Irregular Shelterwood Cuttings Promote Viability of European Yew Population Growing in a Managed Forest: A Case Study from the Starohorské Mountains, Slovakia
Abstract
:1. Introduction
- The intensity of small-scale shelterwood cuttings influences the seed production and growth processes of mature yew trees.
- The density of naturally regenerated yew is related to the size and radial growth rate of the parent trees.
- Reproduction (masting) reduces the diameter increment, and therefore sex-related differences in radial growth should be expected.
- Different life strategies of male and female trees are likely to result in gender-specific sensitivity to variation in climate.
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design and Measurements
2.3. Climate Records
2.4. Data Analysis
3. Results
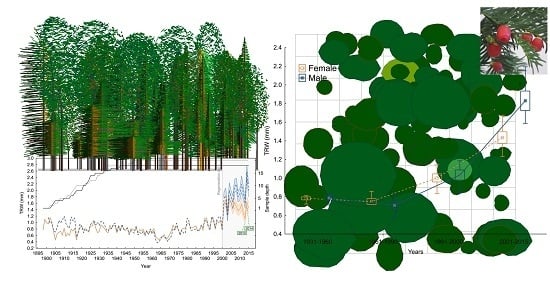
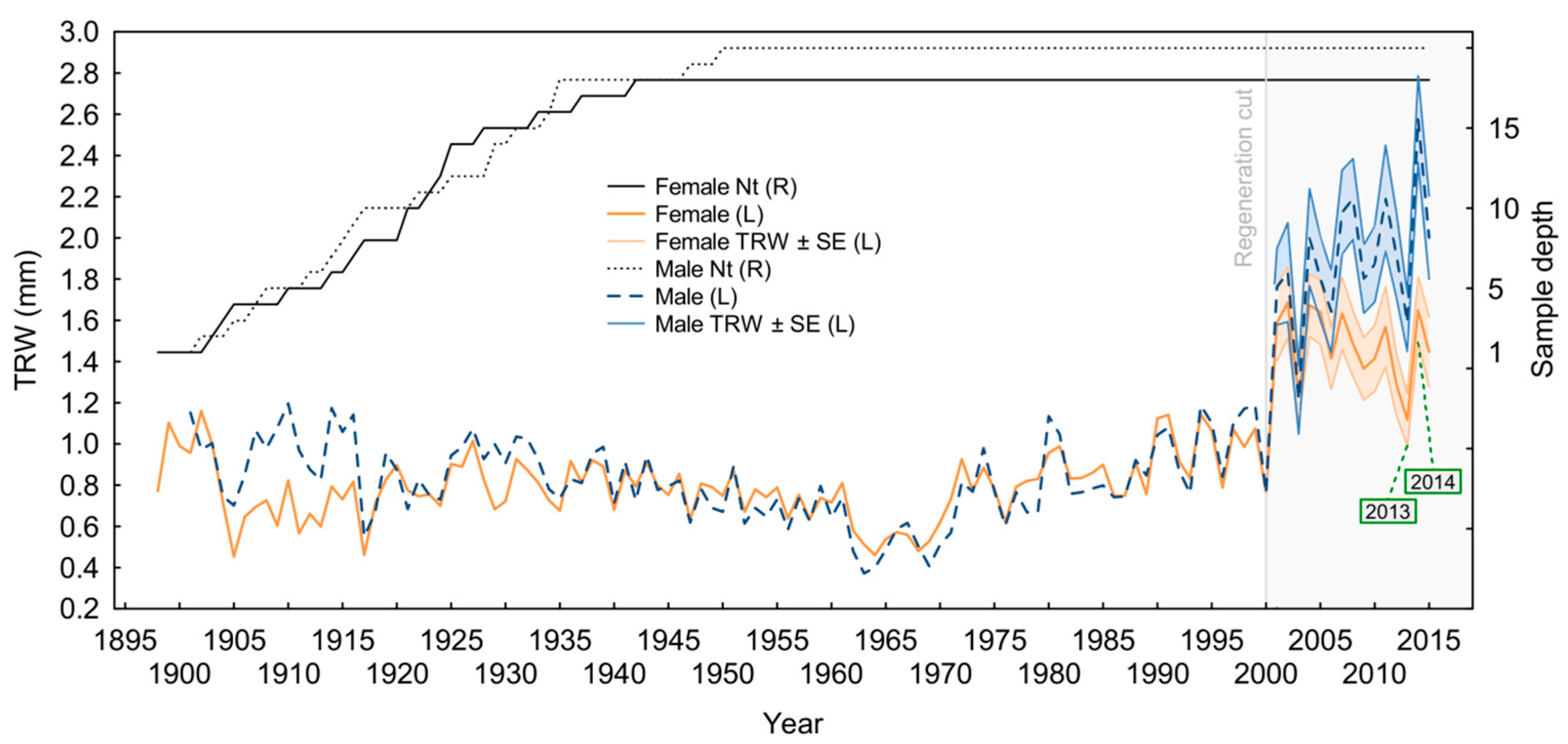
3.1. Yew Tree Characteristics
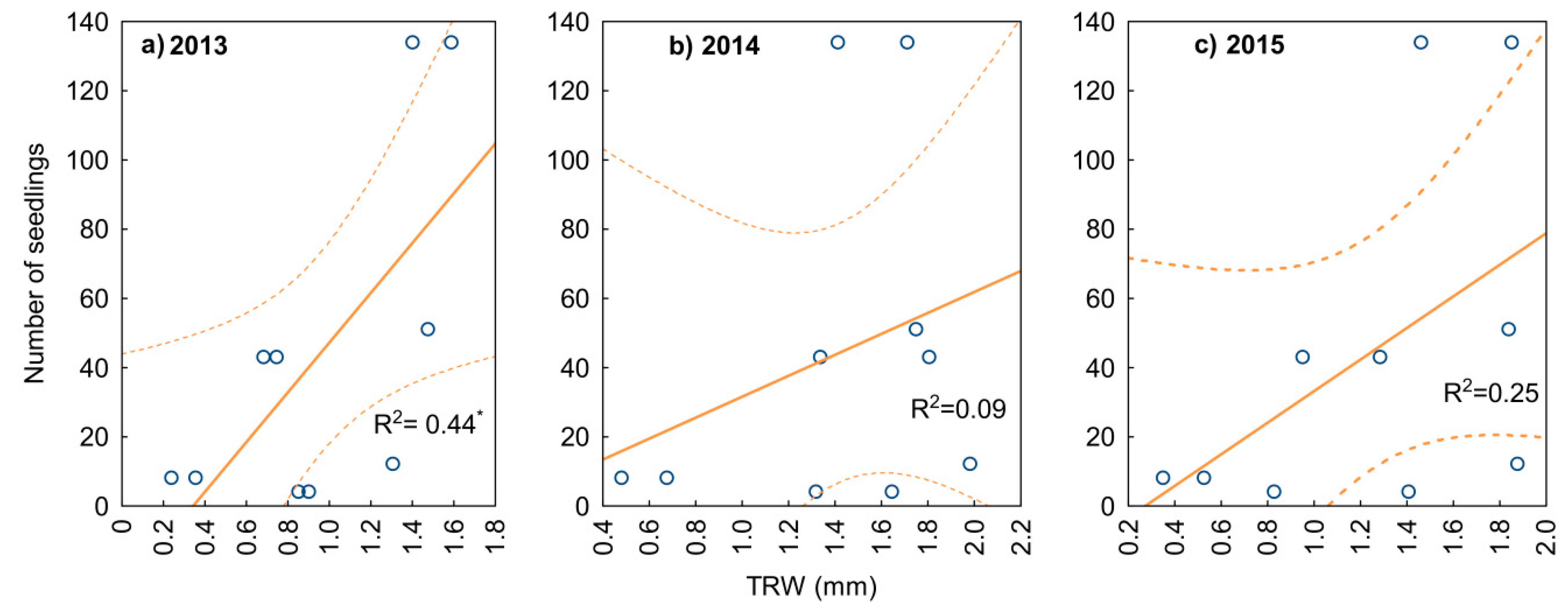
3.2. Yew Natural Regeneration
3.3. Growth–Climate Relationship and Patterns of Yew Radial Growth
4. Discussion
4.1. Stand Structure
4.2. Yew Natural Regeneration
4.3. Regeneration in Relation to Mother Trees
4.4. Sex-Related Patterns of Radial Growth and Climate Sensitivity
5. Conclusions and Conservation Management Strategy Suggestions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Piovesan, G.; Saba, E.P.; Biondi, F.; Alessandrini, A.; Di Filippo, A.; Schirone, B. Population ecology of yew (Taxus baccata L.) in the Central Apennines: Spatial patterns and their relevance for conservation strategies. Plant Ecol. 2009, 205, 23–46. [Google Scholar] [CrossRef]
- Thomas, P.A.; Garcia-Martí, X. Response of European yews to climate change: A review. For. Syst. 2015, 24, 1–11. [Google Scholar] [CrossRef]
- Linares, J.C. Shifting limiting factors for population dynamics and conservation status of the endangered English yew (Taxus baccata L., Taxaceae). For. Ecol. Manag. 2013, 291, 119–127. [Google Scholar] [CrossRef]
- Dhar, A.; Ruprecht, H.; Vacik, H. Population viability risk management (PVRM) for in situ management of endangered tree species—A case study on a Taxus baccata L. population. For. Ecol. Manag. 2008, 255, 2835–2845. [Google Scholar] [CrossRef]
