Differential Effects of Coarse Woody Debris on Microbial and Soil Properties in Pinus densiflora Sieb. et Zucc. Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
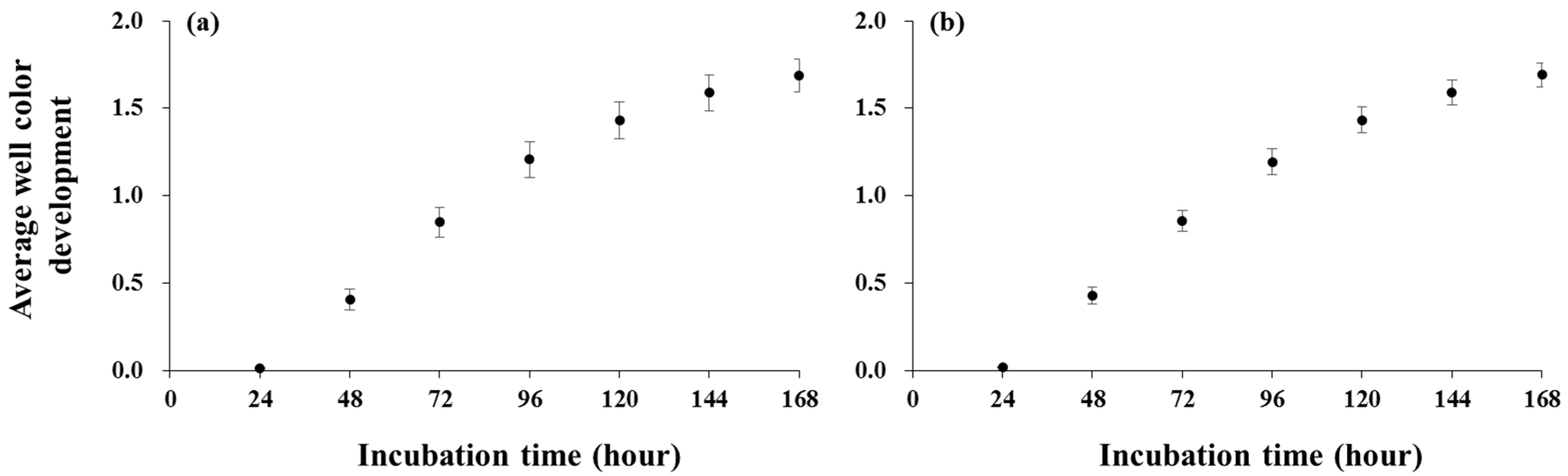
2.2. Sampling and Field Measurement
2.3. Soil Analysis
2.4. Statistical Analysis
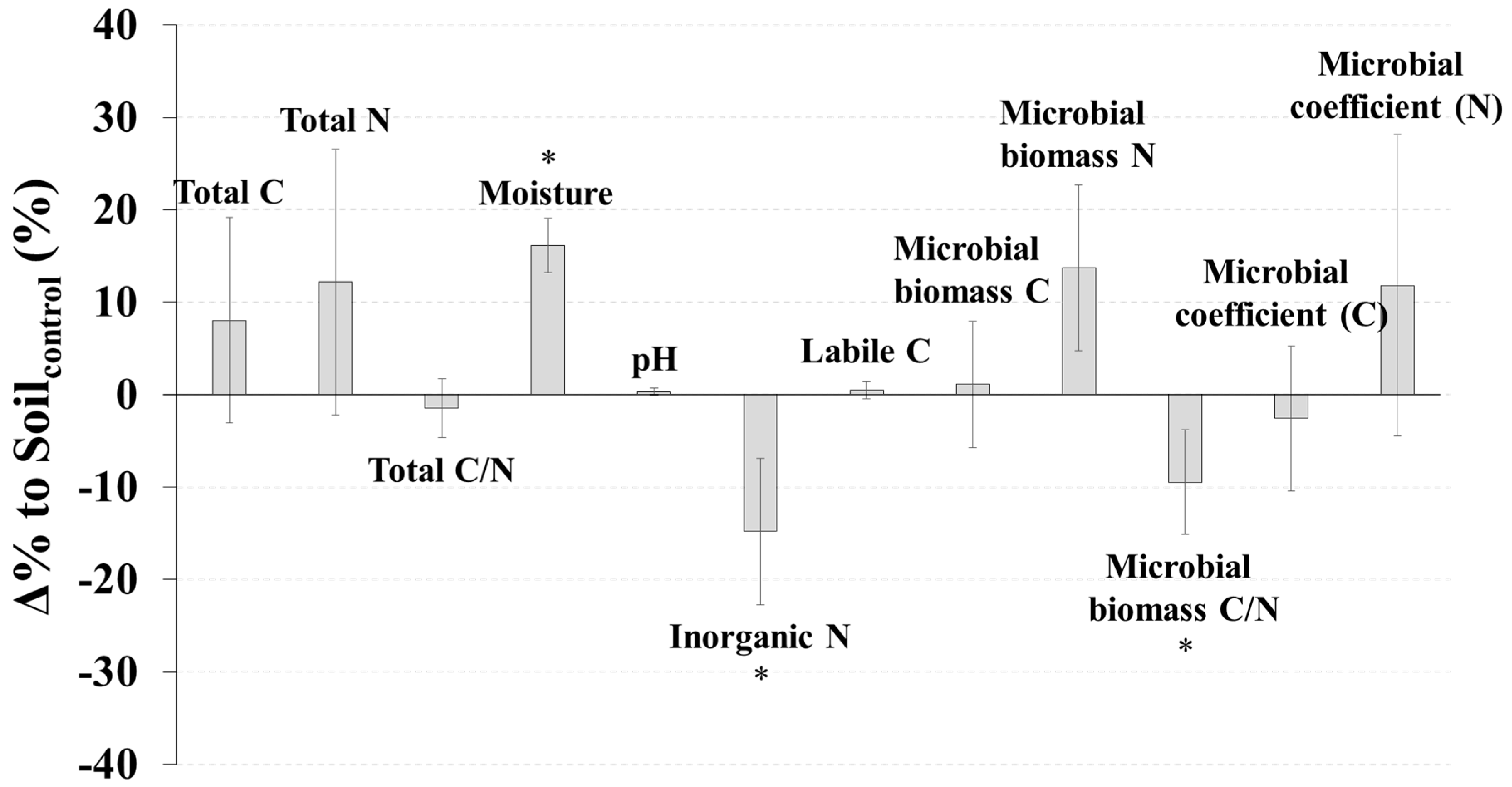
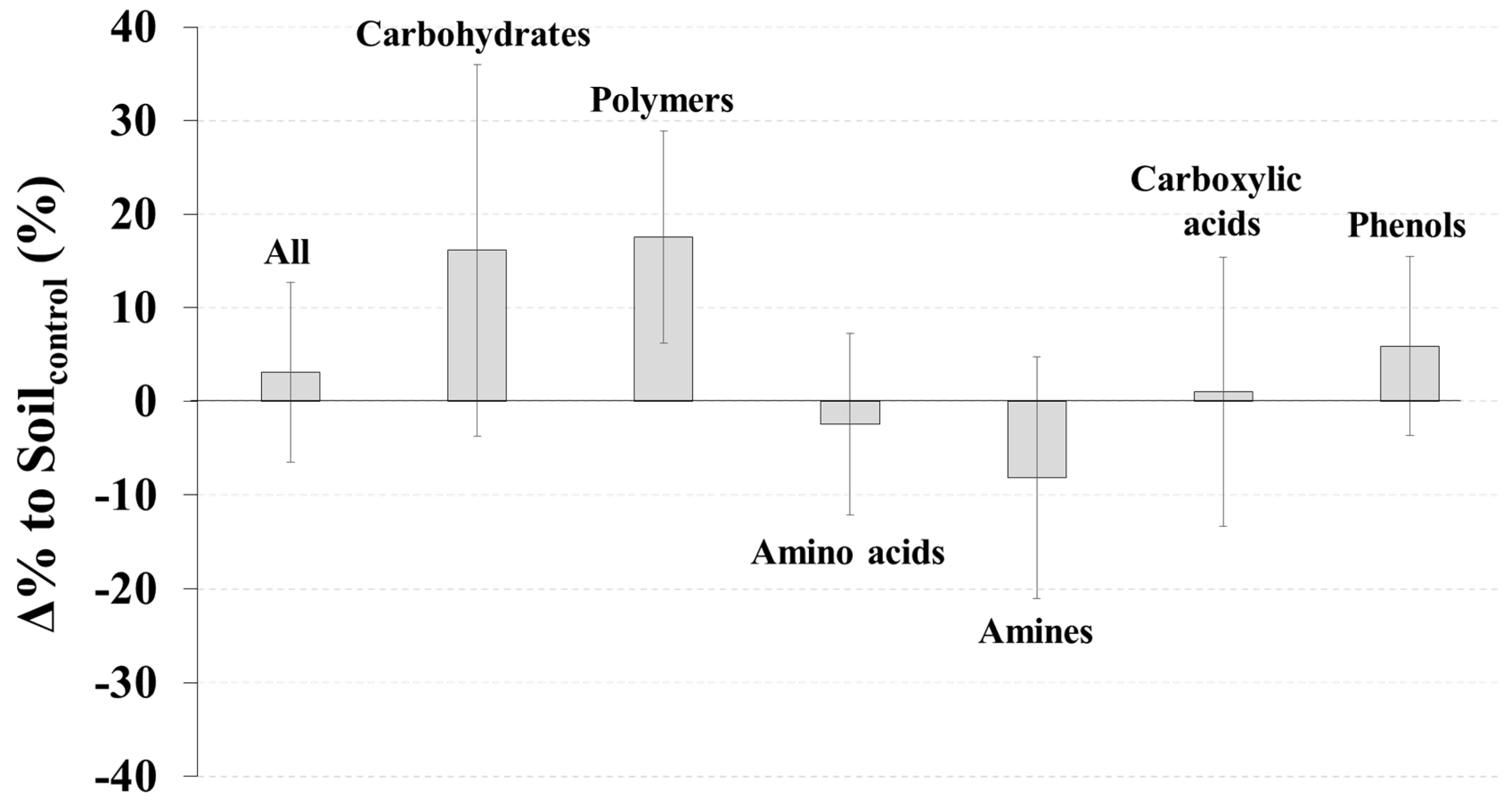
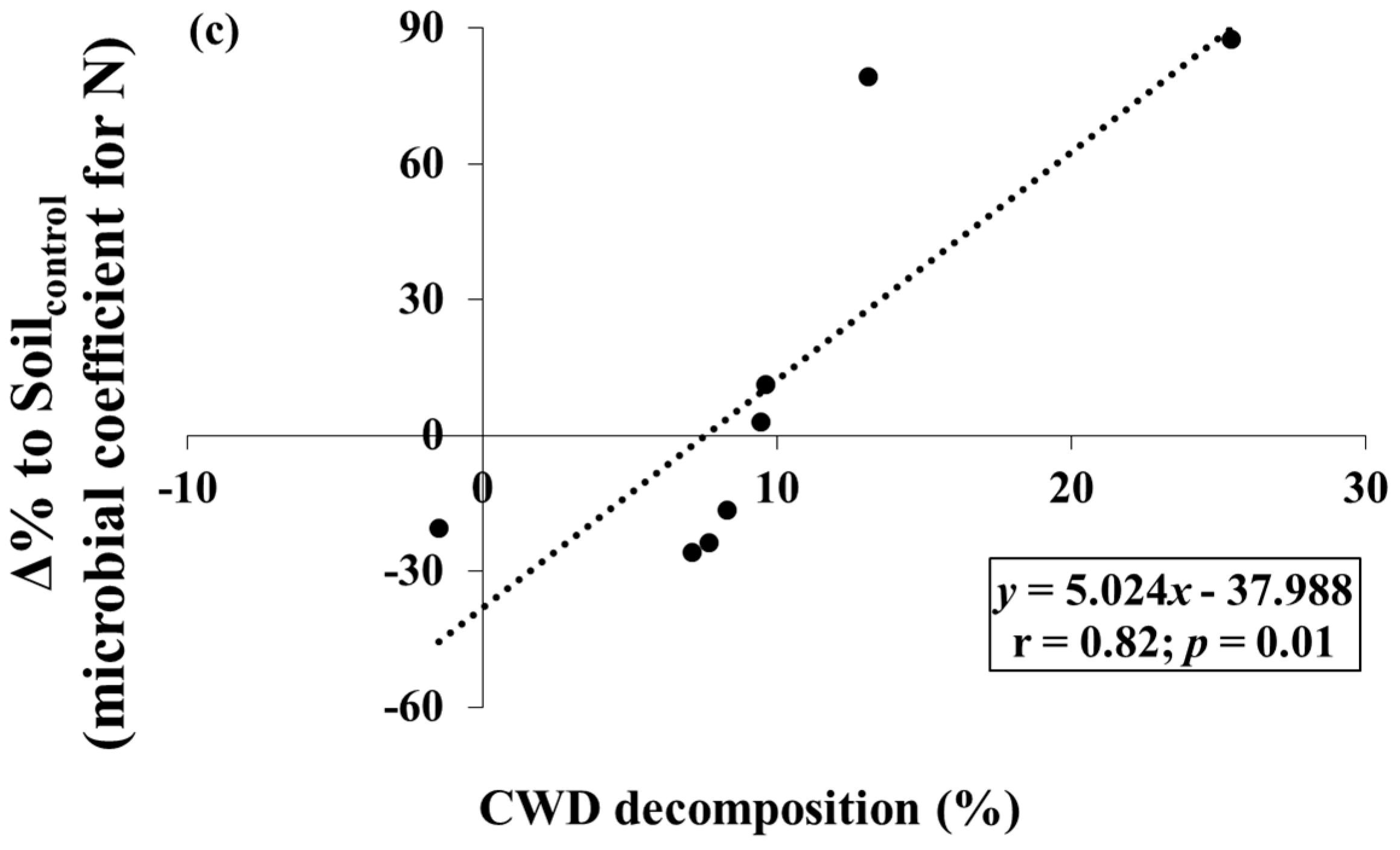
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Diameter (cm) | Length (cm) | Volume (cm−3) | Dry Mass (g) | Density (g cm−3) | C Concentration (%) | N Concentration (%) |
|---|---|---|---|---|---|---|
| 11.1 (0.1) | 10.2 (0.0) | 995.7 (16.0) | 776.4 (6.7) | 0.79 (0.0) | 48.9 (0.3) | 0.04 (0.0) |
References
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Noh, N.J.; Yoon, T.K.; Kim, R.-H.; Bolton, N.W.; Kim, C.; Son, Y. Carbon and nitrogen accumulation and decomposition from coarse woody debris in a naturally regenerated Korean red pine (Pinus densiflora S.et Z.) forest. Forests 2017, 8, 214. [Google Scholar] [CrossRef]
- Yoon, T.K.; Noh, N.J.; Kim, S.; Han, S.; Son, Y. Coarse woody debris respiration of Japanese red pine forests in Korea: Controlling factors and contribution to the ecosystem carbon cycle. Ecol. Res. 2015, 30, 723–734. [Google Scholar] [CrossRef]
- Laiho, R.; Prescott, C.E. The contribution of coarse woody debris to carbon, nitrogen, and phosphorus cycles in three Rocky Mountain coniferous forests. Can. J. For. Res. 1999, 29, 1592–1603. [Google Scholar] [CrossRef]
