The Impact of Water Content on Sources of Heterotrophic Soil Respiration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil and Litter Sampling
2.3. RHM and RHL Laboratory Measurements
2.4. Statistical Analysis
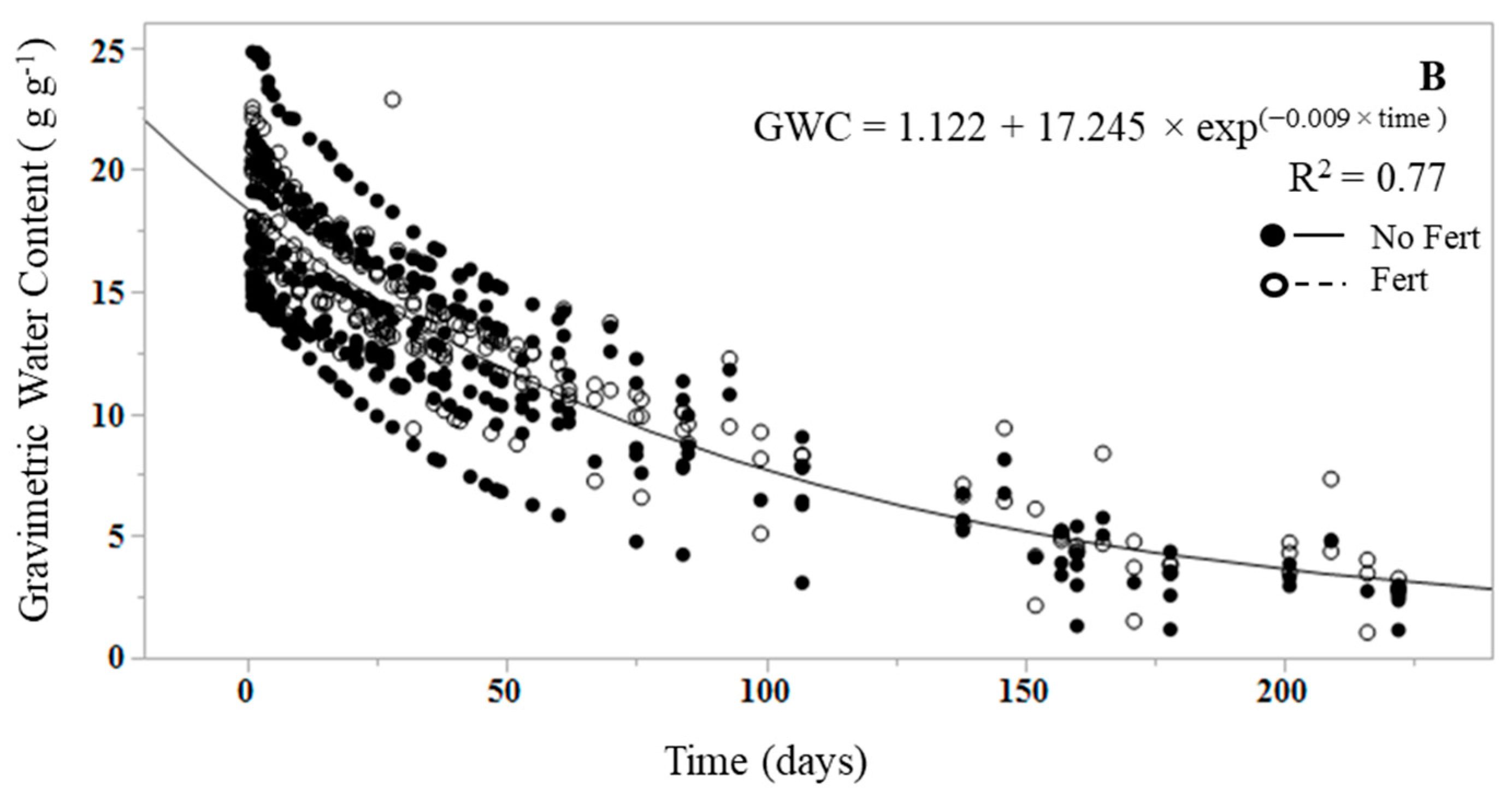
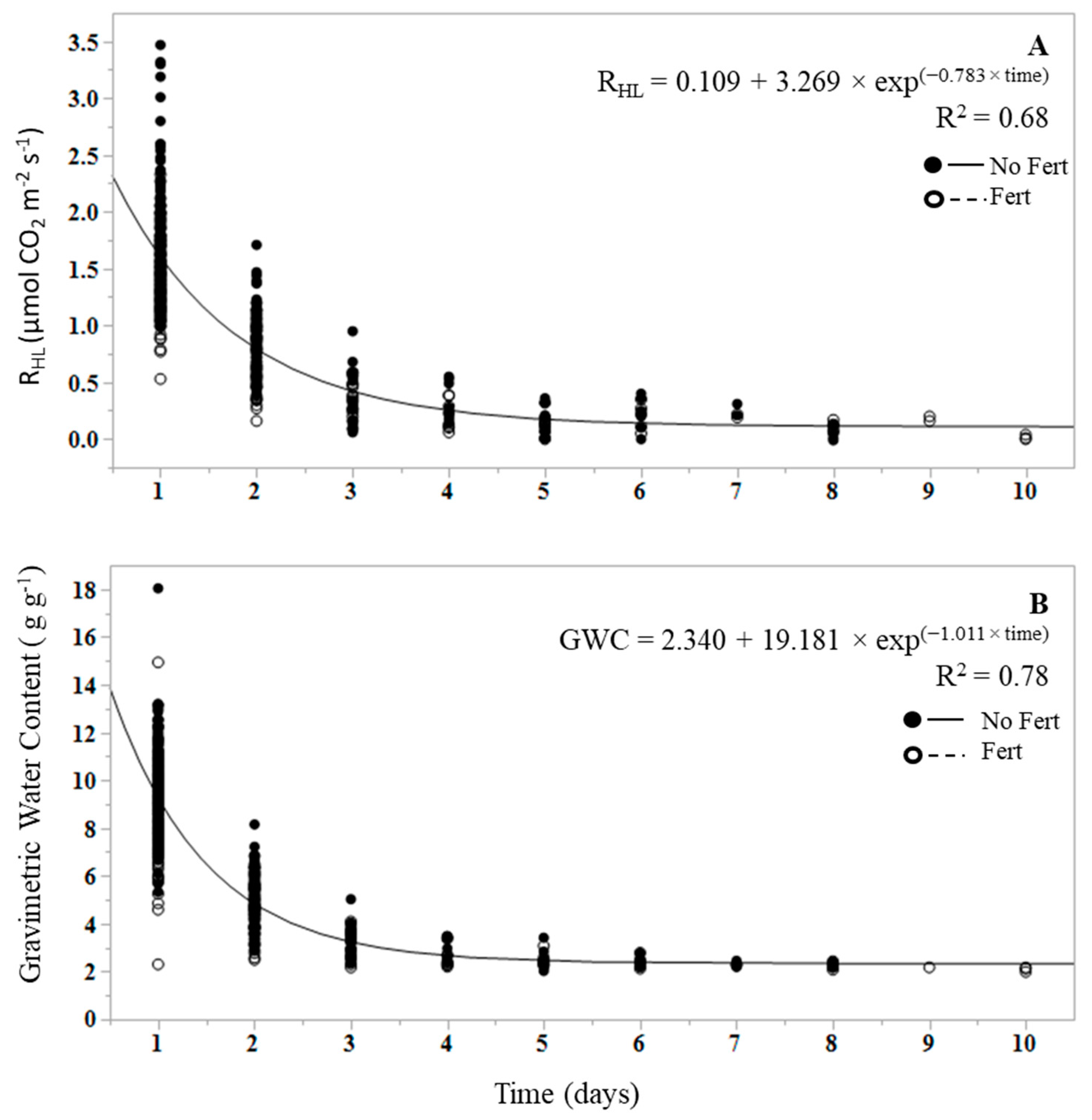
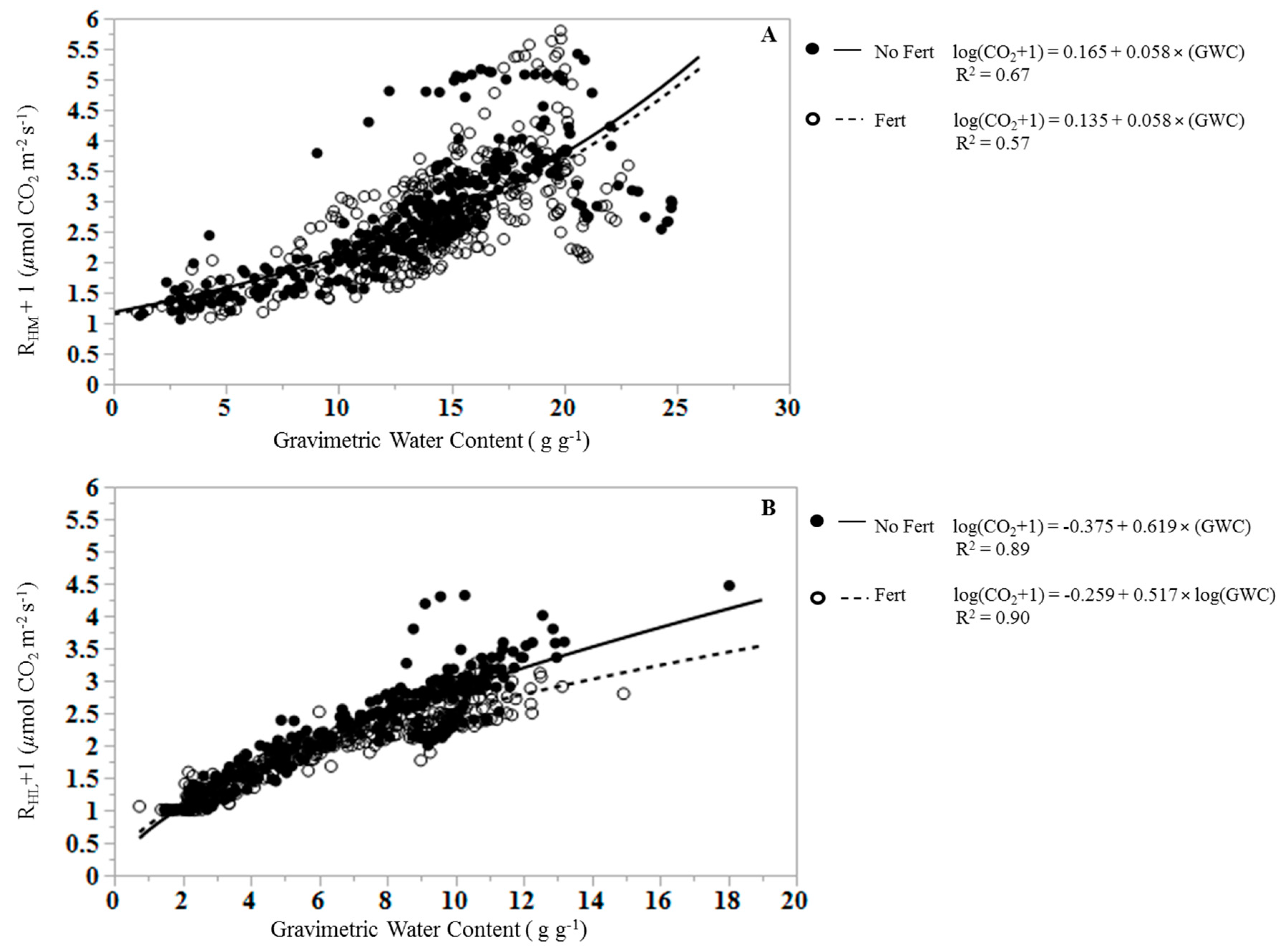
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitchell, R.J.; Liu, Y.; O’Brien, J.J.; Elliott, K.J.; Starr, G.; Miniat, C.F.; Hiers, J.K. Future climate and fire interactions in the southeastern region of the United States. For. Ecol. Manag. 2014, 327, 316–326. [Google Scholar] [CrossRef]
- Rocca, M.E.; Miniat, C.F.; Mitchell, R.J. Editorial. Introduction to the regional assessments: Climate change, wildfire, and forest ecosystem services in the USA. For. Ecol. Manag. 2014, 327, 265–268. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J. Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Jokela, E.J.; Martin, T.A.; Vogel, J.G. Twenty-five years of intensive forest management with southern pines: Important lessons learned. J. For. 2010, 108, 338–347. [Google Scholar]
- Wear, D.N.; Greis, J.G. Southern Forest Resource Assessment—Technical Report; General Technical Report SRS-53; USDA Forest Service; Southern Research Station: Asheville, NC, USA, 2002.