- Iszkuło, G.; Didukh, Y.; Giertych, M.J.; Jasińska, A.K.; Sobierajska, K.; Szmyt, J. Weak competitive ability may explain decline of Taxus baccata. Ann. For. Sci. 2012, 69, 705–712. [Google Scholar] [CrossRef]
- Korpeľ, Š. The Importance of European Yew, Taxus Baccata, in Forest Ecosystems of Slovakia and Possibilities to Improve Its Status (in Slovak); SEA: Banská Bystrica, Slovak, 1995; 68p. [Google Scholar]
- Seidling, W. Eibenvorkommen in siedlungsnahen Forstgebieten und im besiedelten Bereich. Schr. R. f. Vegetationskde 1995, 27, 441–449. [Google Scholar]
- Saniga, M. Impact of silvicultural treatments and deer game on structure and regeneration processes of European yew (Taxus baccata L.) in protected area Pavelcovo (in Slovak). Folia Ecol. 2000, 27, 55–70. [Google Scholar]
- Saniga, M. Structure, production and regeneration processes of English yew in the State Nature Reserve Plavno (in Slovak). J. For. Sci. 2000, 46, 76–90. [Google Scholar]
- Vacik, H.; Oitzinger, G.; Georg, F. Evaluation of in situ conservation strategies for English yew (Taxus baccata L.) in Bad Bleiberg by the use of population viability risk management (PVRM). Forstwiss. Cent. 2001, 120, 390–405. [Google Scholar] [CrossRef]
- Saniga, M.; Jaloviar, P. The effect of natural processes, silvicultural and protective measures on the conservation of common yew in the Nature Reserve Pavelcovo, Slovakia (in German). Schweiz. Z. Forstwes 2005, 156, 487–495. [Google Scholar] [CrossRef]
- Vessella, F.; Salis, A.; Sciré, M.; Piovesan, G.; Schirone, B. Natural regeneration and gender-specific spatial pattern of Taxus baccata in an old-growth population in Foresta Umbra (Italy). Dendrobiology 2015, 73, 75–90. [Google Scholar] [CrossRef]
- Iszkuło, G. Influence of biotic and abiotic factors on natural regeneration of European yew (Taxus baccata L.): A review. Span. J. Rural Dev. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Givnish, T.J. Ecological constraints on the evolution of breeding systems in seed plants: Dioecy and dispersal in gymnosperms. Evolution (N. Y.) 2017, 34, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Ariel, H.M.; Alejandro, R.J.F. Sex-related climate sensitivity of Araucaria araucana Patagonian forest-steppe ecotone. For. Ecol. Manag. 2016, 362, 130–141. [Google Scholar] [CrossRef]