- Marañón-Jiménez, S.; Castro, J. Effect of decomposing post-fire coarse woody debris on soil fertility and nutrient availability in a Mediterranean ecosystem. Biogeochemistry 2013, 112, 519–535. [Google Scholar] [CrossRef]
- Hafner, S.D.; Groffman, P.M. Soil nitrogen cycling under litter and coarse woody debris in a mixed forest in NewYork State. Soil Biol. Biochem. 2005, 37, 2159–2162. [Google Scholar] [CrossRef]
- Brais, S.; Drouin, P. Interactions between deadwood and soil characteristics in a natural boreal trembling aspen–Jack pine stand. Can. J. For. Res. 2012, 42, 1456–1466. [Google Scholar] [CrossRef]
- Stutz, K.; Lang, F. Potentials and unknowns in managing coarse woody debris for soil functioning. Forests 2017, 8, 37. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Adamczyk, S.; Kitunen, V.; Lindroos, A.-J.; Adamczyk, B.; Smolander, A. Soil carbon and nitrogen cycling processes and composition of terpenes five years after clear cutting a Norway spruce stand: Effects of logging residues. For. Ecol. Manag. 2016, 381, 318–326. [Google Scholar] [CrossRef]
- Cardenas, E.; Kranabetter, J.M.; Hope, G.; Maas, K.R.; Hallam, S.; Mohn, W.W. Forest harvesting reduces the soil metagenomics potential for biomass decomposition. ISME J. 2015, 9, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Lenoir, F.; Vegerfors, B. Long-term effects of stump harvesting and site preparation on pools and fluxes of soil carbon and nitrogen in central Sweden. Scand. J. For. Res. 2017, 32, 222–229. