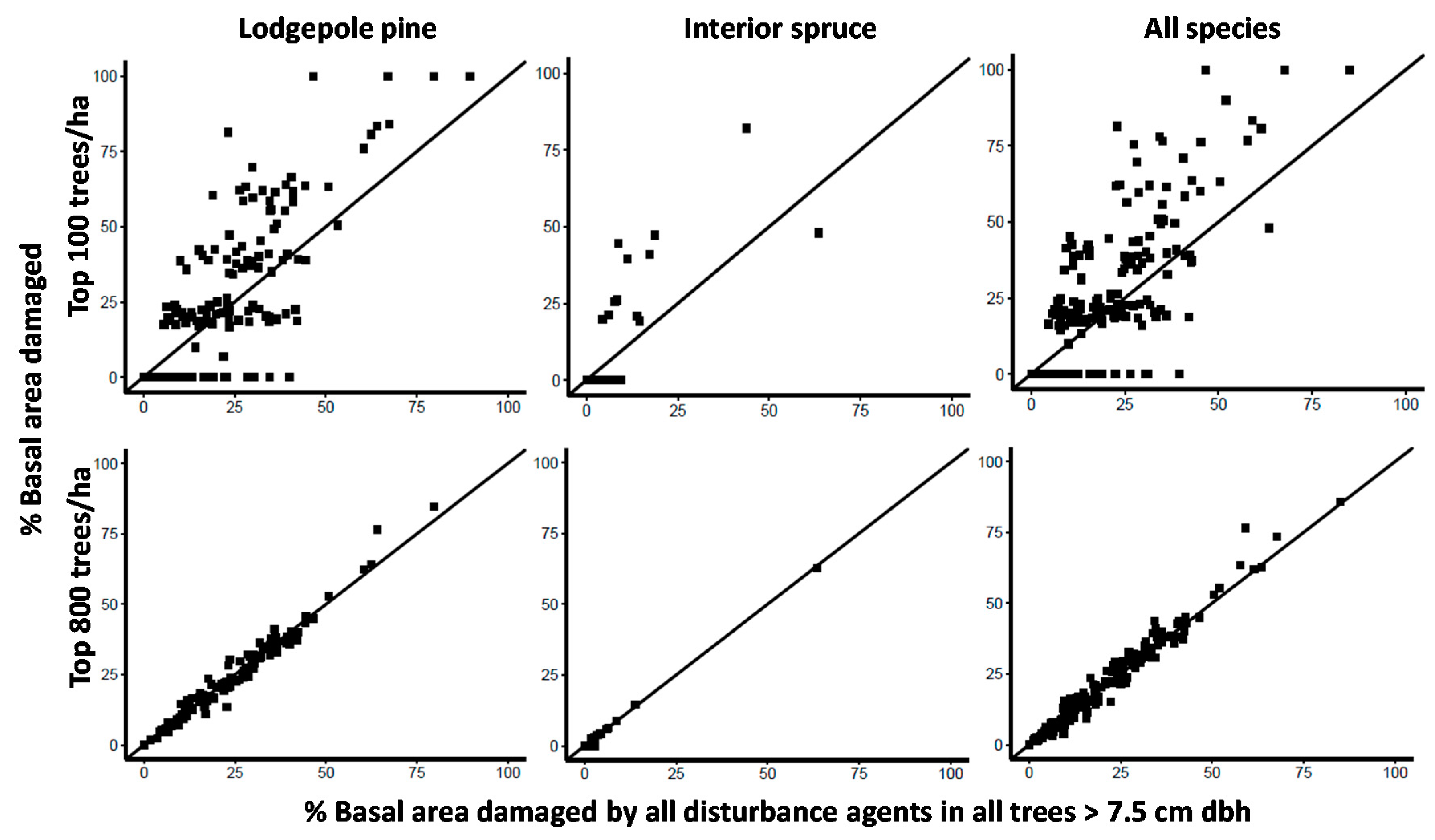
We looked more carefully at a subset of lodgepole pine and interior spruce trees for evidence of size dependence in damage incidence among the largest diameter 100 trees per ha, a grouping of trees commonly used to predict stand productivity in even-aged stands (top height trees). Top height trees are typically assumed to suffer little damage [
54] but we found this not to be the case in approximately half (85 of 175 stands) of the stands we assessed. We also found strong evidence of greater variability in damage and, in more than one third (64 of 175 stands) of the stands, more damage in top height trees. The high incidence of unacceptable damage to larger diameter trees in general, and the unexpected high damage levels observed on top height trees compared to the general population is completely contrary to expectations based on the foundational tenets of stand productivity for even-aged stands [
36].