- Kelly, D. The Evolutionary ecology of mast seeding. Trends Ecol. Evol. 1994, 9, 465–470. [Google Scholar] [CrossRef]
- Koenig, W.D.; Knops, J.M.H. Patterns of annual seed production by Northern Hemisphere trees: A global perspective. Am. Nat. 2000, 155, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T.; Pallardy, S.G. Growth Control in Woody Plants; Academic Press: San Diego, CA, USA, 1997; 643p. [Google Scholar]
- Mitchell, M.G.E.; Antos, J.A.; Allen, G.A. Modules of reproduction in females of the dioecious shrub Oemleria cerasiformis. Can. J. Bot. 2004, 82, 393–400. [Google Scholar] [CrossRef]
- DeSoto, L.; Olano, J.M.; Rozas, V. Secondary growth and carbohydrate storage patterns differ between sexes in Juniperus thurifera. Front. Plant Sci. 2016, 7, 723. [Google Scholar] [CrossRef] [PubMed]
- Iszkuło, G.; Boratyński, A. Initial period of sexual maturity determines the greater growth rate of male over female in the dioecious tree Juniperus communis subsp. communis. Acta Oecologica 2011, 37, 99–102. [Google Scholar] [CrossRef]
- Garbarino, M.; Weisberg, P.J.; Bagnara, L.; Urbinati, C. Sex-related spatial segregation along environmental gradients in the dioecious conifer, Taxus baccata. For. Ecol. Manag. 2015, 358, 122–129. [Google Scholar] [CrossRef]
- Rozas, V.; DeSoto, L.; Olano, J.M. Sex-specific, age-dependent sensitivity of tree-ring growth to climate in the dioecious tree Juniperus thurifera. New Phytol. 2009, 182, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Rovere, A.E.; Aizen, M.A.; Kitzberger, T. Growth and climatic response of male and female trees of Austrocedrus chilensis, a dioecious conifer from the temperate forests of southern South America. Ecoscience 2003, 10, 195–203. [Google Scholar] [CrossRef]
- Farjon, A. Taxus baccata. The IUCN Red List of Threatened Species 2013. Available online: http://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T42546A2986660.en (accessed on 24 April 2017).