- McNulty, S.; Vose, J.; Swank, W. Loblolly pine hydrology and productivity across the southern United States. For. Ecol. Manag. 1996, 86, 241–251. [Google Scholar] [CrossRef]
- Mearns, L.O.; Gutowski, W.; Jones, R.; Leung, R.; McGinnis, S.; Nunes, A.; Qian, Y. A regional climate change assessment program for North America. Eos 2009, 90, 311. [Google Scholar] [CrossRef]
- Ingram, K.T. Climate of the Southeast United States: Variability, Change, Impacts, and Vulnerability; Ingram, K.T., Dow, K., Carter, L., Anderson, J., Eds.; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Groisman, P.Y.; Knight, R.W.; Karl, T.R.; Easterling, D.R.; Sun, B.; Lawrimore, J.H. Contemporary changes of the hydrological cycle over the contiguous United States: Trends derived from in situ observations. J. Hydrometeorol. 2004, 5, 64–85. [Google Scholar] [CrossRef]
- Angert, A.; Biraud, S.; Bonfils, C.; Henning, C.; Buermann, W.; Pinzon, J.; Tucker, C.; Fung, I. Drier Summers Cancel Out the CO2 Uptake Enhancement Induced by Warmer Springs. Proc. Natl. Acad. Sci. USA 2005, 102, 10823–10827. [Google Scholar] [CrossRef] [PubMed]
- Laseter, S.H.; Ford, C.R.; Vose, J.M.; Swift, L.W. Long-term temperature and precipitation trends at the Coweeta Hydrologic Laboratory, Otto, North Carolina, USA. Hydrol. Res. 2012, 43, 890–901. [Google Scholar] [CrossRef]
- Noormets, A.; Gavazzi, M.J.; McNulty, S.G.; Domec, J.C.; Sun, G.; King, J.S.; Chen, J. Response of carbon fluxes to drought in a coastal plain loblolly pine forest. Glob. Chang. Biol. 2010, 16, 272–287. [Google Scholar] [CrossRef]
- Hanson, P.; Edwards, N.; Garten, C.; Andrews, J. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Subke, J.-A.; Inglima, I.; Francesca Cotrufo, M. Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Glob. Chang. Biol. 2006, 12, 921–943. [Google Scholar] [CrossRef]
- Woodwell, G.M.; Whittaker, R.H. Primary production in terrestrial ecosystems. Am. Zool. 1968, 8, 19–30. [Google Scholar] [CrossRef]
- Templeton, B.S.; Seiler, J.R.; Peterson, J.A.; Tyree, M.C. Environmental and stand management influences on soil CO2 efflux across the range of loblolly pine. For. Ecol. Manag. 2015, 355, 15–23. [Google Scholar] [CrossRef]
- Heim, B.C. Partitioning Soil Respiration in Response to Drought and Fertilization in Loblolly Pine: Laboratory and Field Approaches. Ph.D. Thesis, Virginia Polytechnic Institute & State University, Blacksburg, VA, USA, 2014. [Google Scholar]
- Noormets, A.; Epron, D.; Domec, J.; McNulty, S.; Fox, T.; Sun, G.; King, J. Effects of forest management on productivity and carbon sequestration: A review and hypothesis. For. Ecol. Manag. 2015, 355, 124–140. [Google Scholar] [CrossRef]
- ArchMiller, A.; Samuelson, L. Partitioning longleaf pine soil respiration into its heterotrophic and autotrophic components through root exclusion. Forests 2016, 7, 39. [Google Scholar] [CrossRef]
- McElligott, K.M.; Seiler, J.R.; Strahm, B.D. Partitioning soil respiration across four age classes of loblolly pine (Pinus taeda L.) on the Virginia Piedmont. For. Ecol. Manag. 2016, 378, 173–180. [Google Scholar] [CrossRef]
- Brown, R.M. Soil Heterotrophic Respiration in Loblolly Pine Plantations: Measuring and Modeling Seasonal Variation and Silvicultural Impacts. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2016. [Google Scholar]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Academic Press: Burlington, MA, USA, 2006. [Google Scholar]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Chang. Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Inglima, I.; Alberti, G.; Bertolini, T.; Vaccari, F.; Gioli, B.; Miglietta, F.; Cotrufo, M.; Peressotti, A. Precipitation pulses enhance respiration of Mediterranean ecosystems: The balance between organic and inorganic components of increased soil CO2 efflux. Glob. Chang. Biol. 2009, 15, 1289–1301. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Piao, S.; Janssens, I.A.; Tang, J.; Liu, W.; Chi, Y.; Wang, J.; Xu, S. Soil respiration under climate warming: Differential response of heterotrophic and autotrophic respiration. Glob. Chang. Biol. 2014, 20, 3229–3237. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.J.; O’Neill, E.G.; Chambers, M.L.S.; Riggs, J.S.; Joslin, J.D.; Wolfe, M.H. Soil respiration and litter decomposition. In North American Temperate Deciduous Forest Responses to Changing Precipitation Regimes; Hanson, P.J., Wullschleger, S.D., Eds.; Springer: New York, NY, USA, 2003; pp. 163–189. [Google Scholar]