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Insect-mediated nitrogen dynamics in decomposing wood. Ecol. Entomol. 2015, 40, 97–112. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, J.-H.; Park, J.-H.; Ewane, E.B.; Lee, D.-H. Correlation between above-ground and below-ground biomass of 13-year-old Pinus densiflora S.et Z. planted in a post-fire area in Samcheok. For. Sci. Technol. 2016, 12, 115–124. [Google Scholar]
- Pyo, J. Developing the site index equation using a generalized algebraic difference approach for Pinus densiflora in central region, Korea. For. Sci. Technol. 2017, 13, 87–91. [Google Scholar] [CrossRef]
- Ko, S.; Yoon, T.K.; Kim, S.; Kim, C.; Lee, S.-T.; Seo, K.W.; Son, Y. Thinning intensity effects on carbon storage of soil, forest floor and coarse woody debris in Pinus densiflora stands. J. Korean For. Soc. 2014, 103, 30–36. (In Korean) [Google Scholar] [CrossRef]
- Shin, S.-C. Pine wilt disease in Korea. In Pine wilt disease; Zhao, K., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 26–32. [Google Scholar]
- Mulvaney, R.L. Nitrogen-inorganic forms. In Methods of Soil Analysis. Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1146–1155. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.R.; Islam, K.R.; Stine, M.