- Svoboda, P. The largest occurrence of yew in Central Europe (in Czech). Ochr. Přír. 1947, 2, 65–70. [Google Scholar]
- Tschermak, L. The yew at the municipal forest enterprise in Banská Bystrica, Slovakia, the largest up to date known yew occurrence in Europe (in German). Forstwiss. Cent. 1949, 68, 4–11. [Google Scholar] [CrossRef]
- Barták, J. On the History of State Forest Management in Banská Bystrica and Staré Hory District (in Slovak); Slovenská grafia: Banská Bystrica, Slovak, 1929; 207p. [Google Scholar]
- Jankov, J.; Gubka, K. Regeneration of yew (Taxus baccatta L.) in forests with antierosive-production function (in Slovak). In Silviculture in Central Europe (in Slovak), Proceedings of the Silviculture in Central Europe, Zvolen, Slovak, 2–4 July 2012; Saniga, M., Kucbel, S., Eds.; TU: Zvolen, Slovak, 2012; pp. 246–254. [Google Scholar]
- Burkhart, H.E.; Tomé, M. Modeling Forest Trees and Stands; Springer Science + Business Media: Dordrecht, The Netherlands, 2012; 458p. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Aplications in the Environmental Sciences; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; 352p. [Google Scholar]
- Yamaguchi, D.K. A Simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991, 21, 414–416. [Google Scholar] [CrossRef]
- Holmes, R.L. Quality control of crossdating and measuring: A user’s manual for program COFECHA. In Tree-Ring Chronologies of Western North American California, Eastern Oregon and Northern Great Bassin; Holmes, R.L., Adams, R.K., Eds.; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1986; pp. 41–49. [Google Scholar]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Čermák, P.; Rybníček, M.; Žid, T.; Andreassen, K.; Børja, I.; Kolář, T. Impact of climate change on growth dynamics of Norway spruce in south-eastern Norway. Silva Fenn. 2017, 51, 1781. [Google Scholar] [CrossRef]
- Neuwirth, B.; Esper, J.; Schweingruber, F.H.; Winiger, M. Site ecological differences to the climatic forcing of spruce pointer years from the Lotschental, Switzerland. Dendrochronologia 2004, 2, 69–78. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Blackburn Press: Caldwell, NJ, USA, 2001. [Google Scholar]
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; International Institute for Applied Systems Analysis, Kluwer: Boston, MA, USA, 1990; pp. 137–152. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Climtol. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Black, B.A.; Abrams, M.D. Development and application of boundary-line release criteria. Dendrochronologia 2004, 22, 31–42. [Google Scholar] [CrossRef]