- Tewary, C.; Pandey, U.; Singh, J. Soil and litter respiration rates in different microhabitats of a mixed oak-conifer forest and their control by edaphic conditions and substrate quality. Plant Soil 1982, 65, 233–238. [Google Scholar] [CrossRef]
- Boone, R.D.; Nadelhoffer, K.J.; Canary, J.D.; Kaye, J.P. Roots exert a strong influence on the temperature sensitivityof soil respiration. Nature 1998, 396, 570–572. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Cisneros-Dozal, L.M.; Trumbore, S.E.; Hanson, P.J. Effect of moisture on leaf litter decomposition and its contribution to soil respiration in a temperate forest. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- DeForest, J.L.; Chen, J.; McNulty, S.G. Leaf litter is an important mediator of soil respiration in an oak-dominated forest. Int. J. Biometeorol. 2009, 53, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ataka, M.; Kominami, Y.; Jomura, M.; Yoshimura, K.; Uematsu, C. CO2 efflux from leaf litter focused on spatial and temporal heterogeneity of moisture. J. For. Res. 2014, 19, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Ge, X.; Zeng, L.; Huang, Z.; Lei, J.; Zhou, B.; Li, M. Rates of litter decomposition and soil respiration in relation to soil temperature and water in different-aged Pinus massoniana forests in the Three Gorges Reservoir area, China. PLoS ONE 2014, 9, e101890. [Google Scholar] [CrossRef] [PubMed]
- Birch, H. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Jarvis, P.; Rey, A.; Petsikos, C.; Wingate, L.; Rayment, M.; Pereira, J.; Banza, J.; David, J.; Miglietta, F.; Borghetti, M. Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: The “Birch effect”. Tree Physiol. 2007, 27, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Tree nutrition and forest fertilization of pine plantations in the southern United States. South. J. Appl. For. 2007, 31, 5–11. [Google Scholar]
- Knorr, W.; Prentice, I.; House, J.; Holland, E. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Mattson, K.G.; Zaerr, J.B.; Marshall, J.D. Root respiration of Douglas-fir seedlings: Effects of N concentration. Soil Biol. Biochem. 1998, 30, 331–336. [Google Scholar] [CrossRef]
- Gough, C.; Seiler, J.; Maier, C.A. Short-term effects of fertilization on loblolly pine (Pinus taeda L.) physiology. Plant Cell Environ. 2004, 27, 876–886. [Google Scholar] [CrossRef]
- Thirukkumaran, C.M.; Parkinson, D. Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol. Biochem. 2000, 32, 59–66. [Google Scholar] [CrossRef]
- Van Gestel, M.; Merckx, R.; Vlassak, K. Microbial biomass responses to soil drying and rewetting: The fate of fast-and slow-growing microorganisms in soils from different climates. Soil Biol. Biochem. 1993, 25, 109–123. [Google Scholar] [CrossRef]
- Borken, W.; Davidson, E.; Savage, K.; Gaudinski, J.; Trumbore, S.E. Drying and wetting effects on carbon dioxide release from organic horizons. Soil Sci. Soc. Am. J. 2003, 67, 1888–1896. [Google Scholar] [CrossRef]
- Will, R.E.; Fox, T.; Akers, M.; Domec, J.C.; Gonzalez-Benecke, C.; Jokela, E.J.; McGuire, M.A. A range-wide experiment to investigate nutrient and soil moisture interactions in loblolly pine plantations. Forests 2015, 6, 2014–2028. [Google Scholar] [CrossRef]
- NCDC.NOAA.gov. Monthly Summaries. Lynchburg, VA, USA. Available online: http://www.ncdc.noaa.gov/cdo-web/search (accessed on 27 November 2013).