The increased variation in the percent of basal area damaged in the largest trees that we observed challenges long-held growth and yield beliefs [
14] and may represent something more fundamental. Increased variance around a stable state, such as stand productivity, caused by chronic stressors, or slow drivers of internal change, may indicate the presence of a regime shift or a tipping point [
1,
55]. Shifts in climate are arguably the most pervasive slow drivers of ecological change and regime shifts [
56]. Pests, pathogens and abiotic agents can be considered forcing factors capable of causing consequences of similar magnitude to climate forcing factors [
16]. The effect of multiple forcing agents on tree community productivity may drive shifts in the relative dominance of the component species, with potential ecosystem-level consequences [
57,
58].
Lodgepole pine-leading stands in northern BC appear to be undergoing a gradual regime shift (c.f., [
56]) from productive high yielding systems to pest and damage prone low yield systems aided and abetted by a changing climate. Extensive pine mortality caused by Dothistroma needle blight (
Dothistroma septosporum (Dorog. Morelet) in pine-leading stands to the west of our study area [
9] have resulted in near complete loss of dominant pine trees in severely affected stands. The combination of physical damage, growth reductions and diffuse mortality observed in pine-leading stands across northern BC are likely to have long-lasting legacies for stand productivity. As multiple chronic stressors overcome the ecological resilience of a system, alternate stable states are possible. The challenge of identifying such regime shifts in forests is difficult due to their slow development.
One of the great problems with the type of gradual regime shift we have described, in contrast with a rapid regime shift, is a social one: convincing enough people to confront business-as-usual before time runs out to reverse unwanted gradual regime shifts [
56]. Gradual regime shifts are much more likely to go unnoticed or to be ignored. Slow regime shifts provide a false sense of security, effectively concealing evidence of poorly thought out management practices and providing little warning of future declines [
56].
In addition to the slow regime shift occurring in pine stands, we think we have also identified evidence of a social ecological tipping point brought on by the interaction of management practices, disturbance agents and climate change that can reduce timber supply and decrease stability in resource dependent communities unless addressed. This tipping point has been driven by management practices that focused on establishing lodgepole pine leading stands with an expectation of low levels of damage and mortality for individual trees once plantations reached the free-growing stage, an administrative test at about 8–12 years post-planting [
59]. Our current study in northern BC and earlier studies in southern BC [
14,
19] have shown troubling surprises in declaring success too early in stand development. The expected crop trees experienced far less damage at the earlier assessments [
14,
19] and in contrast to forest management expectations, disturbance agents enhanced by a changing climate have caused the greatest damage to some of the largest diameter trees in mid-rotation stands. Our results highlight the importance of considering the different demographic stages of stand development to understand the effects that climate change may exert on individual trees, species population dynamics, susceptibility to disturbance agents and loss of productivity. This unpredictability of productivity expectations in mid-rotation stands coincides with a major decline in timber supply caused by the mountain pine beetle epidemic [
60], adding to an already difficult situation for timber supply in the near term throughout pine dominated management units in the interior regions of BC.
The interactions between stand development and pests, pathogens and abiotic events are complex and poorly understood in the context of climate change. Current yield models used in forestry typically do not account for these processes and interactions and often ignore the effect of multiple slow stressors that are having a cumulative impact on growth rates, physical damage and mortality in managed stands. Unless these impacts are accounted for in models, their yield projections will be increasingly unreliable as climate continues to change [
4]. The prediction of the future states of trees, stands and landscapes cannot be made with precision. The tools that we use or develop should incorporate and accept this inherent inability to predict precisely the future and acknowledge that changes or adaptation to known and unknown future conditions are not only something that we must accept, but rather something that has to be promoted and planned from the outset in management plans [
61]. Under this new paradigm, management interventions would not be aimed at reaching a precise objective or goal in the future but instead aimed at making sure the system (or the forest) has all of the elements to continue to change and adapt to produce desirable goods and services in the future [
62]. This new take on sustainability would focus on the avoidance of irreversible change and loss of resiliency and would ensure future options remain open [
63].
Our results clearly point to higher levels of uncertainty associated with the productivity of managed forests and thus future timber supply. The fate of sub-lethally damaged trees and the timber volume they represent adds considerably to this uncertainty. Although we cannot definitively link the extensive damage that we found to the climatic changes that have already occurred we hope our work can serve as a warning which if heeded will lead to more proactive forest management decisions. Forest managers need to consider and evaluate the short- and long-term viability of specific practices in a framework that minimizes risk and reduces the chance of undesirable future outcomes.