- Saniga, M.; Pittner, J.; Kucbel, S.; Jaloviar, P.; Sedmáková, D; Vencurik, J. Structure, Growth, and Regeneration Processes of European Yew in the Selected Nature Reserves and a Managed Forest: A Study from Starohorské Mts., Slovakia (in Slovak); TU: Zvolen, Slovak, 2017; 58p. [Google Scholar]
- Thomas, P.A.; Polwart, A. Taxus baccata L. Biological flora of the British Isles 229. J. Ecol. 2003, 91, 489–524. [Google Scholar] [CrossRef]
- Dhar, A.; Ruprecht, H.; Klumpp, R.; Vacik, H. Stand structure and natural regeneration of Taxus baccata at “Stiwollgraben” in Austria. Dendrobiology 2006, 56, 19–26. [Google Scholar]
- Svenning, J.-C.; Magård, E. Population ecology and conservation status of the last natural population of English yew Taxus baccata in Denmark. Biol. Conserv. 1999, 88, 173–182. [Google Scholar] [CrossRef]
- Iszkuło, G.; Boratyński, A. Different age and spatial structure of two spontaneous subpopulations of Taxus baccata as a result of various intensity of colonization process. Flora 2005, 200, 195–206. [Google Scholar] [CrossRef]
- Dhar, A.; Ruprecht, H.; Klumpp, R.; Vacik, H. Comparison of ecological condition and conservation status of English yew population in two Austrian gene conservation forests. J. For. Res. 2007, 18, 181–186. [Google Scholar] [CrossRef]
- Hulme, P.E. Natural regeneration of yew (Taxus baccata L.): Microsite, seed or herbivore limitation? J. Ecol. 1996, 84, 853–861. [Google Scholar] [CrossRef]
- Devaney, J.L.; Jansen, M.A.K.; Whelan, P.M. Spatial patterns of natural regeneration in stands of English yew (Taxus baccata L.); negative neighbourhood effects. For. Ecol. Manag. 2014, 321, 52–60. [Google Scholar] [CrossRef]
- Niemczyk, M.; Żółciak, A.; Piotr, W. The influence of stand canopy openness on the growth of common yew (Taxus baccata L.). For. Res. Pap. 2015, 76, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Izskulo, G. Success and failure of endangered tree species: Low temperatures and low light availability affect survival and growth of European yew (Taxus baccata L.) seedlings. Pol. J. Ecol. 2010, 58, 259–271. [Google Scholar]
- Vaško, Ľ.; Finďo, S.; Pirchala, M. Damage of English yew (Taxus baccata) by ungulate (In Slovak). In Current Issues of Forest Protection 2010 (In Slovak); Proceedings of the International Workshop, Nový Smokovec, Slovak, 15–16 April 2010; Kunca, A., Hlaváč, P., Eds.; NFC: Zvolen, Slovak, 2010; pp. 121–126. [Google Scholar]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 2014, 346, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Kaštier, P. Ungulate density and its impact on Slovak forests. In The Current Issues in Forest Establishment and Silviculture 2016-4th International Conference (in Slovak); National Forest Centre: Zvolen, Slovak, 2016; pp. 88–95. [Google Scholar]