- Soil Series Classification Database, USDA Natural Resources Conservation Service. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/class/?cid=nrcs142p2_053583 (accessed on 27 November 2015).
- Heim, B.C.; Seiler, J.R.; Strahm, B.D. Root nonstructural carbohydrates and their relationship with autotrophic respiration of loblolly pine (Pinus taeda L.). Commun. Soil Sci. Plan. 2015, 46, 888–896. [Google Scholar] [CrossRef]
- Saxton, K.; Rawls, W.J.; Romberger, J.; Papendick, R. Estimating generalized soil-water characteristics from texture. Soil Sci. Soc. Am. J. 1986, 50, 1031–1036. [Google Scholar] [CrossRef]
- Van Genuchten, M.T.; Leij, F.J.; Yates, S.R. The RETC Code for Quantifying the Hydraulic Functions of Unsaturated Soils; U.S. Environmental Protection Agency: Washington, DC, USA, 1991.
- Pikul, J., Jr. Soil water, gravimetric measurement. In Encyclopedia of Water Science; Stewart, B.A., Howell, T., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 879–881. [Google Scholar]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Fierer, N.; Allen, A.S.; Schimel, J.P.; Holden, P.A. Controls on microbial CO2 production: A comparison of surface and subsurface soil horizons. Glob. Chang. Biol. 2003, 9, 1322–1332. [Google Scholar] [CrossRef]
- Schnürer, J.; Clarholm, M.; Boström, S.; Rosswall, T. Effects of moisture on soil microorganisms and nematodes: A field experiment. Microbial Ecol. 1986, 12, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Brookes, P. The proportional mineralisation of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol. Biochem. 2005, 37, 507–515. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Chang. Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Kieft, T.L. Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol. Biochem. 1987, 19, 119–126. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci. Soc. Am. J. 2003, 67, 798–805. [Google Scholar] [CrossRef]
- Unger, S.; Máguas, C.; Pereira, J.S.; David, T.S.; Werner, C. The influence of precipitation pulses on soil respiration—Assessing the “Birch effect” by stable carbon isotopes. Soil Biol. Biochem. 2010, 42, 1800–1810. [Google Scholar] [CrossRef]
- Wu, H.-J.; Lee, X. Short-term effects of rain on soil respiration in two New England forests. Plant Soil 2011, 338, 329–342. [Google Scholar] [CrossRef]
- Griffiths, E.; Birch, H. Microbiological changes in freshly moistened soil. Nature 1961, 189, 424. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.-L.; Wang, H.; Guo, C.; Bao, W. Rainfall pulse primarily drives litterfall respiration and its contribution to soil respiration in a young exotic pine plantation in subtropical China. Can. J. For. Res. 2012, 42, 657–666. [Google Scholar] [CrossRef]
- Šnajdr, J.; Valášková, V.; Merhautová, V.; Herinková, J.; Cajthaml, T.; Baldrian, P. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol. Biochem. 2008, 40, 2068–2075. [Google Scholar] [CrossRef]
- Butterly, C.; Bünemann, E.; McNeill, A.; Baldock, J.; Marschner, P. Carbon pulses but not phosphorus pulses are related to decreases in microbial biomass during repeated drying and rewetting of soils. Soil Biol. Biochem. 2009, 41, 1406–1416. [Google Scholar] [CrossRef]
- Foster, R. Microenvironments of soil microorganisms. Biol. Fertil. Soils 1988, 6, 189–203. [Google Scholar] [CrossRef]
- Lado-Monserrat, L.; Lull, C.; Bautista, I.; Lidón, A.; Herrera, R. Soil moisture increment as a controlling variable of the “Birch effect”: Interactions with the pre-wetting soil moisture and litter addition. Plant Soil 2014, 379, 21–34. [Google Scholar] [CrossRef]
- Tyree, M.C.; Seiler, J.R.; Maier, C.A. Contrasting genotypes, soil amendments, and their interactive effects on short-term total soil CO2 efflux in a 3-year-old Pinus taeda L. plantation. Soil Biol. Biochem. 2014, 69, 93–100. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Fahey, T.J.; Horton, T.R.; Lovett, G.M. Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 2002, 83, 104–115. [Google Scholar] [CrossRef]