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Perakis, S.S.; Hedin, L.O. Fluxes and fates of nitrogen in soil of an unpolluted old-growth temperate forest, southern Chile. Ecology 2001, 82, 2245–2260. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the KEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Park, M.J.; Son, Y. Short-term effects of experimental warming and precipitation manipulation on soil microbial biomass C and N, community substrate utilization patterns and community composition. Pedoshpere, 2017, 27, 714–724. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [PubMed]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Lee, J.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Kwak, J.-H.; Chang, S.X.; Naeth, M.A.; Schaaf, W. Coarse woody debris increases microbial community functional diversity but not enzyme activities in reclaimed oil sands soils. PLoS ONE 2015, 10, e0143857. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Jeong, S.-Y. Ecological characteristics of termite (Reticulitermes speratus kyushuensis) for preservation of wooden cultural heritage. Conserv. Stud. 2004, 37, 327–348. (In Korean) [Google Scholar]
- Bonde, T.A.; Schnurer, J.; Rosswall, T. Microbial biomass as a fraction of potentially mineralizable nitrogen in soils from long-term field experiments. Soil Biol. Biochem. 1988, 20, 447–452. [Google Scholar] [CrossRef]

| Location | Tree Density (stem ha−1) | Altitude (m) | Air Temperature (°C) | Relative Humidity (%) | CWD Decomposition (%) | |
|---|---|---|---|---|---|---|
| Central 1 | 37°44′N, 127°10′E | 2500 | 500 | 11.7 | 75.5 | −1.5 |
| Central 2 | 37°46′N, 127°10′E | 1140 | 470 | 10.8 | 73.1 | 7.1 |
| Central 3 | 37°47′N, 127°10′E | 650 | 420 | 11.1 | 73.1 | 9.5 |
| Central 4 | 37°30′N, 128°56′E | 610 | 690 | 9.4 | 77.9 | 8.3 |
| Central 5 | 38°02′N, 128°22′E | 1050 | 610 | 8.8 | 80.8 | 7.7 |
| Southern 1 | 35°12′N, 128°10′E | 1560 | 180 | 14.5 | 71.8 | 9.6 |
| Southern 2 | 35°12′N, 128°10′E | 600 | 170 | 14.4 | 73.1 | 13.1 |
| Southern 3 | 35°21′N, 128°10′E | 1200 | 430 | 13.1 | 74.4 | 25.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Li, G.; Han, S.H.; Chang, H.; Kim, H.-J.; Son, Y. Differential Effects of Coarse Woody Debris on Microbial and Soil Properties in Pinus densiflora Sieb. et Zucc. Forests. Forests 2017, 8, 292. https://doi.org/10.3390/f8080292
Kim S, Li G, Han SH, Chang H, Kim H-J, Son Y. Differential Effects of Coarse Woody Debris on Microbial and Soil Properties in Pinus densiflora Sieb. et Zucc. Forests. Forests. 2017; 8(8):292. https://doi.org/10.3390/f8080292
Chicago/Turabian StyleKim, Seongjun, Guanlin Li, Seung Hyun Han, Hanna Chang, Hyun-Jun Kim, and Yowhan Son. 2017. "Differential Effects of Coarse Woody Debris on Microbial and Soil Properties in Pinus densiflora Sieb. et Zucc. Forests" Forests 8, no. 8: 292. https://doi.org/10.3390/f8080292