- Iszkuło, G.; Nowak-Dyjeta, K.; Sękiewicz, M. Influence of initial light intensity and deer browsing on Taxus baccata saplings: A six years field study. Dendrobiology 2014, 71, 93–99. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Kienast, F. Classifying the life-history strategies of trees on the basis of the Grimian model. For. Ecol. Manag. 1994, 69, 167–187. [Google Scholar] [CrossRef]
- Karlsson, C.; Örlander, G. Mineral nutrients in needles of Pinus sylvestris seed trees after release cutting and their correlations with cone production and seed weight. For. Ecol. Manag. 2002, 166, 183–191. [Google Scholar] [CrossRef]
- Lavabre, J.E.; García, D. Geographic consistency in the seed dispersal patterns of Taxus baccata L. in the Iberian Peninsula. For. Syst. 2015, 24. [Google Scholar] [CrossRef]
- García, D.; Obeso, J.R.; Martínez, I. Spatial concordance between seed rain and seedling establishment in bird-dispersed trees: Does scale matter? J. Ecol. 2005, 93, 693–704. [Google Scholar] [CrossRef]
- García, D.; Martínez, I.; Obeso, J.R. Seed transfer among bird-dispersed trees and its consequences for post-dispersal seed fate. Basic Appl. Ecol. 2007, 8, 533–543. [Google Scholar] [CrossRef]
- Martínez, I.; García, D.; Obeso, J.R. Differential seed dispersal patterns generated by a common assemblage of vertebrate frugivores in three fleshy-fruited trees. Ecoscience 2008, 15, 189–199. [Google Scholar] [CrossRef]
- Tittensor, R.M. Ecological history of yew Taxus baccata L. in southern England. Biol. Conserv. 1980, 17, 243–265. [Google Scholar] [CrossRef]
- Dovčiak, M. Population Dynamics of the Endangered English Yew (Taxus Baccata L.) and Its Management Implications for Biosphere Reserves of the Western Carpathians; MAB UNESCO: Paris, France, 2002; 43p. [Google Scholar]
- Massei, G.; Watkins, R.; Hartley, S.E. Sex-related growth and secondary compounds in Juniperus oxycedrus macrocarpa. Acta Oecologica 2006, 29, 135–140. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Ježík, M.; Blaženec, M.; Střelcová, K.; Ditmarová, Ľ. The impact of the 2003–2008 weather variability on intra-annual stem diameter changes of beech trees at a submontane site in central Slovakia. Dendrochronologia 2011, 29, 227–235. [Google Scholar] [CrossRef]
- Zywiec, M.; Zielonka, T. Does a heavy fruit crop reduce the tree ring increment? Results from a 12-year study in a subalpine zone. Trees Struct. Funct. 2013, 27, 1365–1373. [Google Scholar] [CrossRef]