- Nilsson, L.O.; Wallander, H. Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol. 2003, 158, 409–416. [Google Scholar] [CrossRef]
- Bittman, S.; Forge, T.A.; Kowalenko, C.G. Responses of the bacterial and fungal biomass in a grassland soil to multi-year applications of dairy manure slurry and fertilizer. Soil Biol. Biochem. 2005, 37, 613–623. [Google Scholar] [CrossRef]
- Burton, A.J.; Pregitzer, K.S.; Crawford, J.N.; Zogg, G.P.; Zak, D.R. Simulated chronic NO3− deposition reduces soil respiration in northern hardwood forests. Glob. Chang. Biol. 2004, 10, 1080–1091. [Google Scholar] [CrossRef]
- Swanston, C.; Homann, P.S.; Caldwell, B.A.; Myrold, D.D.; Ganio, L.; Sollins, P. Long-term effects of elevated nitrogen on forest soil organic matter stability. Biogeochemistry 2004, 70, 229–252. [Google Scholar] [CrossRef]
- Olsson, P.; Linder, S.; Giesler, R.; Högberg, P. Fertilization of boreal forest reduces both autotrophic and heterotrophic soil respiration. Glob. Chang. Biol. 2005, 11, 1745–1753. [Google Scholar] [CrossRef]
- Gallardo, A.; Schlesinger, W.H. Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol. Biochem. 1994, 26, 1409–1415. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Moscatelli, M.C.; De Angelis, P.; Grego, S. Labile substrates quality as the main driving force of microbial mineralization activity in a poplar plantation soil under elevated CO2 and nitrogen fertilization. Sci. Total Environ. 2006, 372, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Gartner, T.B. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Ajwa, H.; Dell, C.; Rice, C. Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol. Biochem. 1999, 31, 769–777. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Nohrstedt, H.-Ö.; Arnebrant, K.; Bååth, E.; Söderström, B. Changes in carbon content, respiration rate, ATP content, and microbial biomass in nitrogen-fertilized pine forest soils in Sweden. Can. J. For. Res. 1989, 19, 323–328. [Google Scholar] [CrossRef]
- Cusack, D.F.; Torn, M.S.; McDowell, W.H.; Silver, W.L. The response of heterotrophic activity and carbon cycling to nitrogen additions and warming in two tropical soils. Glob. Chang. Biol. 2010, 16, 2555–2572. [Google Scholar] [CrossRef]
- Orchard, V.A.; Cook, F. Relationship between soil respiration and soil moisture. Soil Biol. Biochem. 1983, 15, 447–453. [Google Scholar] [CrossRef]
- Carbone, M.S.; Still, C.J.; Ambrose, A.R.; Dawson, T.E.; Williams, A.P.; Boot, C.M.; Schaeffer, S.M.; Schimel, J.P. Seasonal and episodic moisture controls on plant and microbial contributions to soil respiration. Oecologia 2011, 167, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wan, S.; Su, B.; Hui, D.; Luo, Y. Response of soil CO2 efflux to water manipulation in a tallgrass prairie ecosystem. Plant Soil 2002, 240, 213–223. [Google Scholar] [CrossRef]
- McIntyre, R.E.; Adams, M.A.; Ford, D.J.; Grierson, P.F. Rewetting and litter addition influence mineralisation and microbial communities in soils from a semi-arid intermittent stream. Soil Biol. Biochem. 2009, 41, 92–101. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McElligott, K.M.; Seiler, J.R.; Strahm, B.D. The Impact of Water Content on Sources of Heterotrophic Soil Respiration. Forests 2017, 8, 299. https://doi.org/10.3390/f8080299
McElligott KM, Seiler JR, Strahm BD. The Impact of Water Content on Sources of Heterotrophic Soil Respiration. Forests. 2017; 8(8):299. https://doi.org/10.3390/f8080299
Chicago/Turabian StyleMcElligott, Kristin M., John R. Seiler, and Brian D. Strahm. 2017. "The Impact of Water Content on Sources of Heterotrophic Soil Respiration" Forests 8, no. 8: 299. https://doi.org/10.3390/f8080299