- Hoch, G.; Siegwolf, R.T.W.; Keel, S.G.; Körner, C.; Han, Q. Fruit production in three masting tree species does not rely on stored carbon reserves. Oecologia 2013, 171, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Knops, J.M.H.; Koenig, W.D.; Carmen, W.J. Negative correlation does not imply a tradeoff between growth and reproduction in California oaks. Proc. Natl. Acad. Sci. USA 2007, 104, 16982–16985. [Google Scholar] [CrossRef] [PubMed]
- Mund, M.; Kutsch, W.L.; Wirth, C.; Kahl, T.; Knohl, A.; Skomarkova, M.V.; Schulze, E.D. The influence of climate and fructification on the inter-annual variability of stem growth and net primary productivity in an old-growth, mixed beech forest. Tree Physiol. 2010, 30, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Müller-Haubold, H.; Hertel, D.; Seidel, D.; Knutzen, F.; Leuschner, C. Climate Responses of Aboveground Productivity and Allocation in Fagus sylvatica: A Transect Study in Mature Forests. Ecosystems 2013, 16, 1498–1516. [Google Scholar] [CrossRef]
- Hacket-Pain, A.; Friend, A.D.; Lageard, J.G.A.; Thomas, P.A. The influence of masting phenomenon on growth-climate relationships in trees: Explaining the influence of previous summers’ climate on ring width. Tree Physiol. 2015, 35, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Cedro, A.; Iszkuło, G. Do females differ from males of European yew (Taxus baccata L.) in dendrochronological analysis? Tree Ring Res. 2011, 67, 3–11. [Google Scholar] [CrossRef]
- Matsushita, M.; Takao, M.; Makita, A. Acta Oecologica Sex-different response in growth traits to resource heterogeneity explains male-biased sex ratio. Acta Oecologica 2016, 75, 8–14. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Gao, L.; Gadow, K. Gender-related distributions of Fraxinus mandshurica in secondary and old-growth forests. Acta Oecologica 2010, 36, 55–62. [Google Scholar] [CrossRef]
- De Frenne, P.; Rodríguez-Sánchez, F.; De Schrijver, A.; Coomes, D.A.; Hermy, M.; Vangansbeke, P.; Verheyen, K. Light Accelerates Plant Responses to Warming. Nat. Plants 2015, 1, 15110. [Google Scholar] [CrossRef] [PubMed]
- Scherere-Lorenzen, M. Conservation through Closure. Nat. Plants 2015, 1, 15116. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef] [PubMed]
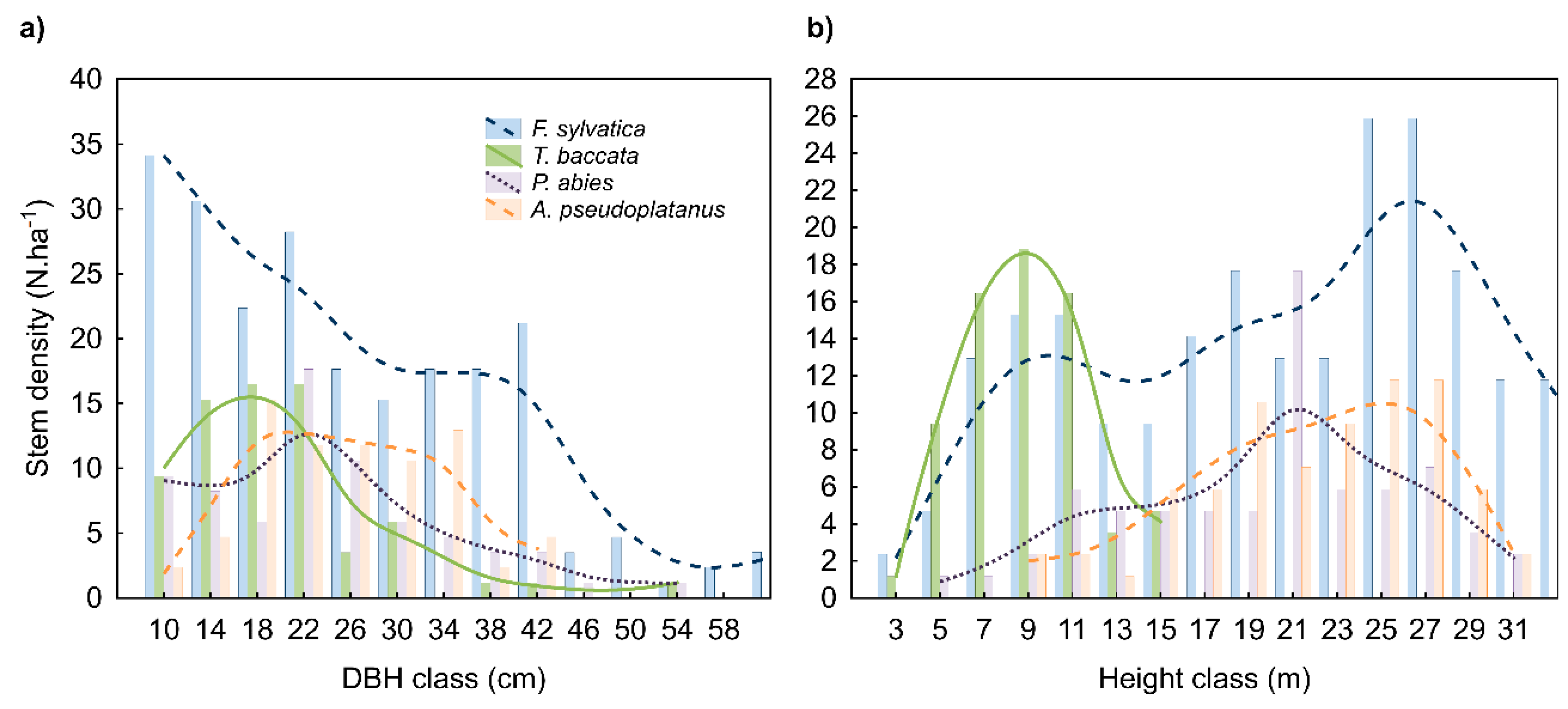
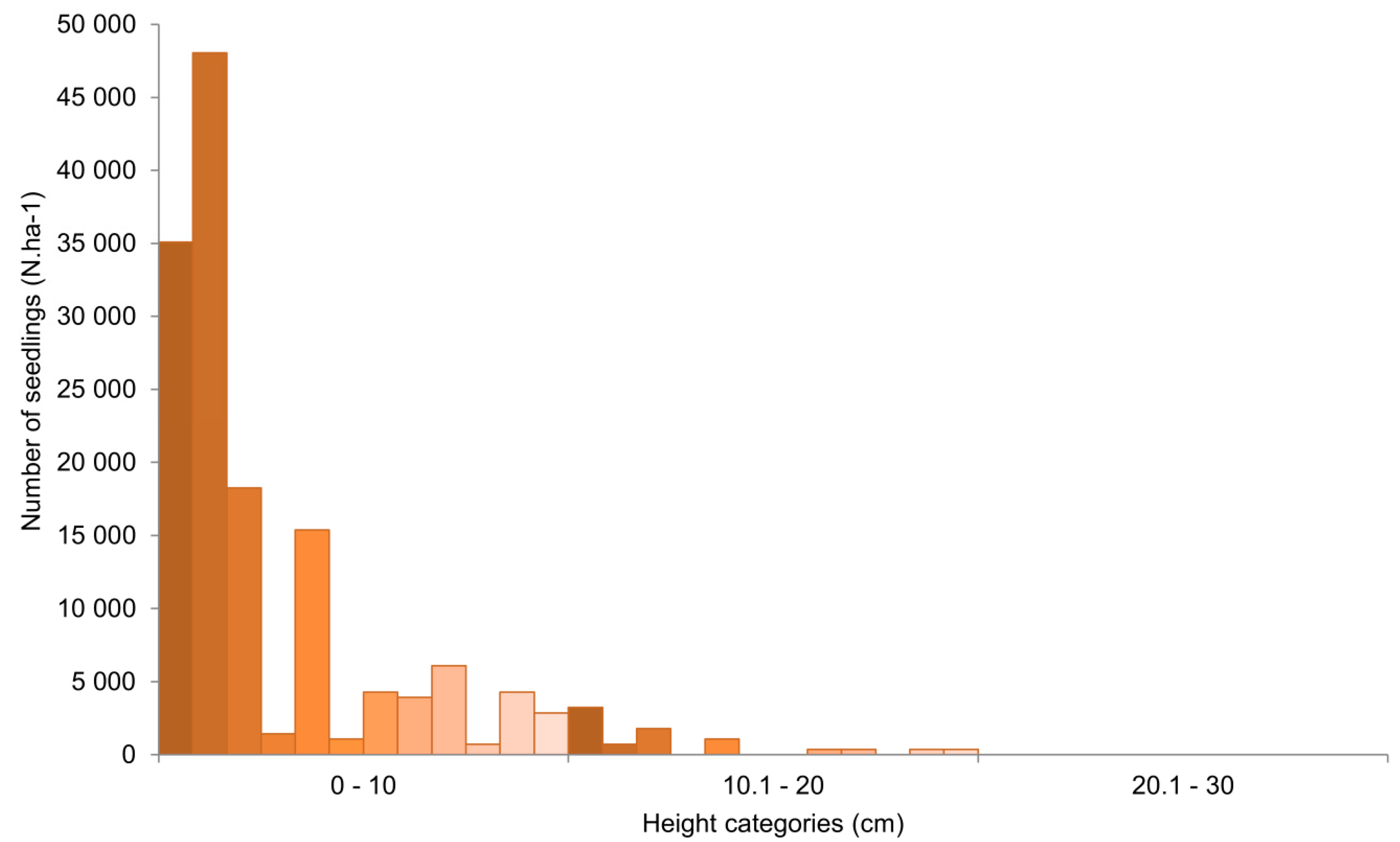
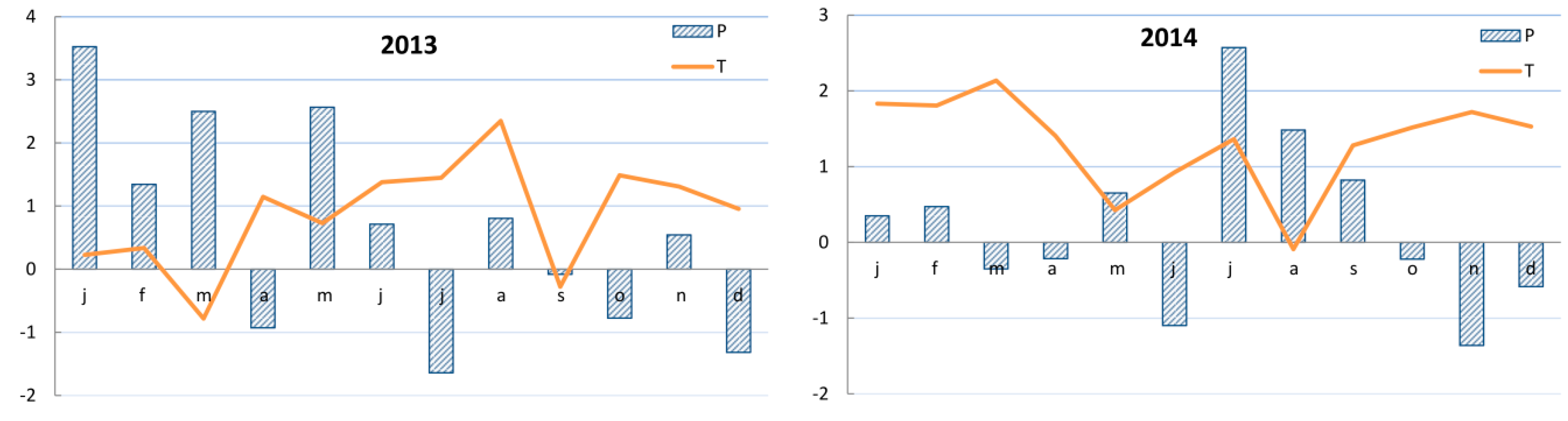


| Single Tree Characteristics (n = 38 Trees) | Female | Male | ||||
|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Mean ± SD | Mean ± SD | ||
| Diameter | DBH (cm) | 11.9 | 22.8 ± 8.14 | 52.7 | 23.5 ± 7.9 | 22.6 ± 8.38 |
| Height | h (m) | 7.0 | 10.7 ± 2.5 | 16.0 | 11.0 ± 2.3 | 10.3 ± 1.68 |
| Stem basal area | g (m2) | 0.01 | 0.05 ± 0.04 | 0.22 | 0.05 ± 0.04 | 0.05 ± 0.04 |
| Stem volume | v (m3) | 0.03 | 0.21 ± 0.19 | 0.96 | 0.23 ± 0.18 | 0.20 ± 0.20 |
| Crown length | CL (m) | 5.1 | 8.3 ± 2.1 | 13.0 | 8.1 ± 2.3 | 7.4 ± 1.76 |
| Crown ratio | CR | 0.56 | 0.73 ± 0.07 | 0.81 | 0.76 ± 0.07 * | 0.70 ± 0.06 |
| Crown projection area | CPA (m2) | 10.2 | 22.0 ± 12.6 | 49.2 | 22.4 ± 13.2 | 21.7 ± 13.1 |
| Crown volume | CV (m3) | 17.3 | 62.8 ± 47.9 | 168.8 | 66.0 ± 41.5 | 59.6 ± 57.5 |
| Crown surface area | CSA (m2) | 30.6 | 70.2 ± 34.0 | 140.5 | 75.7 ± 27.7 | 64.8 ± 41.4 |
| Age | (years) | 62 | 94 ± 13 | 118 | 103 ± 12 | 95 ± 14 |
| Female | Male | All | |||
|---|---|---|---|---|---|
| Raw series | Time span | 1898–2015 | 1901–2015 | 1898–2015 | |
| Number of sampled trees—Nt | 18 | 20 | 38 | ||
| Mean series length—MSL | 97 | 94 | 96 | ||
| Mean tree-ring width (mm)—TRW | 0.94 | 1 | 0.97 | ||
| Standard deviation—SD | 0.46 | 0.6 | 0.53 | ||
| Mean sensitivity of individual series—MSs | 0.23 | 0.25 | 0.24 | ||
| First-order autocorrelation coefficient—AC1 | 0.75 | 0.77 | 0.76 | ||
| Residual chronology | Between trees correlation—RBT | 0.38 | 0.45 | 0.44 | |
| Expressed population signal—EPS | 0.91 | 0.94 | 0.96 | ||
| Climate response | TMP | May–June—mj | 0.26 | 0.18 | 0.22 |
| November–March—ndJFM | 0.48 | 0.37 | 0.39 | ||
| May–June—MJ | −0.2 | −0.19 | −0.21 | ||
| PRE | June–August—JJA | 0.26 | 0.34 | 0.37 | |
| September–S | 0.22 | 0.23 | 0.19 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedmáková, D.; Saniga, M.; Kucbel, S.; Pittner, J.; Kýpeťová, M.; Jaloviar, P.; Bugala, M.; Vencurik, J.; Lukáčik, I. Irregular Shelterwood Cuttings Promote Viability of European Yew Population Growing in a Managed Forest: A Case Study from the Starohorské Mountains, Slovakia. Forests 2017, 8, 289. https://doi.org/10.3390/f8080289
Sedmáková D, Saniga M, Kucbel S, Pittner J, Kýpeťová M, Jaloviar P, Bugala M, Vencurik J, Lukáčik I. Irregular Shelterwood Cuttings Promote Viability of European Yew Population Growing in a Managed Forest: A Case Study from the Starohorské Mountains, Slovakia. Forests. 2017; 8(8):289. https://doi.org/10.3390/f8080289
Chicago/Turabian StyleSedmáková, Denisa, Milan Saniga, Stanislav Kucbel, Ján Pittner, Mariana Kýpeťová, Peter Jaloviar, Michal Bugala, Jaroslav Vencurik, and Ivan Lukáčik. 2017. "Irregular Shelterwood Cuttings Promote Viability of European Yew Population Growing in a Managed Forest: A Case Study from the Starohorské Mountains, Slovakia" Forests 8, no. 8: 289. https://doi.org/10.3390/f8080289